The metabolic syndrome (Mets) is characterised by the coexistence of risk factors predisposing to type 2 diabetes mellitus (T2DM) and CVD. It can contribute to a 5-fold increase in the risk of T2DM and double the risk of developing CVD over the next 5–10 years( Reference Kaur 1 ). These underlying risk factors which are deemed as Mets components include central obesity, hyperglycaemia, hypertension, raised serum TAG and decreased HDL-cholesterol( Reference Takami, Nakamoto and Uemura 2 ). It is a rampant and burgeoning health issue globally that affects about 20–25 % of the world’s adult population( 3 ). Several elements are implicated in the development of the Mets but insulin resistance and abdominal obesity have been identified as the major aetiological factors( 3 ). As chemical drugs have adverse side effects( Reference Razavi and Hosseinzadeh 4 ) and no single treatment has been identified for treating the Mets, there has been a growing interest in finding natural substances to curb the Mets( Reference Santana-Galvez, Cisneros-Zevallos and Jacobo-Velazquez 5 ).
Coffee is one of the most widely consumed beverages around the world( Reference Shang, Li and Jiang 6 ). It is rich in phenolic compounds which are acknowledged as protective agents against chronic degenerative diseases( Reference Ahmed, El-Ghamery and Samy 7 ). There is mounting evidence from epidemiological studies that coffee consumption correlates with a lower risk of developing the Mets( Reference Shang, Li and Jiang 6 ) and T2DM( Reference Meng, Cao and Feng 8 ). Chlorogenic acids (CGA) are the major phenolic compounds in coffee. In fact, CGA which are esters of certain cinnamic acids (caffeic, ferulic or coumaric acid) with quinic acid naturally occur in many plant foods( Reference Upadhyay and Mohan Rao 9 ) but coffee beans are their primary dietary source. As a substantial amount of CGA are lost during the roasting process, green coffee (GC) beans are a richer source of CGA( Reference Ludwig, Mena and Calani 10 ). It has been extensively demonstrated in animal studies that CGA possess anti-diabetes( Reference Meng, Cao and Feng 8 ), anti-obesity( Reference Cho, Jeon and Kim 11 ) and anti-lipidaemic properties( Reference Cho, Jeon and Kim 11 , Reference Ong, Hsu and Tan 12 ) and also could exert ameliorating effects on insulin resistance( Reference Ma, Gao and Liu 13 ). In addition, CGA have been reported to be capable of reducing blood pressure( Reference Onakpoya, Spencer and Thompson 14 , Reference Mubarak, Bondonno and Liu 15 ) and postprandial glucose absorption in human studies( Reference Thom 16 ).
GC has been proposed to have the potentiality to prevent from the Mets( Reference Sarriá, Martínez-López and Sierra-Cinos 17 ) and T2DM( Reference Varghese, Ho and Wang 18 ). Despite some null findings( Reference Li Kwok Cheong, Croft and Henry 19 ), some studies have demonstrated alleviating effects of GC on some of the Mets components such as blood pressure( Reference Revuelta-Iniesta and Al-Dujaili 20 – Reference Kozuma, Tsuchiya and Kohori 22 ), blood glucose( Reference Sarriá, Martínez-López and Sierra-Cinos 17 , Reference Choi, Park and Lee 23 , Reference Song, Choi and Park 24 ), lipid profile( Reference Ahmed, El-Ghamery and Samy 7 , Reference Choi, Park and Lee 23 , Reference Song, Choi and Park 24 ) and also main Mets aetiological factors including insulin resistance( Reference Sarriá, Martínez-López and Sierra-Cinos 17 , Reference Song, Choi and Park 24 , Reference Ho, Varghese and Wang 25 ) and obesity( Reference Choi, Park and Lee 23 , Reference Song, Choi and Park 24 , Reference Dellalibera, Lemaire and Lafay 26 , Reference Shimoda, Seki and Aitani 27 ). For instance, a recent study investigated effects of high-fat diet (HFD) ingestion with 50, 100 or 200 mg/kg green coffee bean extract (GCE) for 6 weeks on HFD-induced obese mice. In this study, a significant reduction in body weight gain, fat mass, glucose, TAG, LDL and total cholesterol (TC) concentration and significant elevation in HDL-cholesterol was seen mainly with 100 or 200 mg/kg GCE plus HFD compared with HFD alone( Reference Choi, Park and Lee 23 ). A cross-over study compared consumption of 40 g/d of green or black coffee in eighteen healthy subjects for 2 weeks. A significant decrease was seen regarding systolic blood pressure (SBP), body weight and BMI with GC compared with black coffee. Also, diastolic blood pressure (DPB), waist circumference and abdominal fat reduced after both interventions( Reference Revuelta-Iniesta and Al-Dujaili 20 ). In a clinical trial conducted on 117 mildly hypertensive males, 93 and 185 mg GCE could significantly reduce SBP and DBP after 28 d compared with placebo. However, this low dose GCE could not improve lipid profile or weight of the subjects( Reference Kozuma, Tsuchiya and Kohori 22 ). Also, a non-blinded and none-randomised clinical trial on fifteen healthy patients taking 600 mg (three capsules of 200 mg) of decaffeinated GCE (DGCE) for 40 d indicated decreased postprandial glycaemia and 3 pounds weight loss( Reference Blum, Lemaire and Lafay 28 ). One contradictory animal study which was conducted on mouse model of the Mets, exhibited no improvement in body weight, glucose tolerance and insulin resistance after a 12 week ingestion of 0·5 % (w/w) GCE plus HFD compared with a HFD-fed group( Reference Li Kwok Cheong, Croft and Henry 19 ).
Data concerning GCE impacts on Mets components are rather inconsistent. Most of the previous studies have been conducted on animal models and have assessed CGA effects rather than GCE impacts. Interventional studies regarding GCE effects are scarce and they have mostly limitations such as being non-randomised, non-blinded and not placebo-controlled or having low sample size and duration. Up to our knowledge, no clinical trial has been conducted on GCE effects on Mets patients so far. Thus, we aimed to carry out this clinical trial to elucidate GCE effects on anthropometric indices, glycaemic control, insulin resistance, blood pressure, lipid profile and appetite in patients with the Mets.
Methods
Green coffee extract characteristics and analytical assays
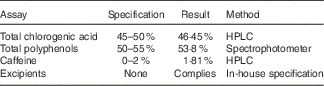
The DGCE and placebo capsules were provided by Arjuna Natural Extracts Ltd. The GCE was standardised with 46·45 % total CGA by HPLC. The total CGA identified comprised of 5-caffeoylquinic acid (5-CQA), 3-caffeoylquinic (3-CQA), 4-caffeoylquinic acid, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid, 3-feruloylquinic acid, 4-feruloylquinic acid and 5-feruloylquinic acid. 5-CQA and 3-CQA were the principal CGA which composed about 35–40 % and 10–15 % of the total CGA content, respectively. The raw material (GC bean) to extract ratio was 6:1. Hence, each 400 mg capsule of GCE was equal to 2400 mg GC bean and contained 186 mg of CGA. The total polyphenols percentage in the GCE supplements was 53·8 %. HPLC for measuring CGA content was performed by combining 25 mg of sample or standard with 15 ml of 70 % methanol in a 25 ml standard flask. Subsequently, the samples were injected into the HPLC system following filtering through a 0·2 μg membrane. Moreover, phenolic compounds were measured by spectrophotometric method by mixing 2 ml of 1N Folin CioCalteu’s reagent and 10 ml of sodium carbonate solution with 2 ml of dissolved sample or standard. Afterwards, UV absorbance was measured at 700 nm against water blank and the following calculation was used: total polyphenol=(sample absorbance×working standard weight×purity of working standard)/(working standard absorbance×sample weight).
The CGA rich extract was prepared by usage of alcohol and caffeine was removed from the extract by using chloroform. For preparing the extract, alcohol–water (70:30) is added to the powdered GC bean and it is stirred at the temperature of 45–55°C for 4 h. The process is repeated twice. Afterwards, the solvent portion is distilled off the alcohol and the aqueous layer is extracted with chloroform to eliminate caffeine. Thereafter, chloroform portion is separated and the aqueous portion is filtered and vaporised by spray drying to produce the dry powder. Table 1 shows the analytical assays of the extract. Placebo capsules were composed of starch with no additives or excipients.
Table 1 Analytical assays of the green coffee bean extract
Participants
This study was a randomised, double-blind, placebo-controlled trial. Men and women aged between 18 and 70 years whom were diagnosed with the Mets and had BMI of over 25 kg/m2 were chosen from the clients of Imam Hossein Hospital diabetes clinic in Tehran. The Mets was diagnosed according to the new International Diabetes Federation definition( 3 ) as having central obesity (waist circumference >102 cm in men or >88 cm in women) in conjunction with two of the following risk factors: fasting blood glucose (FBS)>100 mg/dl (>5·55 mmol/l), TAG>150 mg/dl (>1·69 mmol/l), HDL-cholesterol<50 mg/dl (<1·29 mmol/l) in women or <40 mg/dl (<1·03 mmol/l) in men, SBP>130 mmHg and DBP>85 mmHg. The subjects which met our exclusion criteria as insulin administration for controlling blood glucose, having hypo- or hyperthyroidism, renal failure, routine coffee consumption, pregnancy or breast-feeding, taking corticosteroids, hormone replacement therapy as taking oestrogen or progesterone, taking weight loss supplements or following unusual weight loss plans, cancer, experiencing cerebrovascular accident and other cognitive problems or chronic diseases that impaired their compliance were not included in the study. Also, the patients who altered the type or dose of the medications they used for controlling blood glucose, blood pressure or lipid profile were excluded. Moreover, if a patient had not consumed over 10 % of the supplements, he or she was eliminated from the study. This was assessed by counting the number of capsules remained in the bottle of supplements at the follow-up visits at the 4th and the 8th week of the study.
Study design
The procedure of the study was described for eligible clients of the diabetes clinic and a written informed consent was signed by the volunteers. A questionnaire regarding smoking status, present illnesses, drug history, prescribed medications, menopause status and duration of diabetes (if diabetic) was filled out by interviewing the volunteer patients. Subjects and investigators were blinded until the end of the study as the bottles of supplements were coded with A or B by the manufacturer before the study. Participants were stratified by sex and randomly allocated to the intervention or placebo group by stratified blocked randomisation method. Blocked randomisation was done with block sizes of four concealed in a container by one of the researchers. The blocks were composed of A and B characters representing bottles of capsules coded with A or B to ensure concealment. The other investigator randomly allocated the participants to one of the two groups. The patients were supposed to consume 400 mg of GCE or placebo twice per d (800 mg/d) with their main meals for 64 d. A bottle of supplements containing sixty-four capsules, adequate for 32 d, was given to both groups at the time of randomisation and the other bottle was given at the time of the 4th-week follow-up visit. All the subjects were instructed not to modify their physical activity and salt intake. In addition, a dietary plan with weight loss recommendations was handed to the participants of both groups to amend their nutritional habits. The proportion of macronutrients in the plan in relation to the total energy was 30 % total fat, 18 % protein and 52 % carbohydrate. This study was approved by the ethics committee of National Nutrition and Food Technology Research Institute of Shahid Beheshti University of Medical Sciences and was registered at ClinicalTrials.gov ID: NCT02764957.
Follow-up
Subjects were followed by making phone calls every 15 d to ensure that they complied with the supplementation protocol. Moreover, a follow-up visit was arranged for each individual in the middle of the study at the 4th week.
Dietary assessment
Dietary intake was assessed at baseline, at the 4th-week and at the end of the study using a 3-d food record. The participants were instructed how to record their food and beverage intake for 3 d at each time. In order to distinguish the accurate portion sizes, the patients were interviewed to report their intake based on household measures. Subsequently, the portion sizes were converted to grams and analysed for energy and nutrients content using Nutritionist 4 software, that was modified using the national composition food tables( Reference Ghaffarpour, Houshiar-Rad and Kianfar 29 ). Physical activity was assessed using the metabolic equivalent of task (MET) questionnaire( Reference Ainsworth, Haskell and Whitt 30 ) at the beginning and at the end of the trial. Also, appetite score was assessed by means of simplified nutritional appetite questionnaire (SNAQ)( Reference Wilson, Thomas and Rubenstein 31 ) which was validated by Wilson et al. for assessing appetite and prediction of weight loss. It consists of four questions about level of appetite, amount of food eaten to feel full, taste of foods and number of meals consumed in a day, each with five responses. Scoring of the questionnaire was based on assigning score of 1–5 to the first to fifth response, respectively. Sum of the scores of all questions were considered as the total appetite score. SNAQ score of 4–14, 15–20 and over 20 were assumed as low, moderate and high appetite level.
Measurement of anthropometric parameters and blood pressure
Weight, waist circumference, SBP and DBP of the subjects were measured and BMI was calculated at baseline and at the end of the intervention. Height was measured barefoot to the nearest 0·5 cm using a tape measure attached to the wall at baseline. Also, weight was measured with a precision of 100 g, wearing light clothes, using Seca digital scale. In addition, waist circumference was measured to the nearest 0·5 cm approximately between the lower margin of the last rib and top of the iliac crest at the level of navel with a tape measure. BMI was calculated by dividing weight (kg) by square of height (m2). Also SBP and DBP were measured twice after a 10-min rest using Citizen digital blood pressure monitor in order to assess blood pressure with 1 mmHg precision. The average of the two measurements was used for analysis.
Measurement of biochemical parameters
Following a 12-h fasting period, 10 ml of venous blood sample was drawn from the subjects before and after the intervention. The glycated Hb (HbA1c) test was performed on the whole blood sample by means of Biosystem commercial kits (Biosystems) using chromatography method on the day of taking the blood samples. The blood serum was obtained by centrifugation at a rate of 2000 round per min and was aliquoted into microtubes. Afterwards, the serums were frozen at −80°C until the time of conducting the experiments. Serum FBS, HDL-cholesterol, TC and TAG concentrations were measured by photometric enzymatic method by means of Pars Azmoon kits (Pars Azmoon). LDL-cholesterol concentration was calculated using Friedwald formula: LDL=TC−HDL−(TAG/5). Fasting insulin levels were assessed by Monobind Elisa kits (Monobind). Furthermore, insulin resistance was evaluated by homoeostatic model assessment (HOMA-IR) method using the following formula: HOMA-IR=(glucose (mg/dl)×insulin (μIU/ml))/405 or (glucose (mmol/l) × insulin (μIU/ml))/22·5( Reference Matthews, Hosker and Rudenski 32 ).
Statistical analyses
Statistical analyses were performed using the 21th version of SPSS Software. The Kolmogorov–Smirnov test was used to determine the normality of data distribution. Qualitative variables were compared between the groups by the χ 2 test. For quantitative variables, the means of the two groups were compared by the independent t test and changes within each group were analysed by the paired sample t test. Also, repeated-measures ANOVA was applied to compare within subjects’ dietary intake values of pre-trial, middle of the trial and post-trial in each group. All the tests were two-tailed, and P value of <0·05 was considered as the significance threshold. The quantitative variables are all expressed as means and standard deviations.
The minimum sample size estimated for each group was 20 at a power (1−β) of 80 % and significance level of 0·05 for a two-arm parallel study with two-tailed testing to detect a difference of 47 mg/dl (2·60 mmol/l) in mean values of FBS with a pooled standard deviation of 53 mg/dl (2·94 mmol/l), obtained from the study of Ebrahimi et al.( Reference Ebrahimi, Ghayour-Mobarhan and Rezaiean 33 ). The hypothesised Cohen’s d effect size calculated by dividing difference of means by pooled standard deviation was 0·8 according to the mentioned study( Reference Ebrahimi, Ghayour-Mobarhan and Rezaiean 33 ).
Results
From fifty subjects who were recruited in this trial (twenty-five in the intervention and twenty-five in the control group), forty-three individuals (twenty-one in the intervention and twenty-two in the control group) completed the study. Due to possibility of participant drop-out, the recruited sample (n 50) exceeded the calculated sample size (n 40). The reason why four persons withdrew the study in the intervention group was not consuming the supplements, having pruritis, getting stomachache and initiating insulin administration. Also, three individuals in the control group were excluded from the study due to not consuming the capsules. No major side effect was observed following taking GCE supplements. However, one of the patients who had stomachache history reported stomach irritation after consuming the capsules and dropped out of the study. Also, another patient reported dizziness which was resolved following increasing the time gap between the two doses taken per d.
The flow chart of study participants is illustrated in Fig. 1. Out of the forty-three participants having the Mets, thirty-four persons (79 %) were diabetic, whereas the other nine patients (21 %) did not suffer from diabetes. Baseline characteristics including sex, smoking status, age, duration of diabetes, menopause status and medications used for controlling blood glucose, blood pressure and lipid profile were not significantly different between the two groups (Table 2). Moreover, physical activity (MET-h/d) of the participants in the two groups did not have a significant difference at baseline and after the study (Table 3). Energy and nutrients intake were not significantly different between the two groups (Table 3).
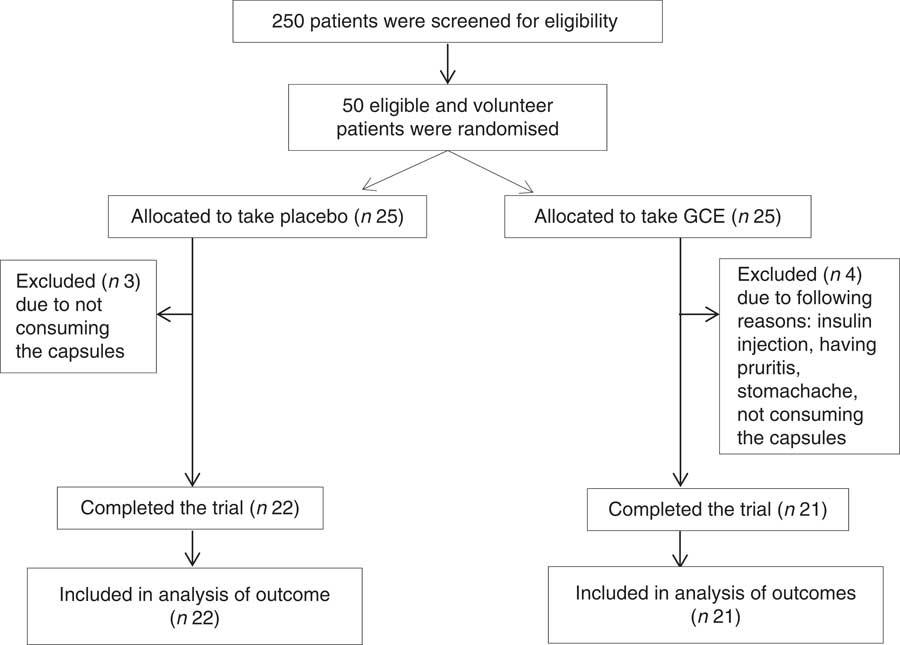
Fig. 1 Flow chart of study participants. GCE, green coffee extract.
Table 2 Baseline characteristics of participants in both study groups (Numbers and percentages; mean values and standard deviations)
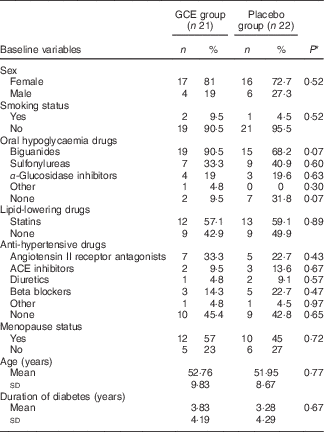
GCE, green coffee extract; ACE inhibitors; angiotensin-converting enzyme inhibitors.
* P values are for comparison of the variables between the two groups (all by χ 2 test, except for age and duration of diabetes which were analysed by independent t test).
Table 3 Dietary intakes and physical activity of the participants during the trial (Mean values and standard deviations)
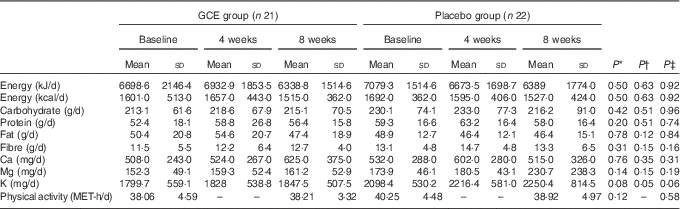
GCE, green coffee extract; MET, metabolic equivalent of task.
* P values are for comparison between the two groups at baseline (independent t test).
† P values are for comparison between the two groups after 4 weeks (independent t test).
‡ P values are for comparison between the two groups after 8 weeks (independent t test).
As expressed in Table 4, analysis indicated that SBP had significantly decreased within both groups compared with the baseline. However, SBP reduction in the intervention group was significantly more than the placebo group (−13·76 (sd 8·48) v. −6·56 (sd 9·58) mmHg) (P=0·01) (effect size: −0·79; 95 % CI −1·41, −0·17). In addition, post-trial mean DBP values of the GCE group were significantly 7·6 % lower than the placebo group (77·02 (sd 7·88) v. 83·36 (sd 11·02) mmHg) (P=0·03). Also, within the intervention group, DBP at the end of the trial was significantly 4·6 % less than the baseline (77·02 (sd 7·88) v. 80·8 (sd 9·56) mmHg) (P=0·02).
Table 4 Dependent variables before and after the study and their changes throughout the trial in both groups before and after the study (Mean values and standard deviations; effect sizes and 95% confidence intervals)
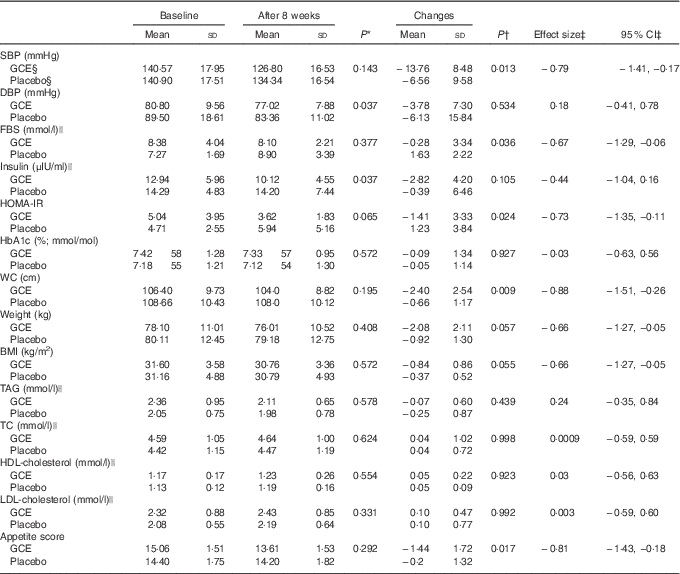
SBP, systolic blood pressure; GCE, green coffee extract; WC, waist circumference; DBP, diastolic blood pressure; FBS, fasting blood sugar; HOMA-IR, homoeostatic model assessment of insulin resistance; HbA1c, glycated Hb; TC, total cholesterol.
* P values are for comparison between the two groups after the intervention (independent t test).
† P values are for comparison of changes of each variable between the two groups (independent t test).
‡ Cohen’s d effect sizes and their 95 % CI are displayed for changes of variables during the study.
§ Total n for GCE group and placebo group were 21 and 22 subjects, respectively.
|| To convert insulin in μIU/ml to pmol/l, multiply by 6·945. To convert TAG, cholesterol and glucose in mmol/l to mg/dl, divide by 0·0113, 0·0259 and 0·0555, respectively.
Changes of FBS were different between the groups as GCE treatment had significantly attenuated FBS compared with the placebo (−5·15 (sd 60·22) v. 29·42 (sd 40·01) mg/dl) (−0·28 (SD 3·34) v. 1·63 (SD 2·22) mmol/l) (P=0·03) (effect size: −0·67; 95 % CI −1·29, −0·06). Furthermore, within the GCE group, fasting insulin levels had significantly diminished by 22 % (12·94 (sd 5·96) v. 10·12 (sd 4·55) μIU/ml) (P=0·006). Also, fasting insulin after GCE consumption was significantly 29 % less than the placebo group at the end of the study (10·12 (sd 4·55) v. 14·20 (sd 7·44) μIU/ml) (P=0·03). In addition, a significant difference existed between the two groups concerning HOMA-IR index changes (−1·41 (sd 3·33) v. 1·23 (sd 3·84)) (P=0·02) (effect size: −0·73; 95 % CI −1·35, −0·11). Nonetheless, HbA1c percentage did not differ between and within the groups after conducting the trial.
A significant reduction was observed in waist circumference of the participants who had consumed GCE compared with the placebo group (−2·40 (sd 2·54) v. −0·66 (sd 1·17) cm) (P=0·009) (effect size: −0·88; 95 % CI −1·51, −0·26). The post-trial mean weight and BMI of the patients had significantly decreased v. the pre-trial values by 2·5 % within the intervention group (weight: P=0·00, BMI: P=0·00) and by 1 % within the placebo group (weight: P=0·009, BMI: P=0·011). Hence, weight and BMI of the subjects consuming GCE had reduced almost twice as much as individuals consuming placebo capsules; however, this difference between the two groups was marginally significant (weight: −2·08 (sd 2·11) v. −0·92 (sd 1·30) kg, P=0·05) (BMI: −0·84 (sd 0·86) v. −0·37 (sd 0·52) kg/m2, P=0·05) (effect size: −0·66; 95 % CI −1·27, −0·05).
No significant discrepancy was observed in terms of lipid profile parameters consisted of serum TAG, TC, LDL-cholesterol and HDL-cholesterol concentrations between and within the groups after conducting the trial.
Appetite score analysis revealed that appetite level of the subjects who had consumed GCE had significantly attenuated in relation to the participants who had taken placebo capsules (−1·44 (sd 1·72) v. −0·2 (sd 1·32)) (P=0·02) (effect size: −0·81; 95 % CI −1·43, −0·18). Appetite score alterations within the GCE-administered group were also significant as the post-trial scores were 9·6 % lower than the baseline (15·06 (sd 1·51) v. 13·61 (sd 1·53)) (P=0·002).
Discussion
The present clinical trial was carried out to examine the effects of 800 mg/d of GCE supplementation for 8 weeks in patients with the Mets. A significant reduction was observed regarding SBP, FBS, HOMA-IR, waist circumference and appetite score after GCE administration compared with the placebo. Also, post-trial DBP and insulin values were lower in the GCE group. Moreover, weight loss and BMI reduction after GCE consumption was about twice as much as the placebo group; however, this discrepancy was marginally significant. GCE had no significant impact on lipid profile parameters and HBA1c.
The GCE dose used in our trial provided 372 mg of CGA/d. This amount of CGA can be achievable in the diet through coffee consumption. It has been estimated that coffee drinkers may have daily intake of 0·5–1·0 g CGA/d( Reference Thom 16 ). Each 1 g of dry Arabica or Robusta GC bean has been reported to provide 68·8 and 88 mg of CGA, respectively( Reference Upadhyay and Mohan Rao 9 ).
Our findings regarding SBP and DBP reduction with GCE are concurrent with previous studies. A trial on twenty-eight patients with mild hypertension who received 125 ml/d of a juice with 0·48 g GCE (containing 140 mg CGA) for 12 weeks indicated a significant decline in SBP and DBP compared with the group taking placebo juice( Reference Watanabe, Arai and Mitsui 21 ). In another clinical trial on 117 mildly hypertensive males, SBP and DBP significantly reduced after consuming 93 or 185 mg GCE for 28 d in relation to placebo( Reference Kozuma, Tsuchiya and Kohori 22 ). Also, a cross-over study( Reference Revuelta-Iniesta and Al-Dujaili 20 ) which compared intake of 40 g GC with 40 g black coffee, each taken for 2 weeks, revealed a significant reduction in SBP after GC intake compared with black coffee. However, DBP reduced after ingestion of both coffees. Meanwhile, GC could decrease urinary cortisol levels in this study by 39 %. It has been postulated that CGA inhibits 11β-hydroxysteroid dehydrogenase which converts cortisone to active cortisol. Cortisol is capable of increasing blood pressure by reducing production and bioavailability of nitric oxide, increasing sensitivity to vasoconstrictors such as endothelin and triggering Na absorption in kidneys to retain water. Hence, the hypotensive feature of GC could be attributed to its cortisol-lowering effect( Reference Revuelta-Iniesta and Al-Dujaili 20 ).
Moreover, GCE in our study was capable of mildly reducing FBS and suppressing its increase compared with placebo. This is consistent with other studies. For instance, Song et al.( Reference Song, Choi and Park 24 ) examined DGCE impact on mice fed a HFD diet with 0·1, 0·3 or 0·9 % GCE. The group with 0·3 % GCE (300 mg/kg diet equivalent to 1460 mg/60 kg for humans) plus the HFD diet indicated a significant decline in FBS compared with the HFD group after 11 weeks. Another study on diabetic rats compared GC effects with light and dark roasted coffee. It was observed that GC had the best effect on attenuating glucose level( Reference Ahmed, El-Ghamery and Samy 7 ). Also, a recent animal study displayed a significant decrease in FBS with 100 mg/kg GCE plus HFD diet in comparison to the HFD group after 6 weeks( Reference Choi, Park and Lee 23 ). The mechanism by which corresponds to lowering FBS by CGA is activation of AMP-activated protein kinase (AMPK). Activation of AMPK contributes to increasing GLUT4 translocation to plasma membrane which augments glucose transport to cells and leads to peripheral glucose disposal( Reference Ong, Hsu and Tan 34 ). Also, CGA in GCE can inhibit glucose-6-phosphatase (Glc-6-pase) by 36 % leading to limited glucose production by gluconeogenesis and glycogenolysis( Reference Henry-Vitrac, Ibarra and Roller 35 ). Our results also revealed that GCE supplementation significantly lowered HOMA-IR index and consequently abated insulin resistance. This has been corroborated by some of previous studies. In one study, 80 mg/kg of DGCE for 14 weeks resulted in improvement of HFD-induced insulin resistance in mice( Reference Ho, Varghese and Wang 25 ). Also, 0·3 % DGCE plus HFD for 11 weeks led to attenuation of HOMA-IR values in mice compared with a control group( Reference Song, Choi and Park 24 ). Nevertheless, one study conducted on the Mets model of mice did not detect any improvement in insulin resistance in mice fed a HFD diet with 0·5 % (w/w) GCE for 12 weeks( Reference Li Kwok Cheong, Croft and Henry 19 ). It has been proposed by Song et al.( Reference Song, Choi and Park 24 ) that GCE exerts its ameliorating effect on insulin resistance by decreasing phosphorylation of c-Jun N-terminal kinase which leads to activation of insulin receptor substrate-1 resulting in GLUT4 translocation to adipocyte membrane and increasing insulin sensitivity.
Our GCE treatment was not effective in reducing HbA1c percentage. On the contrary, in one study it was observed that 80 mg/kg per d of CGA administration for 12 weeks decreased HbA1c along with FBS in db/db mice by modulating adiponectin receptor signalling pathways( Reference Jin, Chang and Zhang 36 ). As the mentioned study appraised solely CGA’s effect on HbA1c, its results might be dissimilar with our study which explored GCE effects. Also, lipid profile parameters in our trial were not improved using GCE. This finding is concordant with some studies. For instance, in the aforementioned study examining GCE effects on HFD-induced the Mets model of mice for 12 weeks, GCE did not affect lipid profile( Reference Li Kwok Cheong, Croft and Henry 19 ). Likewise, 0·48 g GCE providing 140 mg CGA/d for 12 weeks did not yield any improvement in lipid profile( Reference Watanabe, Arai and Mitsui 21 ). However, some animal studies have shown positive effects. For example, in HFD-induced obese mice 200 mg/kg GCE plus HFD for 4 weeks could decrease TC, TAG and LDL-cholesterol while increasing HDL-cholesterol level compared with a HFD group( Reference Choi, Park and Lee 23 ).
Our study demonstrated that GCE could decrease weight and BMI about twice as much as the placebo; however, this difference was marginally significant. Furthermore, GCE in our study was capable of significantly reducing waist circumference compared with the placebo. Weight loss recommendations were given to both groups. Thus, this difference is attributed to GCE consumption. Some other studies have shown anti-obesity property of GCE. In a pilot clinical study on fifteen patients, 600 mg of DGCE for 40 d resulted in 1·36 kg weight loss( Reference Blum, Lemaire and Lafay 28 ). The aforementioned cross-over study comparing 40 g/d of black and GC, which is eight times more than the amount of GC used in our study, demonstrated a significant reduction of 1·4 kg in weight and 0·28 kg/m2 in BMI with GC after 2 weeks. Waist circumference and abdominal fat also decreased after both interventions. However, these effects may be partially due to caffeine aside from CGA( Reference Revuelta-Iniesta and Al-Dujaili 20 ). Also, a clinical trial conducted on thirty overweight patients detected a significant attenuation in weight after 12 weeks of consuming 11 g GCE-enriched coffee providing 1000 mg GCE and 500 mg CGA/d compared with an instant coffee group (5·4 (sd 0·6) kg v. 1·7 (sd 0·9) kg). The greater weight loss in this trial compared with our study might be due to the higher dose of GCE consumed( Reference Thom 16 ). In a recent study on HFD-induced obese mice 100 or 200 mg/kg GCE for 4 weeks plus HFD significantly reduced weight and body fat compared with the HFD group( Reference Choi, Park and Lee 23 ). However, 0·5 % (w/w) GCE plus HFD in a mouse model of the Mets could not lead to losing weight after 12 weeks( Reference Li Kwok Cheong, Croft and Henry 19 ). It has been assumed by Song et al. that GCE exerts its weight-lowering effects by inhibiting adipogenesis which is mainly regulated by transcription factors such as PPARγ2. This was corroborated by down-regulation of adipogenic target genes of PPARγ2 such as adipocyte lipid binding protein, cluster of differentiation 36, fatty acid synthase and lipoprotein lipase by GCE( Reference Song, Choi and Park 24 ). Also, inhibition of Glc-6-pase by GCE which leads to decreased glucose release by glycogenolysis, makes lipids to be utilised as energy source( Reference Henry-Vitrac, Ibarra and Roller 35 ). Furthermore, our study for the first time unveiled that GCE consumption contributes to controlling appetite which can ultimately lead to losing weight.
Limitations of our study encompass, first, the short time of the trial. Second, we did not have access to professional scales and a body composition analyser to assess alterations in body fat percentage. Third, due to budget deficit, measuring other factors such as appetite-related hormones was not possible. Further studies with longer durations and larger sample sizes are required to establish potential GCE effects in patients with the Mets.
Conclusion
In conclusion, the GCE in this trial could attenuate SBP, FBS, HOMA-IR, waist circumference and appetite in comparison to the placebo in patients with the Mets. Hence, GC extract supplementation could feasibly be an effective approach for management of some of the Mets features and Mets major causes such as insulin resistance and abdominal obesity.
Acknowledgements
The authors express their gratitude to the subjects for their participation in this research and the Arjuna Natural Extracts Ltd for generously providing the supplements.
This study was financially supported by a grant from the National Nutrition and Food Technology Research Institute of Shahid Beheshti University (G. S., grant no. 655). The GCE and placebo capsules were provided by Arjuna Natural Extracts Ltd as a donation. The funders had no role in the design, analysis or writing of this article.
G. S., H. R. and O. N. designed the study. M. S. contributed to diagnosing and referring the patients with the metabolic syndrome. H. R. and O. N. carried out the trial and followed the patients. H. R analysed the data and wrote the article.
The authors declare that there are no conflicts of interest.








