A synergistic effect of reductant and complexant is observed in the dissolution of goethite by dithionite and citrate or EDTA. The rate data are interpreted using the surface complexation approach to describe the interface of the reacting oxide. Adsorption of both S2O42− (D) and complexant (L) generates three surface complexes that define the dissolution behavior: ≡ Fe-D, ≡ Fe-L, and dimeric 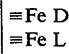 surface complexes. The initial rate increases at lower pH values because of increased surface complexation conditional formation constants. At pH values below 4, however, the fast decomposition of S2O42− gives rise to a rapid depletion of reductant, and total dissolution is not observed. It is shown that for best analytical results in soil analysis, EDTA is a better complexant than citrate; the iron extracted in one dithionite-EDTA treatment at pH 5–6, under N2 at 315 K is not increased by increasing the number of extractions, and is equivalent to the total extractable iron found by previous procedures.
surface complexes. The initial rate increases at lower pH values because of increased surface complexation conditional formation constants. At pH values below 4, however, the fast decomposition of S2O42− gives rise to a rapid depletion of reductant, and total dissolution is not observed. It is shown that for best analytical results in soil analysis, EDTA is a better complexant than citrate; the iron extracted in one dithionite-EDTA treatment at pH 5–6, under N2 at 315 K is not increased by increasing the number of extractions, and is equivalent to the total extractable iron found by previous procedures.

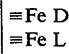 surface complexes. The initial rate increases at lower pH values because of increased surface complexation conditional formation constants. At pH values below 4, however, the fast decomposition of S
surface complexes. The initial rate increases at lower pH values because of increased surface complexation conditional formation constants. At pH values below 4, however, the fast decomposition of S