Case Presentation
J.T. is a 38-year-old female P0 with a clinical stage IA, grade 2, left breast intraductal carcinoma ER + (65%), PR + (95%) HER2-, Ki-67 15% left breast intraductal carcinoma that presents two weeks after her initial diagnosis. Her treatment plan involves upfront surgery, with adjuvant therapy to be determined based on her intraoperative findings. She is referred for an oncofertility consultation to discuss the potential impact of her upcoming cancer treatment on her fertility, as she may be interested in having children in the future.
Introduction
Worldwide, approximately 9 million women were diagnosed with cancer in 2020, and 9.6% were under the age of 40 [1]. Younger patients with cancer commonly present with more advanced disease due to delays in diagnosis, higher uninsured rates, and higher prevalence of aggressive disease [Reference Miller, Fidler-Benaoudia and Keegan2]. Fortunately, cancer mortality rates in adolescent and young adults (AYAs; typically defined as ages 15–39 years) and all age groups, have been declining since at least 1975. The AYA five-year relative survival rates are 94% or greater for many of the most common cancers such as thyroid, melanoma, and Hodgkin’s lymphoma.
As survival rates improve, there is an increased focus on the complex issues surrounding cancer survivorship, particularly for younger women of reproductive age. Among young women diagnosed with cancer, concerns regarding future fertility are secondary only to concerns regarding survival [Reference Loscalzo and Clark3, Reference Carvalho, Kliemchen and Woodruff4]. One study found that among childless cancer survivors, over 75% endorsed wanting children in the future, yet only 6% had undergone fertility treatment [Reference Schover, Rybicki, Martin and Bringelsen5]. Guidelines from the American Society of Clinical Oncology (ASCO) and American Society for Reproductive Medicine (ASRM) state that healthcare providers should discuss the risk of infertility and fertility preservation options with all reproductive age patients diagnosed with cancer [6, Reference Oktay, Harvey and Partridge7]. While the majority of males diagnosed with cancer receive information regarding treatment impact on fertility, 40–62% of reproductive age women diagnosed with cancer have reported not receiving fertility counseling at diagnosis or report unmet fertility needs [Reference Chin, Howards, Kramer, Mertens and Spencer8–Reference Armuand, Rodriguez-Wallberg and Wettergren11]. Unaddressed fertility concerns may significantly impact quality of life among reproductive-aged female cancer survivors [Reference Benedict, Thom and Friedman9]. One qualitative study exploring survivor preference surrounding fertility concerns asked women how they would like to learn about fertility issues, and one prevalent theme was that survivors wanted their doctors or another healthcare provider to initiate a discussion about their options [Reference Gorman, Bailey, Pierce and Su12]. Receipt of counseling regarding fertility preservation has been shown to reduce long-term regret and dissatisfaction amongst cancer survivors and may be associated with both improved physical and psychological quality of life [Reference Letourneau, Ebbel and Katz13–Reference Benedict, Thom and Kelvin15].
Fortunately, current oncology care has evolved from a primary focus on cancer treatment to a comprehensive model that includes survivorship issues. With advancements in screening, diagnostic tools and effective treatments, life after cancer becomes increasingly important for optimizing patient care. The term oncofertility was coined in the early 2000s and represents an interdisciplinary approach to closing the information, data, and option gaps in the care of reproductive age cancer survivors [Reference Woodruff and Snyder16]. Through oncofertility counseling, life-preserving treatments are balanced with fertility preservation options with a focus on reproductive survivorship care. The psychological, social, physical, and emotional changes that occur following cancer treatment are now a focus, as patients are expected to live well beyond their cancer treatment years.
Cancer treatment may include a combined approach of surgery, radiation therapy (proton and photon), and chemotherapy. These treatment modalities may have a detrimental effect on fertility based on the type of treatment and agent(s), number of treatment cycles, cumulative dosing, and timing between treatment cycles [17]. Additional factors such as patient age at time of cancer treatment and the patient’s baseline fertility status also influence future reproductive capacity. The relative probability of a childhood female cancer survivor having a child is reduced by approximately 50% compared to siblings, and the 10-year cumulative postdiagnosis parenthood rate is only 14% among patients diagnosed with cancer at the age of 15–45 [Reference Madanat, Malila and Dyba18, Reference Magelssen, Melve, Skjaerven and Fossa19]. Therefore, it is imperative that healthcare providers counsel patients about the impact of cancer treatment on future fertility and provide options for fertility preservation.
Fertility Assessment
A baseline fertility assessment includes obtaining a thorough history, targeted physical examination, bloodwork, and pelvic ultrasound. Comprehensive medical and surgical history should be obtained including gynecologic history such as a detailed menstrual history, date of last menstrual cycle, current contraception, menarche, cycle interval and length, ovulation and obstetric history (gravidity, parity, time to previous pregnancies, and mode of delivery). Prior fertility attempts, testing, and treatment history should be elicited. Physical examination should include basic vital signs, body mass index (BMI), thyroid, breast, and pelvic examinations with attention to uterine size, shape, position, adnexal masses, or tenderness.
While age remains the most important overall predictor of reproductive potential and live birth rates, laboratory and ultrasound evaluation to determine ovarian reserve is a key aspect of a baseline fertility evaluation in cancer patients (Table 1.1). Ovarian reserve represents the total number of healthy, immature oocytes available within the ovaries. Ovarian reserve testing includes both ultrasound imaging and hormonal measures to predict reproductive potential. Transvaginal ultrasound examination is performed to determine the antral follicle count (AFC), ovarian volume, and uterine characteristics. AFC describes the total number of small follicles that measure between 2 and 10 mm in diameter with transvaginal ultrasound [20]. Normal range for antral follicle count is broad, depending on the patient’s history. For example, women with polycystic ovary syndrome may have an AFC of >40 on transvaginal ultrasound assessment, which would be normal for that disease physiology. AFC of at least 7 is considered normal. AFC of less than 5–7 is consistent with diminished ovarian reserve [20]. This value is especially important when considering treatment options for fertility preservation, as it correlates with expected oocyte recruitment during in vitro fertilization (IVF).
| Test | Values indicative of DORa | Timing in menstrual cycle | Advantage | Effect of hormone therapies |
|---|---|---|---|---|
| AFCb | <5–7 | Days 2–5 | Good predictive value for response to ovarian stimulation | AFC levels may be slightly decreased |
| AMHc | <1 ng/mL | Any day | High sensitivity; good predictive value for response to ovarian stimulation; minimal variability during menstrual cycle | Levels decrease with GnRHa, but effect of other hormone therapies is low |
| FSHd | >10 IU/L | Days 2–5 | High specificity for poor response to ovarian stimulation response; readily available | FSH serum levels decrease |
| Estradiole | >60–80 pg/mLd | Days 2–5 | Increases sensitivity of FSH in predicting diminished ovarian reserve | Levels may be altered |
aDOR, diminished ovarian reserve;
bAFC, antral follicle count, the number of follicles that are 2–10mmin both ovaries as assessed by transvaginal ultrasonography;
cAMH, antimullerian hormone;
dFSH, follicle stimulating hormone, can be used as an alternative to AMH in combination with estradiol;
eElevated estradiol levels can be indicative of DOR when abnormal FSH is masked, thereby increasing sensitivity for detecting DOR
Hormonal measures of ovarian reserve include serum follicle-stimulating hormone (FSH), estradiol, inhibin B, and anti-mullerian hormone (AMH). AMH is a glycoprotein product of small ovarian follicles. FSH, estradiol and inhibin B must be measured in the early follicular phase (typically days 2–5 of a menstrual cycle) to provide an accurate assessment of ovarian reserve. These tests are not reliable while taking combination oral contraceptive pills, and often require a several-week “wash out’ before values can be interpretable. In contrast to these traditional markers of ovarian reserve, AMH levels are independent of menstrual cycle phase; although may be slightly reduced in women taking combined oral contraceptive pills [Reference Landersoe, Larsen and Forman21]. A recent cross-sectional study that reviewed AMH levels in over 27,000 women found lower AMH levels with women utilizing oral contraceptive pills, as well as the vaginal ring, hormonal intrauterine device, implant and progesterone-only when compared to women not using any hormonal contraceptive [Reference Hariton, Shirazi and Douglas22]. An AMH greater than 1.0 ng/mL in an adult female indicates good ovarian reserve while values less than 1.0 ng/mL indicate diminished ovarian reserve. Individuals with higher AMH values before cancer treatment may be more likely to regain ovarian function following cancer treatment [Reference Dezellus, Barriere and Campone23–Reference Dillon, Sammel and Prewitt26]. While AMH is the gold-standard marker of ovarian reserve and a valuable predictor of response to ovarian stimulation in women undergoing IVF, there is conflicting data on its ability to predict future live birth rates. Not surprisingly, AMH levels and live birth rates diminish remarkably at age 40 and beyond. However, for younger women (under age 35) AMH levels have little influence on live birth rate prediction [Reference Goswami and Nikolaou27]. Prospective data have also confirmed that there is no association with AMH and natural fecundity in the general population [Reference Zarek, Mitchell and Sjaarda28]. Thus, AMH must be interpreted in conjunction with other indices including age and AFC to provide a more informative assessment of ovarian reserve. Despite its limitations, baseline ovarian reserve testing is helpful to counsel patients regarding the expected success of oocyte/embryo banking and for comparison after cancer treatment.
Reproductive Effects of Treatment
Chemotherapy
Chemotherapy administration may result in permanent cessation of menses, and the risk is typically quantified as high (>80%), intermediate (20%–80%), or low (<20%). Patient-related factors such as age and baseline fertility, as well as cumulative dose and cycle schedule, affect this risk [Reference Nelson29]. For traditional chemotherapies, the alkylating agents pose the highest risk for deleterious effects on fertility by inducing double-stranded DNA breaks in oocytes, resulting in direct ovarian toxicity [Reference De Vos, Devroey and Fauser30, Reference Schüring, Fehm and Behringer31]. Alkylating agents with high ovarian toxicity include chlorambucil, cyclophosphamide, ifosfamide, melphalan B sulfate, nitrogen mustard, and procarbazine (Figure 1.1) [Reference Wan, Gai, Li, Tao and Zhang32, Reference Dolmans33]. Platinum agents (cisplatin, carboplatin, oxaliplatin) also carry a moderate to high risk for oocyte/ovarian damage. In contrast, the antimetabolites and vinca alkaloids, such as vincristine and methotrexate, are classified as low risk for ovarian toxicity [Reference De Vos, Devroey and Fauser30, Reference Bedoschi, Navarro and Oktay34]. Specific chemotherapy agents can also vary in their level of ovarian toxicity, based on patient age. For example, women older than 40 years of age receiving doxorubicin are at higher risk of ovarian toxicity, whereas those younger than 30 years are at lower risk [Reference Reynolds and McKenzie35].
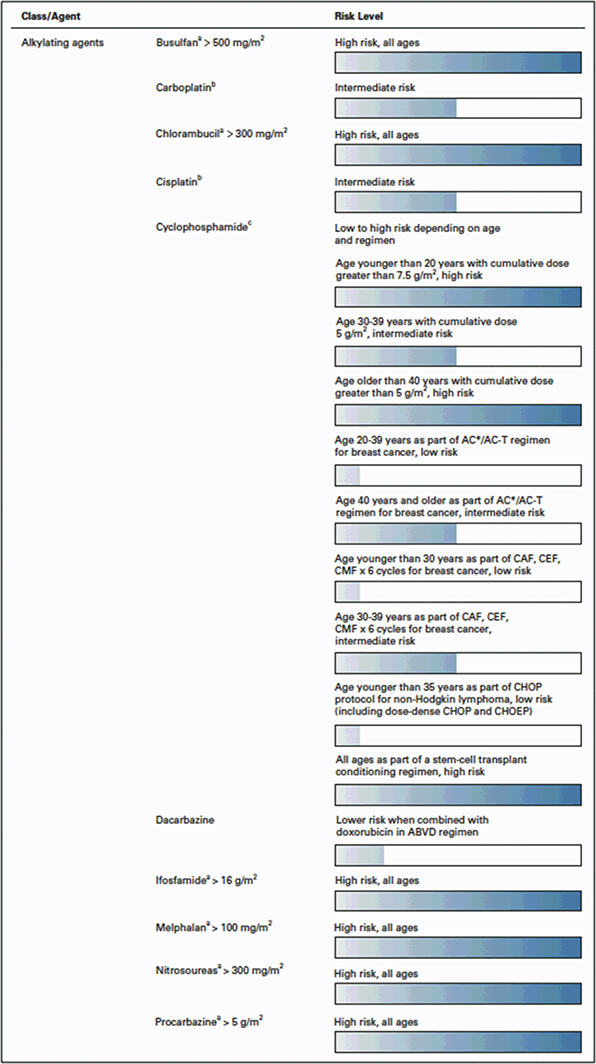

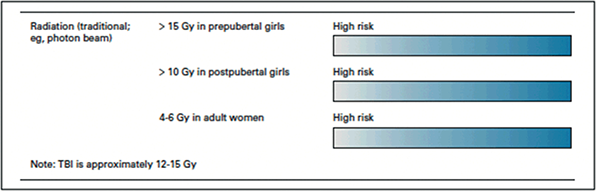
Figure 1.1 Chemotherapy-induced ovarian toxicity [Reference Reynolds and McKenzie35] Reprinted by permission by Reynolds et al. JCO 2023 [Reference Reynolds and McKenzie35]
Several methods can be utilized to assess ovarian function following treatment with systemic chemotherapeutic agents. Women with ovarian toxicity following chemotherapy will often present with menstrual irregularities (oligomenorrhea and amenorrhea) and vasomotor symptoms, including hot flushes and night sweats. However, regular menstrual cycles do not necessarily indicate normal fertility potential. Patients with diminished ovarian reserve may have regular menstrual cycles for a time period prior to experiencing menstrual cycle changes. Diminished ovarian reserve may initially present as hormonal abnormalities such as an increase in FSH or decrease in AMH before progressing into clinical symptoms of irregular or absent menses [Reference Reynolds and McKenzie35]. Patients who have undergone gonadotoxic therapy may develop primary ovarian insufficiency (POI). POI (formerly known as premature ovarian failure) is a diagnosis defined by menstrual irregularity for at least three to four consecutive months and a FSH >40 IU/L on 2 separate measurements (spaced at least one month apart) prior to age 40 [Reference De Vos, Devroey and Fauser30, Reference Reynolds and McKenzie35, Reference Mauri, Gazouli and Zarkavelis36].
Proposed Mechanisms for Chemotherapy-Induced Ovarian Toxicity
It is difficult to predict the effect of chemotherapy on fertility, however, multiple mechanisms have been proposed regarding the underlying pathophysiology of chemotherapy-induced gonadotoxicity. Ovarian tissue ischemia may occur as a result of vasoconstriction or inhibition of angiogenesis [Reference Mauri, Gazouli and Zarkavelis36]. DNA cross-linking/intercalation or inhibition of protein synthesis through DNA methylation inhibition leads to ovarian follicle apoptosis. Follicular death may also arise from a disruption in follicular cycling, as inhibition of microtubule assembly can arrest follicles in metaphase [Reference Bedoschi, Navarro and Oktay34]. Destruction of larger follicles may decrease AMH, which is responsible for suppression of the primordial (early) follicular pool. With the drop in AMH, primordial follicles are then activated and subsequently recruited in an effort to replace the loss of growing follicles. The primordial follicular pool represents a finite, nongrowing population; therefore, once it is depleted, follicles are not replaced [Reference Reynolds and McKenzie35, Reference Mauri, Gazouli and Zarkavelis36].
Radiation
The human oocyte is exquisitely sensitive to radiation. It is estimated that the lethal dose of radiation required to eliminate 50% of oocytes (LD50) is 2 Gray (Gy) [Reference Arian, Goodman, Flyckt and Falcone37]. A radiotherapy dose of 45–50 Gy induces POI in greater than 90% of patients [Reference Schüring, Fehm and Behringer31]. Prescribed radiation doses vary based on cancer type and stage at diagnosis. For example, in colorectal cancer (CRC) treatment, cumulative radiation doses can typically approximate 50 Gy [Reference Shandley and McKenzie38]. For cancer that require treatment with total body irradiation, radiation doses can be estimated at 12–14 Gy. POI would be expected with both of these examples.
Pituitary or hypothalamic exposure to radiation can result in gonadotropin deficiency, precocious puberty and/or hyperprolactinemia. The degree of neurotoxicity depends on the total dose, fraction size and duration of radiation. Gonadotropin deficiency is typically a late complication of radiation administration with a cumulative incidence of approximately 20–30% during long term follow up, irrespective of childhood versus adulthood exposure [Reference Darzy and Shalet39]. Fortunately, however, gonadotropin(s) can be replaced exogenously and allow a pregnancy to occur. Proton beam as opposed to photon radiation may confer less neurotoxicity, although reproductive outcomes are lacking.
While the ovaries are much more susceptible to radiation-induced damage compared to the uterus, the uterus can also be negatively impacted. Histological changes following radiation include edema of the uterine serosa, uterine atrophy, and abnormal vascularity particularly in the prepubertal patient [Reference Teh, Stern, Chander and Hickey40]. However, the adult uterus is susceptible as well. Uterine radiotherapy doses of more than 5 Gy in a postpubertal females confer a relative risk of 2.48 for infertility, increase in the incidence of premature births (OR = 3.5, 95% CI: 1.5–8.0), low birth weight (OR = 6.8, 95% CI: 2.1–22.2) and small for gestational age (OR = 4.0, 95% CI: 1.6–9.8) when compared to women with no history of radiotherapy [Reference Barton, Najita and Ginsburg41]. Unfortunately, there is no consensus on the dose above which a pregnancy is not sustainable. It has been suggested that pregnancy is contraindicated with doses exceeding 45 Gy in adulthood and 25 Gy in childhood [Reference Teh, Stern, Chander and Hickey40].
Immunotherapies/Other
Evidence of ovarian toxicity from immunotherapy and targeted agents, such as small-molecule inhibitors, is limited and mixed. Immunotherapies, particularly ipilimumab (either alone or in combination with other immune checkpoint inhibitors), have been linked to risk of hypophysitis, which can lead to menstrual cycle irregularity and infertility [Reference Azem, Amit, Merimsky and Lessing42–44]. Small-molecule inhibitors, such as tyrosine kinase inhibitors and mammalian target of rapamycin (mTOR) inhibitors, have limited reproductive data in humans, but may have a negative impact on ovarian reserve. Tyrosine kinase inhibitors (TKI) specifically have shown varied ovarian effects; however, most clinical case series suggest that they cause reversible changes in ovarian function. A recent study assessing 361 childhood outcomes following TKI exposure in women at the time of conception, found twice the baseline incidence of teratogenicity. Clinical observational studies show an association of menstrual disorders and ovarian cysts during treatment with mTOR inhibitors, specifically sirolimus [Reference Kerr, Hutt and Cook45–Reference Braun, Young and Reiner47]. Reassuringly, normal menstrual cycles were restored within a few months after treatment cessation [Reference Reynolds and McKenzie35].
Monoclonal antibodies, such as the anti-angiogenic agent of bevacizumab, have unknown impact on long-term fertility. In 2011, the FDA required the addition of a revised package insert warning of detrimental fertility effects as a result of a study involving 179 colon cancer patients exposed to bevacizumab [Reference Azem, Amit, Merimsky and Lessing42]. Those utilizing bevacizumab + FOLFOX (combination of leucovorin calcium, fluorouracil and oxaliplatin) experienced a 34% ovarian failure rate compared to 2% that did not utilize bevacizumab [43]. Ovarian function returned in approximately 20% of women after discontinuation of bevacizumab therapy. In contrast, trastuzumab, a monoclonal antibody targeting human epidermal growth factor receptor, has not been shown to increase risk of ovarian failure [44].
There is a notable increase in the use of immunomodulatory drugs, and the data regarding fertility impact is exceedingly scarce. Hopefully the next several years will yield data regarding reproductive outcomes in humans.
Fertility-Sparing Approaches for Gynecologic Malignancies
Endometrial Cancer
Endometrial cancer is the most common gynecologic cancer affecting women. While it more commonly affects postmenopausal women, the overall prevalence continues to rise among women younger than age 45. Standard-of-care approach includes surgical staging with hysterectomy, bilateral salpingo-oophorectomy (BSO) and pelvic and para-aortic lymph node assessment. Current society guidelines permit the consideration of fertility-sparing management with progestin therapy in patients with stage IA, grade 1 (well-differentiated) endometrioid type endometrial cancer. Pretreatment magnetic resonance imaging (MRI) should be obtained confirming no evidence of metastatic disease or myometrial invasion. In addition, if the initial diagnosis is based on an office endometrial biopsy, dilation and curettage should then be performed as it has been shown to have a better diagnostic performance in determining cancer grade [Reference Leitao, Kehoe and Barakat48]. Fertility-sparing treatment of grade 2 endometrioid endometrial cancer is only reported in very small case studies and should only be considered in highly selected individuals with a shared decision-making approach [Reference Falcone, Leone Roberti, Maggiore and Di Donato49].
A progestin-containing regimen with or without hysteroscopic resection is the recommended fertility-sparing treatment of appropriately selected endometrial cancer patients. Regimens include the use of a levonorgestrel-releasing intrauterine system (LNG-IUS) alone and/or in combination with oral progestins (most commonly medroxyprogesterone acetate or megestrol acetate). Serial surveillance endometrial biopsies are obtained every three months to monitor treatment response. The majority of patients will respond with reported complete response rates ranging from 64–88% with median time to response two to nine months [Reference Obermair, Janda and Baker50–Reference Simpson, Feigenberg and Clarke53]. Risk of recurrence is 20–40% and once childbearing is complete, definitive standard-of-care surgical treatment should be strongly considered [Reference Gunderson, Fader and Carson51, Reference Ramirez, Frumovitz and Bodurka54].
In patients undergoing definitive surgical staging including hysterectomy and lymph node dissection, ovarian preservation is a reasonable option in patients with early-stage, low grade tumors. A large recent study reported that ovarian preservation in women under age 50 at time of endometrial cancer surgery was a safe option, and was associated with decreased risk of death due to cardiovascular disease and improved overall survival [Reference Matsuo, Machida and Shoupe55]. In patients wishing to pursue pregnancy, IVF could later be performed to produce embryos for transfer into a gestational carrier.
Endometrial cancer patients encompass a group of women who often have other baseline health and fertility challenges such as obesity, diabetes, hypertension, polycystic ovarian syndrome (PCOS), anovulation, and irregular menses. These same factors may also negatively impact one’s ability to conceive and maintain a healthy pregnancy. Oral progestin therapy is associated with weight gain; therefore, for these women, a LNG-IUS may be a better first line treatment choice. Lifestyle counseling regarding diet, exercise, nutrition, and weight loss are important and patients may benefit from referral to a weight loss team and/or bariatric surgeon. Optimizing overall health and metabolic status increases the likelihood of conception and decreases the risk of miscarriage, fetal anomalies, and maternal morbidity during pregnancy [56].
Cervical Cancer
Cervical cancer is often diagnosed in reproductive age women, with 37% of new cervical cancer cases in women younger than age 45 [57]. Radical trachelectomy with lymph node assessment is an acceptable alternative in those who desire future fertility [Reference Koh, Abu-Rustum and Bean58]. Potential candidates for a fertility-sparing surgical approach include the following: squamous or adenocarcinoma histology, lesion less than or equal to 2 cm, no deep stromal invasion, and no evidence of lymph node involvement or distant metastatic disease [Reference Machida, Iwata and Okugawa59, Reference Sonoda, Abu-Rustum and Gemignani60]. Overall, oncologic outcomes are excellent for fertility-sparing surgery in cervical cancer with no reported difference in overall or disease-free survival rates [Reference Zhang, Li and Kanis61, Reference Prodromidou, Iavazzo and Fotiou62]. A large systematic review including 2,777 fertility-sparing procedures for cervical cancer and 944 pregnancies reported fertility, live birth, and prematurity rates of 55%, 70%, and 38%, respectively [Reference Bentivegna, Maulard and Pautier63]. Routine completion hysterectomy is not recommended after childbearing is complete, but given lack of data, it may be considered based on individual patient factors.
It is important to stress that a radical trachelectomy procedure does not ensure future conception, and increased rates of preterm births have been reported. Infertility rates post-procedure range from 14–41%, and many patients will require insemination of sperm or IVF to conceive [Reference Plante, Gregoire and Renaud64, Reference Shah, Jooya and Woodard65]. Cervical stenosis is a major cause of post-trachelectomy infertility. Management of cervical stenosis can be challenging and require additional procedures to correct the issue, which are not always successful. If the stenosis is severe, hematometra may develop resulting in the need for chronic menstrual suppression and/or completion hysterectomy. With appropriate patient selection, radical trachelectomy in early-stage cervical cancer is safe with acceptable reproductive outcomes.
Ovarian Cancer
Most ovarian cancer cases are diagnosed in postmenopausal women, with only 12% of new cases arising in women under 45 years of age [66]. Ovarian cancer is the most lethal gynecologic cancer as it is often diagnosed at advanced stages, however younger women are more likely to present with earlier-stage disease and have a better prognosis [Reference Hanatani, Yoshikawa and Yoshida67]. For patients with tumors of low malignant potential, nonepithelial ovarian tumors and stage I epithelial ovarian cancer, fertility-sparing surgery is an acceptable option. A fertility sparing approach may include an ovarian cystectomy or unilateral salpingo-oophorectomy, omentectomy, peritoneal washings, pelvic and paraaortic lymphadenectomy, and peritoneal biopsies with preservation of the uterus and contralateral ovary. The extent of surgical staging varies depending on the individual ovarian tumor histology. Unlike endometrial and cervical cancer, the pathology of the tumor is typically unknown preoperatively. Patients must be counseled on the potential etiologies including benign, borderline, and malignant tumors as well as standard treatment options including both conventional and fertility-sparing treatment options. Intraoperative decision-making is required based on operative findings and frozen section pathology so it is essential to obtain as much information as possible preoperatively about a patient’s desire to preserve fertility. It is also imperative that a patient understands that frozen section pathology may differ from final pathology and a two-step procedure may be necessary in some cases [Reference Shah, Mackelvie and Gershenson68, Reference Park, Lee and Kim69].
Oncologic outcomes after fertility-sparing treatment in select patients with ovarian cancer are reassuring based on observational data. A prospective analysis of fertility sparing surgery in patients with nonepithelial ovarian cancer was not associated with worse oncologic outcomes and demonstrated equivalent five-year overall and progression free survival rates [Reference Johansen, Dahm-Kähler and Staf70]. In epithelial ovarian cancer however, the safety of fertility sparing surgery in patients with high-risk features such as stage IC disease or other high grade histologies is debated [Reference Sinno, Fader and Roche71, Reference Fruscio, Ceppi and Corso72]. A large cohort study using the National Cancer Database demonstrated that fertility sparing surgery in stage IA or unilateral stage IC epithelial ovarian cancer was not associated with an increased risk of death when compared to conventional surgery; however, the total number of patients with high-risk histology was relatively low [Reference Melamed, Rizzo and Nitecki73]. Patients with stage IC epithelial ovarian cancer or other high-risk features should be counseled with caution given the paucity of oncologic safety data. Completion hysterectomy and bilateral salpingo-oophorectomy should be considered after childbearing is complete based on individual tumor characteristics.
Fertility Preservation Options: Surgical Approaches
Ovarian Transposition
One surgical option for women undergoing pelvic radiation therapy who desire future fertility is ovarian transposition (oophoropexy). Ovarian transposition involves mobilizing one or both ovaries and attaching them to the sidewall of the abdomen at the pelvic brim. Ovarian transposition may be performed either open or laparoscopically, although the laparoscopic approach is increasingly preferred due to less postoperative pain, faster recovery, and shorter hospital stay [Reference Ben-Aharon, Granot and Meizner74, Reference Kye and Cho75]. Even with oophoropexy, the ovaries are not without risk of damage, as they can still receive 8–15% of the prescribed dose of radiation due to scatter and transmission through a pelvic shield [Reference Tulandi and Al-Took76,Reference Wo and Viswanathan77].
Methods to assess success of oophoropexy vary. Scant data exist regarding pregnancy rates and outcomes in those attempting pregnancy after oophoropexy and subsequent pelvic radiotherapy for CRC. Spontaneous pregnancies following ovarian transposition in patients with CRC have been reported, although the cases are exceedingly limited [Reference Farber, Ames, Rush and Gal78]. A series of 11 women who underwent ovarian transposition prior to pelvic radiotherapy for Hodgkin’s lymphoma reported 14 pregnancies among the 11 women, with 12 live births [Reference Terenziani, Piva, Meazza, Gandola, Cefalo and Merola79]. Separate meta-analyses have reported that ovarian transposition in women younger than 40 years is associated with an 70–88.6% chance of fertility preservation [Reference Bisharah and Tulandi80, Reference Iwase, Nakamura, Nakahara, Goto and Kikkawa81]. Once the ovaries are mobilized out of the pelvis, future conception may require an abdominal approach to access the ovaries for assisted reproductive technologies or a second surgery to restore the ovaries to their original anatomic position.
Uterine Transposition and Fixation
Ovarian transposition may move the ovaries out of the radiation field but still leave the uterus vulnerable to radiotoxicity. An emerging fertility-sparing technique is uterine transposition and fixation [Reference Köhler, Marnitz, Biel and Cordes82]. Uterine transposition involves repositioning the uterus into the upper abdomen to avoid radiation exposure and then repositioning it in the pelvis after treatment [Reference Mossa, Schimberni, Di Benedetto and Mossa83, Reference Nezhat and Falik84]. This surgery can be performed laparoscopically and involves transecting the round ligament at the pelvic sidewall, separating the broad ligament, ligating the uterine arteries, and separating the cervix from the vagina. The uterus is then transposed to the upper abdomen and fixed to the anterior abdominal wall, followed by attaching the cervix to the fascia near an umbilical incision. While the technique for uterine transposition has been demonstrated, it is considered experimental and data regarding subsequent pregnancy outcomes are exceedingly limited.
Ovarian Suppression
Gonadotropin-releasing hormone (GnRH) agonists, such as leuprolide acetate, are often used as a means of fertility preservation in patients with cancer who are undergoing chemotherapy. Several mechanisms have been proposed by which GnRH agonists may be protective of fertility, including FSH suppression leading to a decreased number of primordial follicles entering development, hypoestrogenism causing a decrease in ovarian perfusion and therefore lower exposure of the ovaries to cytotoxic chemotherapeutic agents, and a direct effect on the ovary that protects the germline stem cells [Reference Harada and Osuga85, Reference Chen, Li, Cui and Hu86]. Despite these proposed mechanisms, GnRH use has not been definitively shown to improve fertility outcomes and remains controversial [Reference Bildik, Akin and Senbabaoglu87]. A meta-analysis of 11 RCTs with 1,062 participants demonstrated a greater number of women treated with a GnRH agonist resumed menses after chemotherapy (pooled OR 2.57, 95% CI 1.65, 4.01), but subgroup analysis failed to show a difference in spontaneous pregnancy rates between those who did and did not receive GnRH agonist during chemotherapy (pooled OR 1.77, 95% CI 0.92, 3.40) [Reference Shen, Zhang and Lv88]. Most of the research assessing GnRH agonists as a protective agent has been performed in women with breast cancer. Consequently, the most recent ASCO guidelines from 2018 recommend that patients be offered GnRH agonist treatment if there is high likelihood of chemotherapy-induced ovarian failure; however, patients should be extensively counseled regarding the conflicting data of its efficacy, and GnRH agonists should not be used to replace other proven fertility preservation methods [Reference Oktay, Harvey and Partridge7].
Although GnRH agonists are most commonly employed for gonadal protection in this patient population, they (in addition to medroxyprogesterone or oral contraceptives) may be used for menstrual suppression during cancer treatments [Reference Coccia, Pappo and Beaupin89, 90]. For hematologic malignancies, avoiding menses is of central importance given the bleeding risks associated with thrombocytopenia. Importantly, menstrual suppression with GnRH agonist therapy is not a reliable form of contraceptive and additional protection against pregnancy should be administered throughout chemotherapy treatment. Multiple contraceptive options are available, depending on the type of malignancy and hormone sensitivity, including barrier contraception, intrauterine device with or without progestin, implants or oral medications (see Chapter 6 for further discussion).
GnRH agonist treatment ideally should begin before the initiation of chemotherapy for best efficacy [Reference Harada and Osuga85, Reference Chen, Li, Cui and Hu86] and can be administered in monthly doses or every 12 weeks via intramuscular injections (Table 1.2). A moderate or heavy menses can occur approximately two weeks after the first injection. This can be alleviated by initiating combination oral contraceptive pills at the same time as the leuprolide. Patients with central nervous system tumors are recommended to avoid use of GnRH agonists due to alteration in the seizure threshold associated with this medication [Reference Coccia, Pappo and Beaupin89]. For patients with breast cancer, the initial administration of medication may result in worsening of cancer symptoms due to a transient increase in estradiol production (i.e. “flare effect”) resulting in bone pain or neuropathies. Patients with vertebral bone lesions should be monitored carefully for signs of spinal cord compression [Reference Coccia, Pappo and Beaupin89]. In patients with thrombocytopenia, the medication may be administered subcutaneously in lieu of the intramuscular injection. Platelet levels of ≥ 50,000 are recommended for intramuscular administration [90]. GnRHa therapy induces a hypoestrogenic state within two weeks of initiation, and the most common adverse side effects are bone density loss with long-term use and vasomotor symptoms.
| Therapy | Dosage |
|---|---|
| Progestin only – Oral Therapy | |
| Medroxyprogesterone acetate | 10–20 mg/day |
| Norethindrone acetate | 5–15 mg/day |
| Drospirenone | 4 mg/day |
| Norethindrone | 0.35 mg/day |
| Gonadotropin-releasing hormone agonists – Injection Therapy | |
| Goserelin acetate | 3.6 mg/28 days |
| Leuprolide acetate | 3.75 mg/month or 11.25 mg/3 months |
Fertility Preservation: Assisted Reproductive Technology (ART)
Oocyte and Embryo Cryopreservation
Current established methods of fertility preservation in postpubertal females include oocyte, embryo, and ovarian tissue cryopreservation. Oocyte and/or embryo cryopreservation are the most established, successful methods of preserving fertility and require an approximate two-week window prior to the initiation of cancer treatments. Typically, 8–12 days of recombinant FSH and luteinizing hormone (LH) are administered to facilitate oocyte recruitment and development followed by transvaginal oocyte harvest. Transvaginal aspiration of the oocytes is a thirty-minute outpatient procedure often performed under conscious sedation. Once the oocytes are harvested, they are assessed for maturity and either cryopreserved as oocytes, or fertilized, cultured and cryopreserved as embryos. Oocyte and/or embryo cryopreservation also allow the opportunity for preimplantation genetic testing (PGT) of embryos for monogenic disorders, which is particularly relevant for those with hereditary cancer.
Random-start IVF protocols allow a patient to initiate fertility treatment immediately regardless of menstrual cycle phase. Random start protocols have been shown to produce similar oocyte and embryo yields when compared to traditional follicular (early menstrual cycle) phase protocols [Reference Cakmak and Rosen91]. A recent comparison of live birth rates between cryopreserved oocytes and embryos revealed a slightly lower survival rate for thawed oocytes than embryos (79.1% versus 90.1%), but similar fertilization rates (76.2% versus 72.8%), clinical pregnancy rates (26.5% versus 30%) and live birth rates (25% versus 25.1%) [Reference Ho, Woo and Louie92]. Given the high circulating estradiol levels that result from fertility medications, and the concern for stimulating estrogen-sensitive tumors, ovarian stimulation protocols have been developed that utilize anti-estrogenic drugs, such as letrozole, to minimize estradiol levels [Reference Oktay, Buyuk and Libertella93]. In addition, careful monitoring is required during ART to avoid complications such as ovarian hyperstimulation syndrome which could further delay cancer treatment.
Ovarian Tissue Cryopreservation
Despite the current use of random start protocols, women diagnosed with cancer who wish to preserve their fertility through embryo or oocyte cryopreservation still require a two-week timeframe to complete a treatment cycle. Ovarian tissue cryopreservation (OTC) exists as an option specifically for patients in whom oocyte harvest is unfeasible due to time constraints and/or for prepubertal females. Previously, OTC was considered an experimental fertility preservation option, but ASRM removed its experimental label in 2019.
Ovarian tissue cryopreservation typically involves laparoscopic removal of a portion of one or both ovaries and then the tissue is sectioned into strips of tissue less than 2 mm thick and cryopreserved [94]. This procedure can often be coordinated with other planned surgeries such as port or central line placement, tumor resection, or bone marrow aspiration, particularly in the prepubertal or pediatric population [Reference Smith, Gracia, Sokalska and Moore95]. The ovarian tissue is then subsequently reimplanted to the patient following completion of treatment with gonadotoxic agents. Transplantation may be performed in an orthotopic manner by reimplantation into the pelvis which is preferable, or a heterotopic (extra pelvic) manner with transplantation to the forearm or abdominal wall [Reference Fritz and Speroff96].
In a 2017 review, just over 130 live births were documented from OTC and reimplantation [Reference Kihara, Yamamoto, Ohshiro and Fujita97–Reference Gellert, Pors and Kristensen100]. A more recent study, published in 2021, reported an additional 95 healthy newborns from a cohort of 285 women who underwent OTC with ovarian tissue transplantation. In this same study by Dolmans et al. ovarian tissue transplantation restored endocrine function in 88.7% (181/204) of patients [Reference Dolmans, von Wolff and Poirot101]. Ovarian function typically returns within 4.5 +/- 2.2 months post-tissue transplant and lasts an average of five years. Pregnancy success rates range from 23–40% in most reports [Reference Gellert, Pors and Kristensen100–Reference Fisch and Abir102]. Of the births and pregnancies where data was available, the conception rate is comparable between those who spontaneously conceived and those that underwent in vitro fertilization.
Although data is limited, there are concerns with the cost of OTC as a surgical option for fertility preservation. The incurred costs are often higher than oocyte cryopreservation, particularly when considering the additional costs of reimplantation of the previously cryopreserved tissue.
There are also specific ethical issues involved when completing fertility preservation in the prepubertal or pediatric population. Minors do not have the ability to truly “consent” for a procedure but instead can only “assent.” Although parents most often have their child’s best interest at heart, there can be discrepancy in future reproductive desires. Additional complex challenges with OTC are chain of custody for the tissue if the patient does not survive. The legal system in the United States has identified gametes as “property”; however, cryopreserved ovarian tissue is an organ rather than a gamete. This is a legal area which is largely unexplored and is currently being handled on an individual basis [Reference Resetkova, Hayashi, Kolp and Christianson103].
Another concern regarding ovarian tissue transplantation in oncology patients is the risk of reintroduction of malignant cells [Reference Dolmans, Luyckx, Donnez, Andersen and Greve104]. In patients with leukemia, ovarian tissue transplantation is not recommended as harvested ovarian tissue can demonstrate infiltration with leukemic cells. In patients with active leukemia, immunohistochemical and genetic markers have been utilized to determine the presence of malignant cells in cryopreserved ovarian tissue through polymerase chain reaction (PCR)-based platforms [Reference Rosendahl, Andersen and Ralfkiær105]. In other cancer types, such as breast cancer, PCR testing did not detect malignant cells in ovarian tissue [Reference Rosendahl, Wielenga and Nedergaard106]. These findings suggest that PCR testing of ovarian tissue prior to reintroduction may be an option. However, the presence of malignant cells through PCR testing in ovarian cortex tissue previously cryopreserved does not appear to fully determine the malignant potential of those cells when reimplanted. At a minimum, histological examination of the cryopreserved ovarian tissue prior to reimplantation is recommended [Reference Rosendahl, Greve and Andersen107]. To avoid the risk of reimplantation of occult tumor cells, in vitro maturation (IVM) techniques hold future promise in the ability to mature oocytes from ovarian tissue ex vivo. In vitro maturation would avoid the need for a reimplantation procedure, however, such technology is not yet widely available [Reference Dolmans, von Wolff and Poirot101].
Third Party Reproduction and Adoption
For those unable to conceive with their own oocytes, third party reproductive options, such as oocyte donation, embryo donation, gestational surrogacy, or child adoption, should be offered. Patients can work with a fertility specialist or adoption agency to pursue their desired options for family-building. Donor and gestational carrier agencies can help patients access donor oocytes, embryos, or gestational carriers based on their needs with regard to family-building. Fertility specialists can facilitate gamete donation using known or anonymous donors. There are specific FDA-regulated requirements for all third-party reproduction, including infectious disease testing of donors and quarantine of certain donor samples. Over 60% of cancer survivors state a willingness to adopt if they could not have a biological child; adoption agencies assist with child adoption and foster-to-adopt programs [Reference Schover, Rybicki, Martin and Bringelsen5]. Counseling with a mental health professional with expertise in third-party reproduction is recommended. In addition to addressing the psychosocial impact associated with cancer-related infertility, this counseling can help patients process the family-building options available to them if they are unable to have a genetic child or carry a pregnancy.
Safety of Assisted Reproductive Technology in Patients with Cancer
Ovarian Stimulation
To develop multiple oocytes during oocyte or embryo banking cycles, recombinant FSH and LH are administered for approximately 8–12 days. During this 8–12-day window, estradiol levels are supraphysiologic often peaking in the range of 1500–3000 pg/ml. For patients with hormonally sensitive or responsive tumors, this supraphysiologic estradiol exposure has presented concerns for increased tumor recurrence/growth. However, adjuncts can be utilized to decrease plasma levels of estradiol throughout fertility treatment. Most commonly, aromatase inhibitors such as letrozole, are given throughout the stimulation to suppress estrogen levels. In the setting of fertility preservation, adding letrozole to gonadotropins during ovarian stimulation decreases serum estradiol levels to a physiologic range without compromising oocyte or embryo outcomes [Reference Cakmak and Rose108]. Other alternatives include natural cycle IVF (no exogenous gonadotropin exposure), which has high cancellation rates and low oocyte yield, or tamoxifen with or without gonadotropins exposure.
Ovarian stimulation for purposes of oocyte or embryo cryopreservation takes approximately two weeks, specifically with random-start protocols. Patients have expressed concern that fertility preservation would negatively impact their outcomes due to delaying the start of treatment, and providers often lack knowledge on the length of time required for fertility preservation. When assessing time to chemotherapy initiation in breast cancer patients, those that underwent fertility preservation started chemotherapy at the same time interval as those that declined fertility preservation [Reference Kitano, Shimizu and Yamauchi109]. Understanding that each cancer diagnosis is unique with regard to time to treatment, with few exceptions, the time required for fertility preservation treatment is often allowable.
Safety of Gonadotropin Exposure and Timing of Subsequent Pregnancy
There does not appear to be an increased risk of cancer recurrence in those who receive fertility medication before, during, or after their cancer treatment, although long-term data are limited [110]. Patients are often counseled to wait at least two years following cancer diagnosis to attempt conception as the vast majority of recurrences will occur within the first two years of diagnosis and treatment [Reference Srikanthan, Amir and Bedard111]. For patients with hormonally responsive breast cancer, adjuvant endocrine therapy, such as tamoxifen, GnRH agonists, and aromatase inhibitors are often recommended for up to 10 years. For women who would like to conceive prior to the completion of endocrine therapy, many clinicians have recommended a “drug holiday” after completion of two years to allow for conception efforts. Recent results of the POSITIVE study (Pregnancy Outcome and Safety of Interrupting Therapy for Women with Endocrine Responsive Breast Cancer) demonstrated that women with early-stage breast cancer who interrupted endocrine therapy had the same breast cancer free interval as women that did not [Reference Partridge, Niman and Ruggeri112]. The median age for this patient sample was 37 years and all received endocrine therapy for at least 18 months but no more than 30 months. Other considerations when planning subsequent conception include overall risk of recurrence, health status including cardiovascular risk profile, adjuvant treatment length, residual fertility, and patient age at delivery.
Resources for Fertility Preservation
There are a variety of resources for patients seeking more information regarding cancer related fertility preservation. The Oncofertility Consortium website (https://oncofertility.msu.edu) provides a resource browser with over 300 patient facing materials on fertility preservation options for both men and women. Additionally, the website also offers access to a clinical navigator through their FertLine in order to locate the closest medical program that can offer fertility services for the patient. Oncofertility Consortium also sponsors SaveMyFertility (www.savemyfertility.org) – an online fertility toolkit for patients and providers. This website provides patient and provider pocket guides with counseling information on fertility preservation resources. Further, these materials are available in multiple languages and the brochures can be downloaded for reading and distribution.
Livestrong Fertility (www.livestrong.org/) provides patients with supportive measures such as access to community programs that discuss the daily struggles cancer patients may face and day-to-day concerns of survivors. Additionally, Livestrong Fertility has a dedicated program to provide financial support for patients pursuing fertility preservation and offers a guidebook to help patients and survivors navigate both emotional and physical needs throughout their cancer journey.
The Young Survival Coalition (https://youngsurvival.org) is a resource targeted to the unique needs of young women with breast cancer; specifically postdiagnosis fertility concerns. This platform offers patients a rich online community of support groups and discussion boards as well as educational materials for survivors. The American Society for Clinical Oncology (ASCO) shares its detailed guidelines and recommendations via its online platform at Cancer Net (www.cancer.net). This resource provides educational materials and support to cancer patients and their caregivers to help them make informed decisions on their therapy and future fertility. Other useful organizations include Chick Mission (www.thechickmission.org), Alliance for Fertility Preservation (www.allianceforfertilitypreservation.org), American Society for Reproductive Medicine (www.reproductivefacts.org) and Stupid Cancer (https://stupidcancer.org).
Conclusion
Approximately 10% of women diagnosed with cancer are of reproductive age, and future childbearing is a significant survivorship issue for many of them. Discussing their risk of cancer-related infertility as well as fertility preservation options will improve reproductive decision making and quality of life, while decreasing distress and long-term regret.
Case Resolution
J.T. reports regular menses with no prior fertility evaluation or treatment. She is not currently in a committed relationship and is inquiring about options for fertility preservation. Baseline ovarian reserve testing was obtained and found to be reassuring with an AMH of 1.2 ng/mL (normal 1.0–4.5 ng/mL) and an antral follicle count of 14 (normal greater than 7). In light of her advanced maternal age, treatment-related delay of pregnancy, and the potential for gonadotoxic adjuvant therapy, oocyte cryopreservation was recommended. Financial resources were provided and the logistics of a typical cycle were reviewed.
J.T. underwent a left lumpectomy with sentinel lymph node biopsy. Surgical pathology revealed an intraductal carcinoma measuring 2.5 cm, with mucinous and micropapillary features grade 2. Micrometastases noted in one of six lymph nodes. Adjuvant therapy with localized radiation was recommended, followed by Adriamycin and Cytoxan every two weeks (x 4 cycles) then weekly paclitaxel (x 12 cycles). Treatment plan included monthly Zoladex for menstrual suppression during adjuvant chemotherapy. Endocrine therapy for a minimum of five years was anticipated.
Two weeks postoperatively J.T. initiated a random start oocyte cryopreservation treatment cycle. She utilized recombinant gonadotropins (FSH and LH) for 10 days with concomitant letrozole to attenuate her estradiol levels. Peak estradiol level during her stimulation was 214 pg/ml and 16 mature oocytes were harvested transvaginally.
Although 16 cryopreserved oocytes does not guarantee her a live birth, it will provide her with an additional option for future conception. Conception is more difficult at maternal age 40 or 41 compared to 38 and dose-dense chemotherapy protocols may induce more ovarian toxicity compared to traditional dosing regimens. Menstrual suppression with a GnRH agonist may attenuate chemotherapy-induced ovarian toxicity but data is mixed in regards to degree of benefit.
Effective nonhormonal contraception is recommended and repeat ovarian reserve testing was scheduled for 18 months after completion of chemotherapy. J.T. expressed that she was very thankful she underwent fertility preservation prior to chemotherapy start, and stated she felt hopeful and better prepared to manage her cancer treatment journey.
Take Home Points
Approximately 10% of women diagnosed with cancer are of reproductive age.
Concerns regarding future fertility are secondary only to concerns regarding survival for many young adult women diagnosed with cancer.
Undergoing fertility preservation via oocyte or embryo banking takes approximately two weeks, which is allowable for the majority of cancer patients prior to initiation of cancer therapy.
For prepubertal girls or women in which a two-week delay in cancer therapy is not appropriate, ovarian tissue cryopreservation can be considered.
For patients with a known germline variant in a hereditary cancer gene, preimplantation genetic testing with IVF can be utilized to decrease the chance of an affected child.
Aromatase inhibitors (letrozole) are recommended in conjunction with gonadotropin administration in women with endocrine responsive tumors undergoing fertility preservation.
Baseline AMH and antral follicle counts correlate with number of oocytes procured with fertility preservation and can be employed for post-treatment assessment of ovarian reserve.






