There is a growing interest on the role of n-3 long chain-PUFA during pregnancy since higher DHA (22:6n-3) plasma concentrations and higher n-3 index have found to be associated with longer duration of gestation and reduced risk for early preterm birth (< 34 weeks of gestation)(Reference Hoge, Donneau and Dardenne1). Moreover, DHA supplementation reduce the risks of perinatal death, neonatal care admission and low birthweight newborns. Maternal DHA supplementation may however prolong gestation and increase birth weights of the newborns with a small increased risk of large for gestational age babies(Reference Olsen, Halldorsson and Thorne-Lyman2). This information is important as preterm birth is a major public health issue(Reference McCormick, Litt and Smith3). The Academy of Nutrition and Dietetics recommends about 500 mg/d of long chain-PUFA in the adult populations(Reference Kris-Etherton, Innis and Ammerican Dietetic4) and Australian health recommendations are 800 mg/d of DHA plus 100 mg/d of EPA in pregnant women(5). The March of Dimes(6), the International Society for the Study of Fatty Acids and Lipids(Reference Koletzko, Cetin and Brenna7), the FAQ of the UN(8) and the World Association of Perinatal Medicine(Reference Koletzko, Lien and Agostoni9) all recommend at least 200 mg/d DHA in pregnant and lactating women from either fish or supplements. However, this advice does not appear to be reaching the population as recent estimates of dietary intakes of DHA in forty-seven developed and 128 developing countries demonstrated that 48 % of the 175 countries have a DHA intake of less than 200 mg/d(Reference Forsyth, Gautier and Salem10).
In addition, during pregnancy there is an elevated DHA requirement for the fetus, as DHA is a critical building block for the brain and the retina(Reference Gustafson, Liao and Mathis11,Reference Lauritzen, Brambilla and Mazzocchi12) . Peak brain deposition of DHA occurs during the third trimester of gestation with a fetal accretion of 67 mg of n-3 fatty acids per day, mainly DHA, and around 5 % is delivered to the brain (3·1 mg/d)(Reference Clandinin, Chappell and Heim13).
Increasing DHA intake during pregnancy, either by diet or supplements, has been the objective of several studies(Reference Carlson, Colombo and Gajewski14–Reference Middleton, Gomersall and Gould17). However, a universal strategy of DHA supplementation may not be optimal because of the regional differences in DHA status of pregnant women(Reference Forsyth, Gautier and Salem10) and because of the possible and likely variable contribution of endogenous DHA biosynthesis during pregnancy(Reference Burdge and Calder18). This likely explains why the clinical effects of DHA correlate less well with intake than with blood levels(Reference Hoge, Donneau and Dardenne1,Reference Olsen, Halldorsson and Thorne-Lyman2,Reference Leventakou, Roumeliotaki and Martinez15,Reference Brantsaeter, Englund-Ogge and Haugen19,Reference Oken, Kleinman and Olsen20) . In addition, in supplemented pregnant women, erythrocytes membrane DHA ranges from 4·1 to 10·1 % with rather large inter-individual variations(Reference Gellert, Schuchardt and Hahn21). Important differences of baseline DHA amount, of dietary intakes during pregnancy, of the contribution of endogenous biosynthesis and finally of the variable dosing of the DHA supplementation are all possibly contributing to the rather large variation of the biochemical indices of DHA status during pregnancy(Reference Gellert, Schuchardt and Hahn21).
Natural abundance isotopic analysis represents a valid, safe and inexpensive alternative to the use of synthetic isotopic tracers for investigating fatty acid metabolism in vivo in humans. Our laboratory has utilised the natural variations in 13C/12C (expressed as δ13C) to estimate the synthesis rates of DHA and arachidonic acid (ARA) in preterm infants(Reference Carnielli, Simonato and Verlato22). Similarly, Koletzko et al. have used variations in natural abundance carbon isotope ratio to investigate the contribution of linoleic acid to ARA synthesis in full-term neonates(Reference Demmelmair, von Schenck and Behrendt23). In plants, carbon isotopic fractionation depends on the mechanism used to fix carbon during the photosynthesis with plants being divided into C3 and C4 plants. C3 plants dominate food supply (e.g. tomato, potato, wheat rice), whereas there are few C4 plants (e.g. maize, sugar cane, sorghum and millet). In C3 plants, CO2 is directly provided to RuBisCO, the key enzyme of the Calvin cycle, whereas in C4 plants CO2 is first concentrated in mesophyll cell before it enters the Calvin cycle, ensuring that RuBisCO is mainly used to fix carbon and not to bind oxygen like in C3 plants. Because C4 plants use CO2 more efficiently C3 plants can fractionate more and are generally ∼ 10 mUr depleted in δ13C compared with C4 plants (–23 to –32 v. –10 to –16 mUr).(Reference Smith and Epstein24). Because the carbon isotopic signature of a molecule is conserved following incorporation from the diet, highly precise measurement in the δ13C by GC-combustion interface high resolution mass spectrometry (GC-IRMS) can provide insight into the fate of selected compounds of interest.
In this study, we aimed at testing the feasibility of using supplement containing fish-derived DHA (carbon source C3 plants) and algae-derived DHA (carbon source C4 plants) to assess the contribution of a 200 mg/d of DHA to the plasma DHA pool in pregnant women.
Methods
This is an observational single-centre pilot study in pregnant women taking a supplement of DHA. We studied two DHA supplements both in the TAG form but from different sources: from fish oil (Fish group) or from an algal source of biotechnological origin (Algae group). Pregnant women took 200 mg of DHA daily from pregnancy week 20 until delivery. Maternal DHA supplementation during the second half of gestation is always recommended in our region. Participation to the study was voluntary. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the local ethical committee (protocol number 1333P). Written informed consent was obtained from all subjects.
The inclusion criteria of the study were single pregnancy, 18–40 years old and gestational age at enrolment below 20th week. A total of nineteen women completed the study (online Supplementary Fig. S1). There were no exclusion criteria based on fish intake; enrolled women were asked not to change DHA supplement type during the study.
Blood samples
Leftover blood samples (1 ml) taken for diagnostic purposes were collected from the antecubital vein in EDTA tubes at baseline (T0), at 10 (T1) and 90 ds (T2) of DHA supplementation and at delivery (T3) as part of the local routine health checks during uncomplicated pregnancies.
Samples were centrifuged within 2 h of collection and plasma aliquots were stored in tubes containing pyrogallol as antioxidant at −80°C.
Sample preparation and compositional fatty acid analysis
Lipids were extracted from 100 µl of sample using a chloroform-methanol solution in accordance with the Folch method(Reference Folch, Lees and Sloane Stanley25). The lipid extract was resolved in classes by thin layer chromatography (for the plasma sample) as previously reported(Reference Correani, Visentin and Cosmi26). The total phospholipid fraction was hydrolysed with HCl-methanol, and the resulting NEFA were transesterified and extracted with hexane. Hexane containing fatty acid methyl esters (FAME) was directly taken for gas chromatographic analysis.
GC analysis was performed with an Agilent 5890 GC equipped with a flame-ionisation detector. FAME were resolved with an Omegawax column (30 m × 0·25 mm internal diameter × 0·25 µm film thickness; Supelco) with 1 µl injection volume running in on-column mode. Oven temperature was programmed as follows: 60°C for 3 min, increased 20°C/min to 205°C, where remained constant for 15 min. Temperature then increased 0·4°C/min up to 213°C, which was maintained for 10 min and finally increased to 240°C at 5·0°C/min and held for 8 min. Peaks were identified in relation to a reference standard mixture (GLC 461, Nuchek Prep). Fatty acid composition was reported as the percentage of fatty acid in total phospholipid fatty acids for plasma sample or as the percentage in total fatty acids for the supplements.
δ13C DHA in supplement and in plasma phospholipids
The δ13C of the FAME derived either from plasma phospholipids or from DHA dietary supplement was analysed by using GC-C-IRMS (Delta V, Thermo Fisher Scientific). The system was externally calibrated with standard mixtures F8–2 for FAME (even chain fatty acid methyl and ethyl esters from n-C14:0 to n-C20:0), obtained from Arndt Schimmelmann. FAME were resolved on a DB225 column (30 m × 0·25 mm internal diameter × 0·25 µm film thickness; Agilent Technologies) with 1µl injection volume running in on-column mode. Oven temperature was programmed as follows: 40°C for 2 min, increased 10°C/min to 120°C, where remained for 1 min. Temperature then increased 1°C/min up to 230°C, which was maintained for 12 min. Peaks were identified in relation to a reference standard fatty acids mixture (GLC 461).
Carbon isotopic analysis was performed in triplicate. The values of δ13C are reported in millUrey (mUr)(Reference Brand and Coplen27). Each 1 mUr change is representative of a one per mill (1 in 1000, ‰) change in the 13C/12C ratio with respect to an international reference standard:
where ‘R’ is the ratio of the heavy to light isotope in the sample or standard.
Vienna Pee Dee Belemnite (V-PDB) is made of inorganic material rich in 13C. As a result, most organic matter is depleted in 13C relative to the standard, resulting in a negative δ13C value. The δ13C value in our study will then have a higher 13C content if the value is less negative (e.g. −15·8 mUr), and a lower 13C content if the value is more negative (e.g. −25·3 mUr). Since the comparison of the δ 13C value was made within the same fatty acid, we did not correct for the 13C contribution of the methyl group.
Throughout the article, ‘supplemental DHA’ was used for the quote of plasma DHA coming from the supplement, whereas ‘non-supplemental DHA’ was used for the quote of plasma DHA both synthesised from its precursor and derived from the free diet.
The percentage of maternal non-supplemental DHA was calculated as follows:
where A is the δ13C value of the plasma phospholipid DHA at T delivery (T3), B is the δ13C value of the plasma phospholipid DHA at T0 and C is the δ13C value of the DHA present in the capsule.
Statistical analysis
All data reported were expressed as mean values and standard deviation unless otherwise stated.
Intra-group differences between pre- and post-supplementation were determined by paired t test. Inter-group and supplement difference in fatty acid percentage and fatty acid enrichment were determined by independent t test. We used one-way ANOVA for the comparison of plasma phospholipids fatty acid composition and DHA enrichment within each study group. The Bonferroni test was used for post hoc analysis.
As this is a pilot study, the sample size was based on participant flow and protocol adherence and on the number of participants needed to reasonably evaluate the feasibility goal. This was estimated on the basis of published data(Reference Carnielli, Simonato and Verlato22) with the same study design, where we found a mean difference in DHA enrichment of about 12·0 mUr between the plasma δ13C value measured at the end of the study in the supplemented group and the δ13C of the algae supplement with a standard deviation of about 0·9 mUr.
Considering an analytical error of 0·2 mUr, we would be able to discriminate an endogenous/exogenous ratio of at least 1/60. Under these premises, we considered thirteen participants in the Algae group an adequate number to demonstrate the feasibility of using the natural variation of 13C content to estimate the contribution of DHA supplement from an algal source.
All tests were two-sided, and a P value lower than 0·05 was considered statistically significant. Statistical analysis was performed using PASW Statistics 18.0 (IBM Corp).
Results
Nineteen pregnant women participated in the study, six took a DHA supplement from fish (Fish group) and thirteen from algae origin (Algae group). No significative difference in pre-pregnancy body weight, BMI, gestational weight gain, length of gestation and newborn birth weight was observed between the two groups (data not shown). All women had uncomplicated full-term pregnancies.
The days with supplementation were T1 9 (sd 2) and 10 (sd 2) d; T2 93 (sd 5) and 89 (sd 4) d; T3 156 (sd 11) and 167 (sd 7) d in Fish and Algae groups, respectively.
DHA supplement composition
The fatty acid composition of the study supplements is reported in Table 1. The percentage of DHA of the two preparations was not different being 45·7 and 45·8 mol% in fish and algae supplements, respectively (P = 0·80). ARA (20:4n-6) was markedly higher in fish than in the algae supplement (P < 0·0001). Neither supplement contained detectable amounts of α-linolenic acid (18:3n-3).
Table 1. Fatty acid composition and δ13C of DHA supplements
(Mean values and standard deviations, n 6 different batches)

δ13C, carbon-13 isotopic abundance.
Fatty acid composition expressed as the percentage of fatty acid in total fatty acids.
P determined by independent t test.
Fatty acids δ13C were measured in six different batches of the supplements to test for constancy of carbon stable isotope abundance. The mean values are depicted in Table 1.
Plasma phospholipid fatty acid quantitative analysis
Plasma phospholipids fatty acid compositions before (T0) and at the end of DHA supplementation (T3) are reported in Table 2.
Table 2. Plasma phospholipids fatty acid composition before and at the end of DHA supplementation
(Mean values and standard deviations)

SFA, short fatty acid.
Results are expressed as mol% of total phospholipid fatty acids.
P determined by paired t test, baseline (T0) v. delivery (T3).
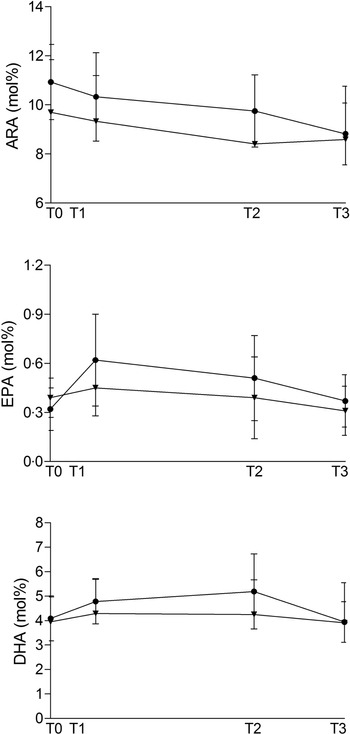
Fish oil supplementation had no significant effect in any of the n-3 PUFA, but ARA (20:4n-6) was significantly decreased between T0 and T3. Algal oil supplementation significantly decreased 22:4n-6, whereas we observed no significant effect on n-3 PUFA.
Fig. 1 shows ARA, EPA and DHA percentage during the study period.

Fig. 1. Maternal plasma phospholipid ARA, EPA and DHA amount (expressed in mol% on total phospholipid fatty acid) at baseline (T0, 20 weeks of gestation), 10 (T1) and 90 days (T2) after the start of supplementation and at delivery (T3) in the Fish and in the Algae group. ![]() , fish;
, fish; ![]() , algae.
, algae.
Plasma DHA isotopic enrichment
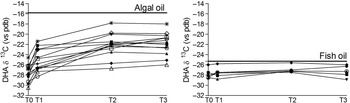
In the Fish group, the DHA δ13C did not change during the study (P = 0·09), whereas in the Algae group we observed a marked and significant (P < 0·001) change towards less negative values, closer to the δ13C of the algal DHA supplement. The difference was significant between T0 and T3 (P < 0·001) and T1 and T3 (P < 0·001) but not between T2 and T3 (Fig. 2).

Fig. 2. Plasma phospholipid δ13C DHA in maternal plasma measured during the time of the study. T0 = before supplementation (20 weeks of gestation); T1 and T2 10 and 90 d after the start of supplementation; T3, delivery. Each symbol corresponds to a different patient. Panel on the left, Algae group, panel on the right Fish group. The bold line represents the δ13C of DHA in the supplement.
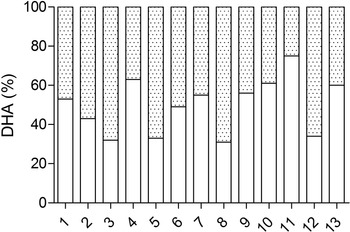
The contribution of supplemental DHA and non-supplemental DHA to the plasma DHA pool in the Algae group is presented in Fig. 3. The supplementation of DHA contributed to the plasma phospholipid DHA pool by a median value of 53 % with a range from 31 to 75 %.

Fig. 3. Individual plasma phospholipids DHA percentage of (![]() ) supplemental and (
) supplemental and (![]() ) non-supplemental DHA in the Algae group.
) non-supplemental DHA in the Algae group.
We were unable to find a significant correlation between the percentage contribution of DHA from the supplement and the DHA mol% of plasma phospholipids.
Discussion
In this study, we demonstrated that 13C natural abundance approach can be used to study the contribution of DHA supplement from algae to the plasma phospholipid DHA pool in human pregnancy, whereas fish oil cannot be used. This method is likely to be safe as it involves the use of the commercially available DHA supplements, uses left-over plasma obtained during the routine pregnancy checks and appears to be easily applicable to large studies of algal DHA supplementation in different populations with different dietary DHA intakes. Under the assumption that after 20 weeks of DHA supplementation, ‘supplemental DHA’ is in equilibrium with the fast and medium turning over pools of our body (plasma lipids, blood cells, endothelium and liver), we can reasonably say that the percentage of ‘supplemental DHA’ found in plasma phospholipids of pregnant women reflects the proportion of the supplement in all the accessible pools including the fetus. This information could be of paramount importance for fetal nutrition.
The natural variation in δ13C of fatty acids in food supply has been previously used to investigate the contribution of endogenous synthesis from 18-carbon fatty acids to ARA and DHA in infants(Reference Carnielli, Simonato and Verlato22,Reference Demmelmair, von Schenck and Behrendt23) and to determine the half-life of brain DHA in mice(Reference Lacombe, Lee and Bazinet28). Metherel et al. used the natural variation in δ13C to assess n-3 fatty acids metabolism in healthy adults following EPA and DHA supplementation(Reference Metherel, Irfan and Klingel29). The EPA supplementation (−23·5 (sd 0·22) mUr) significantly changed the plasma value of EPA from −31·5 (sd 0·2) mUr to −25·7 (sd 0·2) mUr and that of DHA from −27·9 (sd 0·2) mUr to −25·6 (sd 0·1) mUr indicating an in vivo synthesis of DHA from EPA. Conversely, DHA supplementation had no detectable effects on plasma δ13C of DHA, because the pre-supplementation plasma δ13C of DHA (−27·6 (sd 0·3) mUr) was almost identical to the δ13C of the supplement (−27·5 (sd 0·03) mUr).
The natural abundance isotopic approach applied to the study of DHA metabolism in human pregnancy offers major and minor advantages. Among the major advantages, there is no need of expensive tracers, which are commonly obtained by chemical synthesis or biosynthesis, and the technique allows for rather long-time studies, which are necessary for lipid metabolism studies in general and, more specifically for slow turning over molecules like DHA. Among other advantages, we wish to comment the analytical precision of the method. The precision in our hand (expressed as standard deviation) for plasma samples processed in a single batch and injected in triplicate is ± 0·2 mUr. Considering a mean difference of 12·1 mUr between natural background DHA enrichment in the study subjects and supplemental DHA (algal DHA with significantly higher 13C), as was the case in this study, and a 0·2 mUr error of the method, we were able to discriminate a non-supplemental/supplemental ratio of 1/59 that corresponds to about 1·7 %.
The major limitation of the natural isotope technique lays on the difficulty of finding suitable compounds of interest (nutrients/preparations) with different isotopic signatures than the background isotopic enrichment of the compound of interest in the study subjects. This condition usually applies to a given geographical area and should apply to most of the individuals participating to the study. This limitation is usually taken care in all study subjects, as the background isotopic enrichment is measured in all baseline samples. Another important limitation is the need for a high precision GC-C-IRMS instrument with a sizable upfront expense and the need of highly skilled technician.
In this pilot study, we were unable to obtain dietary records to estimate DHA intakes nor were we able, for technical reasons, to measure DHA in erythrocyte. Erythrocyte DHA may be a more stable indicator of the incorporation of the DHA from the algal source and may better represent the fatty acid composition of the cell membranes of the pregnant woman(Reference Gellert, Schuchardt and Hahn21). Furthermore, in this study we did not enroll control pregnant women on a free diet. The reason was twofold: this was not necessary for this study and in addition it would have been difficult to recruit such control women as nearly all pregnant women in our region were motivated to take DHA supplements as recommended by scientific societies(6,8,30) .
In conclusion, our study should be regarded as a proof of concept and showed the feasibility of measuring the long-term changes of plasma phospholipid δ13C DHA in pregnant women in response to dietary DHA supplementation. We were able to estimate the contribution of DHA from supplements to the plasma DHA pool or vice versa estimate the quote of DHA coming from the diet and from the endogenous biosynthesis. These results obtained after long period of equilibration highlight the usefulness of the natural abundance approach to monitor DHA metabolism in human pregnancy. Adherence to long-time study could represent a limitation; however, pregnant women are highly motivated participants and the compliance with the DHA supplement, which we evaluated by pills count at control visit and by the plasma phospholipid δ13C DHA trend, appears to have been fully addressed. We speculate that our method could be used to customise the DHA supplementation during pregnancy in women from different geographical areas with different dietary intakes of DHA and possibly to better identify women who could benefit from higher or lower doses of DHA supplementation. Moreover, it could be useful to take advantage of this technique to compare pregnant v. non-pregnant women to address if and how pregnancy can alter the contribution of supplemental DHA to the plasma pool. Measuring δ13C DHA in cord blood plasma could also be important to gain more information on fetal DHA origin.
Acknowledgements
The authors are grateful to the nursing and medical staff.
None.
The authors’ contributions were as follows: V. P. and P. C. designed the research; S. V., E. C., G. V. and M. S. conducted the research; M. S. and A. C. analysed the samples and performed the data analysis. All authors read and approved the final version of the manuscript.
The authors declare no conflict of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114522001088