To the Editor—The International Classification of Diseases, Ninth revision, Clinical Modification (ICD-9-CM) coding system is often used to conduct surveillance for various infections.Reference Olsen, Ball, Nickel, Wallace and Fraser 1 Unfortunately, ICD-9-CM coding is subject to error and does not always reflect the true level of comorbid and acute illnesses.Reference Bouza, Lopez-Cuadrado and Amate-Blanco 2 Little research has been done to determine the accuracy of ICD-9-CM codes to identify multidrug-resistant organism (MDRO) infections.Reference Schweizer, Eber and Laxminarayan 3 Inaccurate coding of MDROs has implications for monitoring of MDRO transmission dynamics, assessments of MDRO epidemiology, calculations of hospital ratings and rankings, and hospital reimbursements. At present, no globally utilized MDRO reporting system exists. Therefore, understanding the sensitivity of ICD-9-CM codes for various MDROs will inform policy decisions regarding hospital rankings and reimbursements and determine the limitations of ICD-9-CM codes for studying MDRO infections from large retrospective administrative databases. Our goal was to determine the correlation between microbiologically confirmed MDRO infection in sterile sites or bronchial wash/bronchoalveolar lavage (BAL) cultures and ICD-9-CM coding for various MDROs.
This study was conducted at Barnes-Jewish Hospital, a 1,250-bed academic medical center in St Louis, Missouri. The study period was January 1, 2006, to October 1, 2015. Hospitalized patients with a positive sterile site or BAL/bronchial wash culture for any of the following MDROs were identified from the hospital clinical data repository and assessed for eligibility: Enterobacteriaceae, Enterococcus spp., Staphylococcus aureus, Pseudomonas aeruginosa, or Acinetobacter spp. Antimicrobial susceptibilities were determined in the clinical microbiology laboratory using disc diffusion methodology, and drug resistance was defined according to accepted definitions.Reference Magiorakos, Srinivasan and Carey 4 – 6 Sterile sites were defined as bloodstream; pleural, intra-abdominal, pericardial, cerebrospinal, and synovial fluids; bone marrow; and surgical specimens collected from lymph nodes, central nervous system, liver, spleen, kidney, pancreas, ovary, or vascular tissue. This study was approved with a waiver of informed consent by the Washington University School of Medicine Institutional Review Board.
All discharge ICD-9-CM diagnosis codes from the index MDRO hospitalization were utilized. Medical coders can assign an ICD-9-CM code for an organism and add a V09 code if drug resistance is present. V09 codes were used to identify drug resistance for all organisms except methicillin-resistant S. aureus (MRSA) after October 1, 2008, when unique MRSA codes were introduced.
The primary end points were the proportion of patients with clinically identified MDROs who had a discharge ICD-9-CM code for the correct organism and the proportion of patients with a discharge ICD-9-CM code for drug resistance. We also examined whether infectious disease (ID) consultation was associated with higher rates of coding for drug resistance.
In total, 4,429 patients met the eligibility criteria. Patients with MDR S. aureus that were not MRSA and with polymicrobial MDRO infections were excluded, leaving 4,005 patients for analysis. MRSA patients were analyzed in 2 groups: (1) patients discharged prior to October 1, 2008, and (2) patients discharged after introduction of MRSA-specific ICD-9-CM codes on October 1, 2008. Rates of organism and drug resistance ICD-9-CM coding are shown in Table 1.
TABLE 1 Organism and Multidrug-Resistant Organism (MDRO) Discharge International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) Codes for Various Sterile Site MDRO Infections
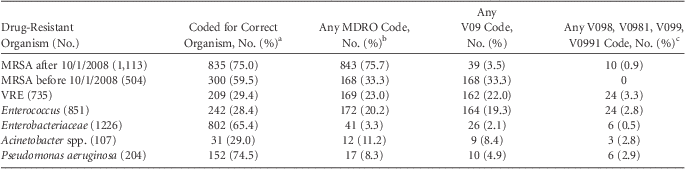
NOTE. MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus.
a For MRSA after 10/1/2008: 038.12, 482.42, 041.12. For MRSA before 10/1/2008: 038.11, 482.41, 041.11. For VRE and Enterococcus: 041.04. For Enterobacteriaceae: 038.4, 038.40, 038.42, 038.44, 038.49, 041.3, 041.4, 041.49, 041.6, 041.85, 48.20, 48.282, 48.283. For Acinetobacter spp.: 038.40, 038.49, 482.83. For Pseudomonas aeruginosa: 038.43, 041.7, 48.21.
b Any of the following: 038.12, 482.42, 041.12 (MRSA codes); V09, V09.0, V09.1, V09.2, V09.3, V09.4, V09.5, V09.50, V09.51, V09.6, V09.7, V09.71, V09.70, V09.8, V09.80, V09.81, V09.9, V09.91, V09.90.
c A distinction was made for V098, V0981, V099, and V0991 because these code for multidrug resistance, rather than single drug or single class resistance of the other V09 codes.
Patients with MRSA infections after introduction of the MRSA-specific ICD-9-CM codes had high rates of appropriately coded organism (75.0%) and MDRO status (75.7%). The proportion of MRSA patients with any drug resistance code increased from 33.3% to 75.7% after the introduction of MRSA-specific codes. Among patients surviving ≥48 hours after cultures were drawn, ID consultation was associated with a higher rate of coding for MRSA (519 of 587, 88.4%) than for patients without ID consultation (306 of 474, 64.6%; P < .001).
Patients with drug-resistant P. aeruginosa had the next highest rate of appropriately coded organism (74.5%) but low rates of drug resistance codes (8.3%). Drug-resistance coding was poor for all non-MRSA pathogens, ranging from 3.3% (Enterobacteriaceae) to 23.0% (vancomycin-resistant Enterococcus) (Table 1).
The correlation between microbiologically confirmed non-MRSA MDRO infection and V09 diagnosis codes for drug resistance was poor. Previous research showed poor correlation between V09 codes and confirmed MRSA infection prior to the introduction of MRSA-specific ICD-9-CM codes.Reference Schweizer, Eber and Laxminarayan 3 Our MRSA coding rates after the introduction of MRSA-specific ICD-9-CM codes were higher than previously reported.Reference Schaefer, Ellingson and Conover 7 We also found that ID consultation increased rates of MRSA coding, likely due to increased recognition and documentation of the presence and importance of MRSA by ID physicians.
In addition, coding rates for MRSA were significantly higher than coding rates of drug resistance for other organisms, suggesting a need for unique codes for other MDROs. This conclusion is reinforced by the fact that for patients with MRSA, introduction of MRSA-specific codes resulted in a dramatic increase in coding for resistant S. aureus. As ICD-9-CM codes are assigned by nonmedical personnel, universal drug resistance definitions and organism-specific drug resistance codes will likely assist in the proper coding of MDROs. Our findings are likely applicable to ICD-10-CM codes because the structure of ICD-10-CM drug resistance codes mimics those from ICD-9-CM.
Our results demonstrate that ICD-9-CM diagnosis codes cannot be used to estimate the burden of MDRO infections in hospitals. Additionally, researchers should be aware of the limitations of ICD-9-CM codes for studying MDRO infections from large retrospective medical databases. More specific MDRO codes are needed to facilitate future research using administrative data, a problem not addressed by ICD-10-CM.
To our knowledge, this study is the first to examine drug resistance coding rates for a variety of MDRO pathogens. The study is limited to a single tertiary-care referral center, and these results may not be generalizable. However, the study draws strength from its large sample size and has implications for hospital rankings, reimbursements, and future MDRO research utilizing large administrative databases.
ACKNOWLEDGMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health (NIH).
Financial support: This work was supported by the Washington University Institute of Clinical and Translational Sciences (grant no. UL1TR000448) from the National Center for Advancing Translational Sciences (NCATS) of the NIH. Dr Marin Kollef is supported by the Barnes-Jewish Hospital Foundation. Dr. Kwon reports that the research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences (grant no. UL1TR000448, sub-award KL2TR000450) from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.



