Montmorillonite-water-cation systems were characterized using high-frequency impedance spectroscopy by studying the influence of the solid concentration and the nature of the exchangeable cation (Na+, K+, Ca2+) on the dielectric characteristics of the dispersions. A new method is proposed to calculate the relaxation frequency (fr) and the dispersion factor (α) from a limited number of impedance measurements. By comparison with rheology, microscopy, X-ray diffraction and immersion calorimetry results, it is shown that impedance spectroscopy is a very powerful technique which yields structural information on a complex system. For Na-montmorillonite, two transitions are observed at 2.5% and 3.6% in solids. The cation mobility and the number of connections between particles are described by fr and α, respectively. The two transitions can then be attributed to the formation of the gel and to the reduction of the macroporosity within the gel, respectively. For Ca-montmorillonite, thick layer-stacks form at the lowest concentrations, and connections between these stacks are observed at 9% in solids, in good aggrement with rheological measurements. The K-montmorillonite displays progressive thickening of the tactoids, and no formation of a unique connected network, as revealed by the smooth evolution of fr and α.

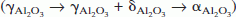 , on annealing. Coarsening of the alumina particles was accompanied by these phase transitions. The phase evolution is attributed to differences in free energies between the metastable and stable phases and a kinetic hierarchy in nucleation, brought about by structural and hence interfacial energy considerations.
, on annealing. Coarsening of the alumina particles was accompanied by these phase transitions. The phase evolution is attributed to differences in free energies between the metastable and stable phases and a kinetic hierarchy in nucleation, brought about by structural and hence interfacial energy considerations.