Several hitherto unknown hydrates of magnesium selenate have been formed by quenching aqueous solutions of MgSeO4 in liquid nitrogen. MgSeO4·11H2O is apparently isostructural with the mineral meridianiite (MgSO4·11H2O), being triclinic, 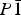 $P{\rm \bar 1}$, Z = 2, with unit-cell parameters a = 6.779 00(8) Å, b = 6.965 16(9) Å, c = 17.4934(2) Å, α = 87.713(1)°, β = 89.222(1)°, γ = 63.121(1)°, and V = 736.15(1) Å3 at −25 °C. MgSeO4·9H2O represents a new hydration state in the MgSeO4–H2O system; it is monoclinic, space-group P21/c, Z = 4, with unit-cell parameters a = 7.270 24(6) Å, b = 10.510 94(9) Å, c = 17.4030(2) Å, β = 109.447(1)°, and V = 1254.02(1) Å3 at −22 °C. The heavy-atom structure of MgSeO4·9H2O has been determined by direct-space methods from X-ray powder diffraction data and consists of isolated Mg(H2O)62+ octahedra and SeO42− tetrahedra linked by hydrogen bonds. The remaining three water molecules occupy the space between the polyhedral ions, contributing to the H-bonded network, which comprises 4-, 5-, and 6-membered rings. A third phase has been observed to crystallise prior to the 11-hydrate upon warming of liquid-nitrogen-quenched glass, but this transforms rapidly to the meridianiite-structured 11-hydrate and the identity of this phase is unclear.
$P{\rm \bar 1}$, Z = 2, with unit-cell parameters a = 6.779 00(8) Å, b = 6.965 16(9) Å, c = 17.4934(2) Å, α = 87.713(1)°, β = 89.222(1)°, γ = 63.121(1)°, and V = 736.15(1) Å3 at −25 °C. MgSeO4·9H2O represents a new hydration state in the MgSeO4–H2O system; it is monoclinic, space-group P21/c, Z = 4, with unit-cell parameters a = 7.270 24(6) Å, b = 10.510 94(9) Å, c = 17.4030(2) Å, β = 109.447(1)°, and V = 1254.02(1) Å3 at −22 °C. The heavy-atom structure of MgSeO4·9H2O has been determined by direct-space methods from X-ray powder diffraction data and consists of isolated Mg(H2O)62+ octahedra and SeO42− tetrahedra linked by hydrogen bonds. The remaining three water molecules occupy the space between the polyhedral ions, contributing to the H-bonded network, which comprises 4-, 5-, and 6-membered rings. A third phase has been observed to crystallise prior to the 11-hydrate upon warming of liquid-nitrogen-quenched glass, but this transforms rapidly to the meridianiite-structured 11-hydrate and the identity of this phase is unclear.

 ,
, 