Book contents
- Frontmatter
- Contents
- Preface to first edition
- Preface to second edition
- 1 Chemical equilibrium
- 2 Chemical thermodynamics
- 3 Chemical kinetics
- 4 Solution chemistry and aqueous equilibria
- 5 Acids and bases
- 6 Oxidation–reduction reactions
- 7 Photochemistry
- Appendix I International system of units (SI)
- Appendix II Some useful numerical values
- Appendix III Atomic weights
- Appendix IV Equilibrium (or dissociation) constants for some chemical reactions
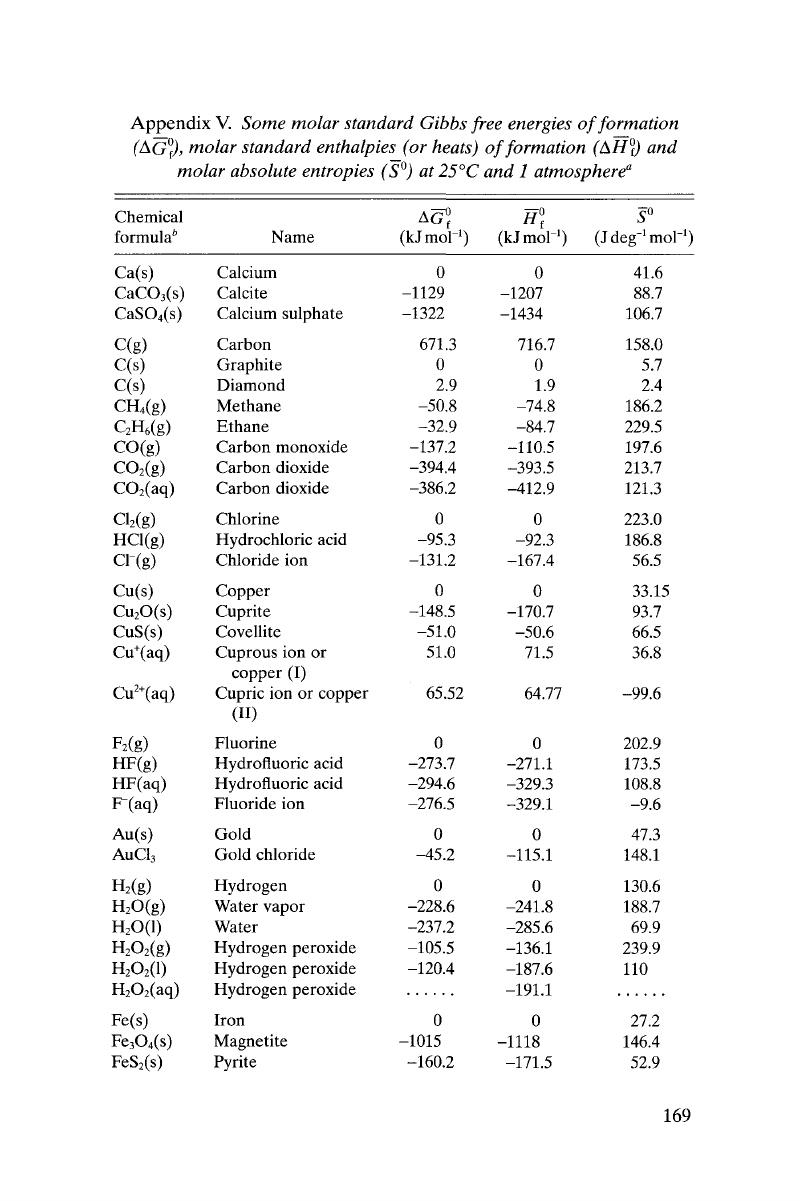
- Appendix V Some molar standard Gibbs free energies of formation, molar standard enthalpies (or heats) of formation, and molar absolute entropies at 25°C and 1 atmosphere
- Appendix VI Names, formulas, and charges of some common ions
- Appendix VII Answers to exercises and hints and solutions to selected exercises
- Index
Appendix V - Some molar standard Gibbs free energies of formation, molar standard enthalpies (or heats) of formation, and molar absolute entropies at 25°C and 1 atmosphere
Published online by Cambridge University Press: 05 June 2012
- Frontmatter
- Contents
- Preface to first edition
- Preface to second edition
- 1 Chemical equilibrium
- 2 Chemical thermodynamics
- 3 Chemical kinetics
- 4 Solution chemistry and aqueous equilibria
- 5 Acids and bases
- 6 Oxidation–reduction reactions
- 7 Photochemistry
- Appendix I International system of units (SI)
- Appendix II Some useful numerical values
- Appendix III Atomic weights
- Appendix IV Equilibrium (or dissociation) constants for some chemical reactions
- Appendix V Some molar standard Gibbs free energies of formation, molar standard enthalpies (or heats) of formation, and molar absolute entropies at 25°C and 1 atmosphere
- Appendix VI Names, formulas, and charges of some common ions
- Appendix VII Answers to exercises and hints and solutions to selected exercises
- Index
Summary
- Type
- Chapter
- Information
- Basic Physical Chemistry for the Atmospheric Sciences , pp. 169 - 171Publisher: Cambridge University PressPrint publication year: 2000

