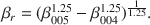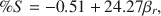The chemical composition of mixed-layer serpentine/chlorite (Sp/Ch) in Tuscaloosa Formation sandstone was analyzed by energy dispersive X-ray spectroscopy (EDX) in the scanning electron miscroscope (SEM) and by X-ray diffraction (XRD). EDX results indicate little depth-controlled variation in composition, whereas XRD results suggest distinct decreases in octahedral Fe and tetrahedral Al. XRD-determined compositions appear to be erroneous and actually reflect progressive changes in Sp/Ch unit-cell dimensions caused by polytype transformations of Ibb layers to Iaa layers in a mixed-layer Ibb/Iaa polytype. The relative lack of variation in Sp/Ch composition, especially when compared to other studies of chlorite minerals over similar temperature ranges, is attributed to a reaction mechanism whereby mineralogic transformations (serpentine layers to chlorite layers and Ibb layers to Iaa layers) occur on a layer-by-layer basis within coherent crystallites, rather than by dissolution-precipitation crystal growth.
The lack of titanium in chlorite minerals is attributed to high levels of octahedral Al3+ that prohibit inclusion of the highly charged Ti4+ in the octahedral sheet. Anatase (TiO2) in the Tuscaloosa Formation apparently formed when Ti was liberated during crystallization of Sp/Ch following the breakdown of a Ti-bearing precursor (detrital ultramafic clasts and/or odinite). Odinite, an Fe-rich 7-Å phyllosilicate that forms in some shallow marine sands, apparently existed as a short-lived, poorly crystallized intermediary between dissolution of the ultramafic clasts and formation of Sp/Ch.