Introduction
Subsistence hunting is a fundamental activity for many communities in tropical regions, especially as a source of dietary protein (Smith, Reference Smith2008; Read et al., Reference Read, Fragoso, Silvius and Luzar2010; Linder & Oates, Reference Linder and Oates2011; Valsecchi et al., Reference Valsecchi, El Bizri and Figueira2014; Constantino, Reference Constantino2015). In Brazil the use of wild animals for food is controlled by the Environmental Crimes Law (Law 9605, of 12 February 1998), which states that it is not a crime for a hunter, when in need, to hunt wildlife to feed himself or his family. However, there is some doubt about the interpretation of ‘state of necessity’ as mentioned in law, which precludes a clear understanding regarding the legality or otherwise of subsistence hunting. Furthermore, there is still a need for scientific studies to support the effective management and conservation of biological resources.
Primates are among the most preferred game species of the people who inhabit the tropical forests of South America, Asia and Africa (Levi et al., Reference Levi, Shepard, Ohl-Schacherer, Wilmers, Peres and Yu2011; Fa et al., Reference Fa, Farfán, Márquez, Duarte and Vargas2013; Quinten et al., Reference Quinten, Stirling, Schwarze, Dinata and Hodges2014; Borgerson, Reference Borgerson2015; Constantino, Reference Constantino2015). However, little is known about the spatial patterns of primate hunting. Most studies on hunting, and the models most frequently used to evaluate them, do not take spatial patterns into consideration, nor do they evaluate temporal distribution or the area affected (Bodmer, Reference Bodmer, Western and Wright1994; Robinson & Bodmer, Reference Robinson and Bodmer1999; Sirén et al., Reference Sirén, Hambäck and Machoa2004), which limits evaluation of the variation in the distribution of hunting grounds and hampers the identification of the factors that influence this variation. However, the spatial patterns of hunting activity are receiving increasing attention, providing new insights into the sustainability of this practice (Sirén et al., Reference Sirén, Hambäck and Machoa2004; Smith, Reference Smith2008; Levi et al., Reference Levi, Shepard, Ohl-Schacherer, Peres and Yu2009; Read et al., Reference Read, Fragoso, Silvius and Luzar2010; Constantino, Reference Constantino2015).
Abiotic factors are among the main factors that influence the spatial distribution of game species, and hunting patterns. Soil type, hydrological conditions, anthropogenic impact, and habitat availability and use all contribute to the heterogeneity of the landscape (Eisenberg & Thorington, Reference Eisenberg and Thorington1973; Tilman & Kareiva, Reference Tilman and Kareiva1997; Collinge, Reference Collinge2001; Valsecchi et al., Reference Valsecchi, El Bizri and Figueira2014; Morcatty & Valsecchi, Reference Morcatty and Valsecchi2015). This heterogeneity, in turn, influences the distribution and abundance of species (Peres, Reference Peres1994) and affects hunting efforts and yields (Balée & Gély, Reference Balée, Gély, Posey and Balée1989; Bodmer, Reference Bodmer1995, Morcatty & Valsecchi, Reference Morcatty and Valsecchi2015). Hunting activity is also influenced by the accessibility of hunting grounds (Peres & Lake, Reference Peres and Lake2003; Sirén et al., Reference Sirén, Hambäck and Machoa2004; Parry et al., Reference Parry, Barlow and Peres2009). For traditional communities in the Amazon, rivers are essential for movement and for access to many hunting areas (Smith, Reference Smith2008; Constantino, Reference Constantino2015).
New technologies have improved the efficiency of hunting practices, resulting in increased yields (El Bizri et al., Reference El Bizri, Morcatty, Lima and Valsecchi2015), and some of these technologies, in particular global positioning systems and geographical information systems, have become important tools for evaluating the spatial–temporal features of hunting activities (Brøseth & Pedersen, Reference Brøseth and Pedersen2000; Smith, Reference Smith2008; Levi et al., Reference Levi, Shepard, Ohl-Schacherer, Wilmers, Peres and Yu2011). A number of studies have shown that variation in hunting effort may affect the spatial–temporal impact of hunting on game species and their natural populations (McCullough, Reference McCullough1996; FitzGibbon, Reference FitzGibbon and Caro1998).
A system for monitoring the use of local fauna was established in 2002 at the Mamirauá and Amanã Sustainable Development Reserves, in Central Amazonia in Brazil, to collect information on the use of local fauna by riverine communities, and evaluate the consumption of bushmeat and the stability of hunting in the two environments (Valsecchi et al., Reference Valsecchi, El Bizri and Figueira2014). In both Reserves hunters move within the forest, walking on country roads and hunting trails, or travelling by canoe along rivers and other water bodies.
We monitored areas used for the hunting of primates over an 11-year period, and compared the spatial patterns of the harvesting of primates in the various environments used by local hunters. We tested the hypothesis that there was no increasing trend in the size of the areas used to hunt primates over time. We predicted that the hunting carried out in the study areas did not affect the populations of the targeted species, and therefore that the size of the hunting grounds had not increased (Valsecchi & Amaral, Reference Valsecchi and Amaral2009; Lopes et al., Reference Lopes, Valsecchi, Vieira, Amaral and Costa2012). Primates are more abundant in the várzea forest (seasonally flooded forest, with nutrient-rich alluvial soils) than in the neighbouring forested environments, which are not subjected to periodic flooding (Peres, Reference Peres1997). Thus, we also tested the hypothesis that the mean distance travelled by hunters in search of primates is shorter in the várzea forest compared to other environments, where primary productivity and primate abundance are lower, and that the hunting grounds are smaller in the várzea environment.
Study area
Mamirauá Sustainable Development Reserve (1,124,000 ha), officially protected since 1982, is covered entirely by várzea forest (Ayres, Reference Ayres1995). It has a population of c. 11,532 inhabitants, in c. 200 small riverine communities (Moura et al., Reference Moura, Nascimento and Corrêa2015b), whose principal subsistence activities are agriculture, fishing and hunting. The Solimões River floods every year, and the water level fluctuates by a mean of 10.6 m (Ramalho et al., Reference Ramalho, Macedo, Vieira, Valsecchi, Calvimontes, Marmontel and Queiroz2009). This flooding pulse results in the deposition of sediments onto the soils of the Mamirauá várzea, which contributes to its high primary productivity (Junk et al., Reference Junk, Piedade, Wittmann, Schöngart and Parolin2010). The Reserve is home to 11 primate taxa: Alouatta juara, Aotus cf. vociferans, Ateles chamek, Cacajao calvus calvus, Cacajao calvus rubicundus, Cebuella pygmaea, Pithecia cazuzai, Saimiri cassiquiarensis, Saimiri macrodon, Saimiri vanzolinii and Sapajus macrocephalus (Valsecchi, Reference Valsecchi2005; Paim et al., Reference Paim, de Silva Júnior, Valsecchi, Harada and Queiroz2013; Cardoso et al., Reference Cardoso, Valsecchi, Vieira and Queiroz2014; Marsh, Reference Marsh2014; Rabelo et al., Reference Rabelo, Silva, Vieira, Ferreira-Ferreira, Paim and Dutra2014). We analysed data from the Fauna Use Monitoring System, a participatory monitoring initiative started in 2002 with the involvement of local inhabitants of riverine communities trained to record all hunting events, for five villages: Barroso, Boca do Mamirauá, São Francisco do Aiucá, São Raimundo do Jarauá and Sítio Fortaleza (Fig. 1; Table 1).
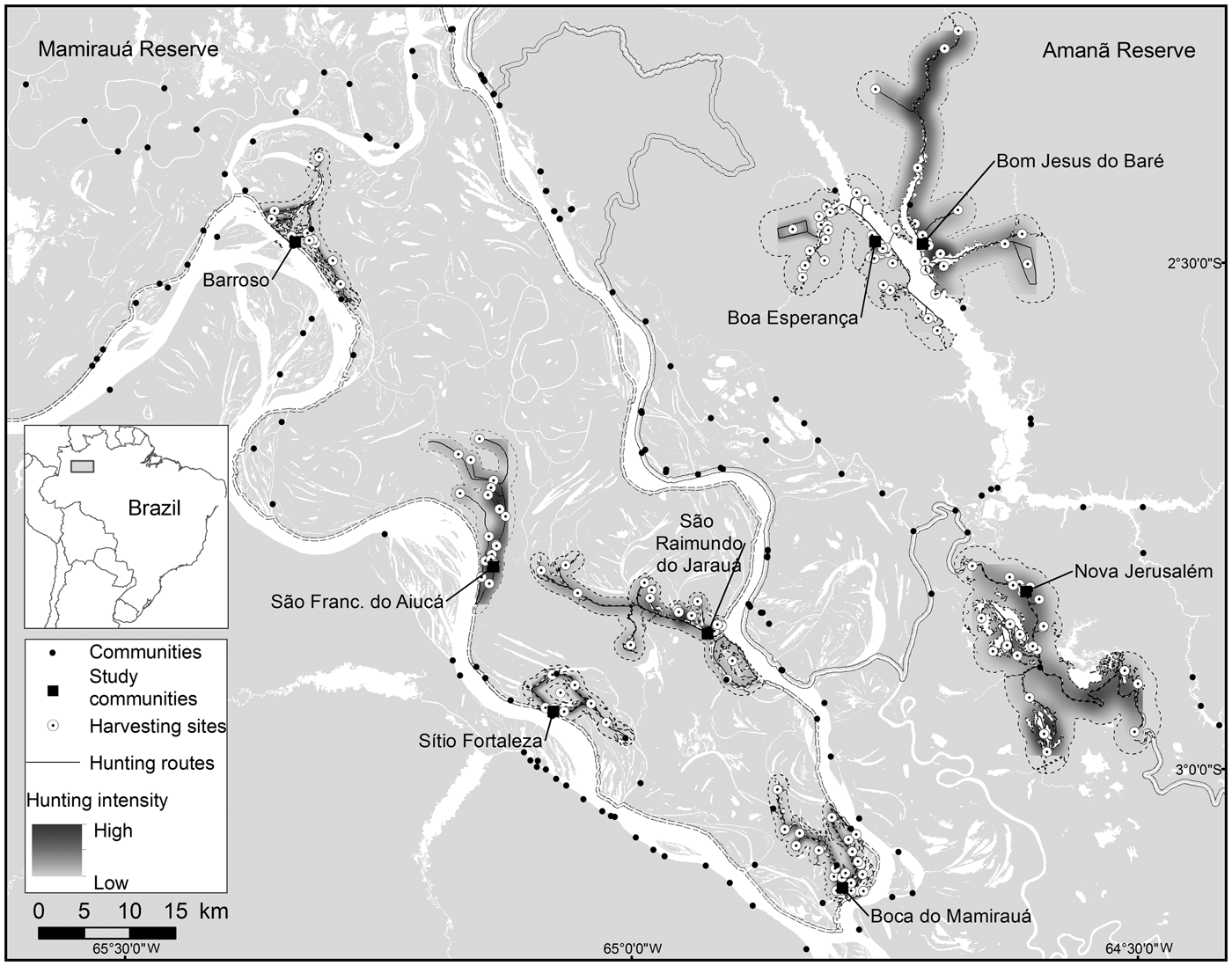
Fig. 1 The communities in Amanã and Mamirauá Sustainable Development Reserves in Central Amazonia, Brazil, monitored for primate hunting between 2003 and 2013, with the neighbouring communities, the hunting travel routes, the harvesting sites, and areas most intensively hunted by the study communities.
Table 1 Characteristics of the communities monitored between 2003 and 2013 in Mamirauá and Amanã Sustainable Development Reserves in Central Amazonia (Fig. 1), and spatial features of primate hunting by these communities (Supplementary Figs S1–S6).
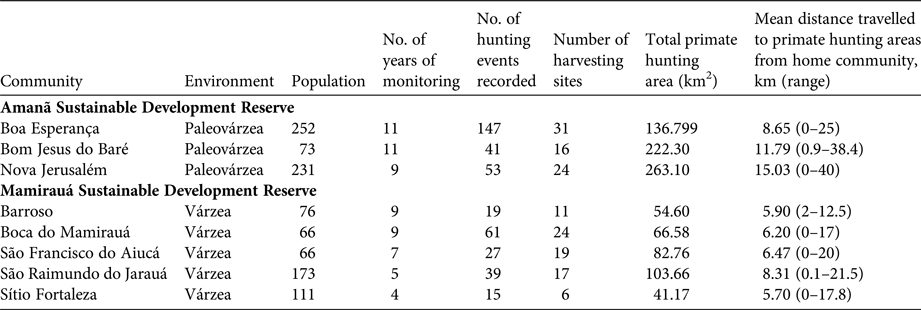
The c. 2,350,000 ha Amanã Sustainable Development Reserve was created in 1998. It has a population of c. 3,860 people, in 86 communities (Moura et al., Reference Moura, Nascimento and Corrêa2015a), whose principal subsistence activities are agriculture, extraction of plant resources (e.g. Brazil nut and açaí, lumber), fishing and hunting. Amanã has a more diverse environment than Mamirauá, with flooded and non-flooded vegetation, including paleovárzea (characterized by a series of low-lying ridges interspersed with flooded depressions, which result from cyclical depositional processes; Irion, Reference Irion1976), igapó (blackwater flood plains), várzea, terra firme (well-drained soils) and campinarana (a type of Amazonian high caatinga, savannah forests on white sandy soil) (Queiroz, Reference Queiroz, Ayres, Fonseca, Rylands, Queiroz, Pinto, Masterson and Cavalcante2005a; Irion et al., Reference Irion, de Mello, Morais, Piedade, Junk, Garming, Junk, Piedade, Wittmann, Schöngart and Parolin2010; Assis et al., Reference Assis, Haugaasen, Schöngart, Montero, Piedade and Wittmann2015). Areas of paleovárzea are used widely to hunt some game species, including primates (Valsecchi et al., Reference Valsecchi, El Bizri and Figueira2014). Paleovárzea areas are adjacent to tracts of igapó that encompass Amanã Lake, which is 45 km long and 2–3 km wide, on average (Queiroz, Reference Queiroz, Ayres, Fonseca, Rylands, Queiroz, Pinto, Masterson and Cavalcante2005a). The paleovárzea is known by local residents as terra firme, a term normally applied in the Amazon Basin to higher grounds that are never flooded. In Amanã, however, the true terra firme habitats are far from the inhabited areas and are not exploited or used as hunting grounds by the communities monitored by the Fauna Use Monitoring System or in this study.
Eight primate species are present in Amanã: A. juara, A. cf. vociferans, Cacajao ouakary, Callicebus lucifer, Cebus albifrons, Saguinus inustus, S. cassiquiarensis and S. macrocephalus (Valsecchi, Reference Valsecchi2005; Valsecchi et al., Reference Valsecchi, Vieira, Silva Júnior, Muniz and Avelar2010; Ferrari et al., Reference Ferrari, Guedes, Figueiredo-Ready and Barnett2014). We analysed data from Fauna Use Monitoring System records for three communities in Amanã: Boa Esperança, Bom Jesus do Baré and Nova Jerusalém (Fig. 1; Table 1).
From data and records from the monitoring system it was possible to identify distinct patterns of hunting, especially in the composition and frequency of the most hunted species in each area. Among the communities in the paleovárzea environments of Amanã, the preferred species are terrestrial mammals of medium and large size, such as white-lipped Tayassu pecari and collared peccaries Pecari tajacu, and pacas Cuniculus paca. In the várzea environments of Mamirauá the inhabitants make use of chelonians, medium and large birds, and howler monkeys Alouatta spp. (Valsecchi & Amaral, Reference Valsecchi and Amaral2009; Lopes et al., Reference Lopes, Valsecchi, Vieira, Amaral and Costa2012). We believe the spatial–temporal patterns of hunting activity are also influenced by the environmental peculiarities that differentiate the two environments monitored.
In Amazonia white water rivers act as geographical barriers for some primate species (Wallace, Reference Wallace1852; Ayres & Clutton-Brock, Reference Ayres and Clutton-Brock1992; Ferrari, Reference Ferrari2004). However, in the riverine communities studied, there are no barriers to hunters or primates in the areas used as hunting grounds. Local hydrography is therefore not a limiting factor for the composition of primate species hunted. However, some species of primates in the study areas have preferences for particular habitats in the landscape (Barnett & Brandon-Jones, Reference Barnett and Brandon-Jones1997; Barnett et al., Reference Barnett, Defler, Oliveira, Queiroz, Bezerra, Veiga, Barnett, Ferrari and Norconk2013), and these preferences or habitat selection patterns can influence the composition of species hunted by the same communities.
Methods
Data collection
Data on the hunting of primates during January 2003–December 2013 were derived from the Fauna Use Monitoring System (Valsecchi & Amaral, Reference Valsecchi and Amaral2009; Valsecchi et al., Reference Valsecchi, El Bizri and Figueira2014). Usually, the hunters declared the information to trained members of each monitored community, who made notes and records. The main data recorded for each hunting event were the location of the harvesting, time invested in the hunt, number of hunters involved, hunting technique used, and details of the specimens harvested, such as their body length and weight, and reproductive condition, such as gestating or lactating (Valsecchi et al., Reference Valsecchi, El Bizri and Figueira2014). The hunters provided this information voluntarily, and in some cases the body measurements of hunted animals were taken directly by the trained resident. Hunting events can involve one or more hunters, and one or more hunted animals, in a single location. Hunting can be intentional, when hunters leave home specifically for this purpose, or opportunistic, when the hunting event happens during unexpected encounters between hunters and wildlife. Usually the latter occurs when hunters are performing some other activity (e.g. agriculture or fishing).
The locations where primates were harvested were georeferenced using a global positioning system, with the assistance of local hunters. The travel routes used by the hunters within their hunting grounds were also georeferenced, which facilitated the determination of the area covered and the distance travelled.
Mapping
We produced maps of the hunting areas, and spatial metrics, in ArcGIS 10.2.2 (ESRI, Redlands, USA). To calculate the hunting areas, buffers were generated using the georeferenced hunting trails and locations of harvesting sites. These buffers varied in size between the two Reserves because of the difference in the mean distance travelled by the hunters from river banks (which are the main transport routes in the area) to the hunting grounds in the forest. The mean distance travelled was 1 km for hunters in the várzea forests and 2 km in the paleovárzea. Kernel density maps (heat maps) were created to identify the intensity of hunting within the study areas.
Data analysis
We used a t-test to compare the differences in the size of the hunting areas between the várzea and paleovárzea environments, as well as the distances travelled by the hunters, using each hunting event as a sample unit. Only intentional hunting events were considered in this analysis. This test was also used to determine whether the area used to hunt primates varied among years. This analysis was conducted separately for each of the communities monitored. The analyses were run in Excel 2007 (Microsoft Inc., Redmond, USA) and R v. 3.1.1 (R Development Core Team, 2011), and results were considered to be significant at P < 0.05.
Results
During 2003–2013 the Fauna Use Monitoring System recorded 4,218 hunting events, with 10,992 animals harvested and consumed in the monitored communities. In 402 of these events the target species was a primate, of which 240 were recorded as harvested in the paleovárzea and 162 in the várzea. These hunting events resulted in the harvesting of 541 primate individuals of nine species. In the várzea, two species dominated, whereas three species dominated in the paleovárzea (Table 2).
Table 2 Primate species hunted in the várzea environment of Mamirauá Sustainable Development Reserve and the paleovárzea environment of Amanã Sustainable Development Reserve, in Central Amazonia (Fig. 1).
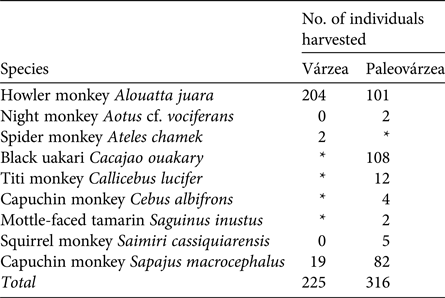
* The species does not occur in the environment.
In the paleovárzea, hunters used a mean hunting area of 105.18 ± SD 57.87 km2, whereas in the várzea the mean hunting area was significantly smaller, at 31.03 ± SD 19.07 km2 (t = 6.65508, df = 57, P < 0.001; Table 1; Supplementary Figs S1–S6). No significant difference was detected in the sizes of the hunting areas used by the monitored communities over the study period. In six of the communities, the comparison of the size of the hunting grounds used during the first and last years of the study period indicated there was no significant change in the area exploited. Data from São Raimundo do Jarauá and Sítio Fortaleza spanned too short a timeframe to conduct this analysis (5 and 4 years, respectively).
The distances travelled by hunters differed significantly between the várzea and paleovárzea environments (t = −2.451, df = 41, P < 0.05; Table 3). The mean distance travelled from the community to the harvesting site was 11.62 km in the paleovárzea environment, and 6.57 km in the várzea environment.
Table 3 Spatial features of hunting recorded in various studies in the Amazon biome, with locality, type of environment, number of communities monitored, population, mean hunting area, and data source.
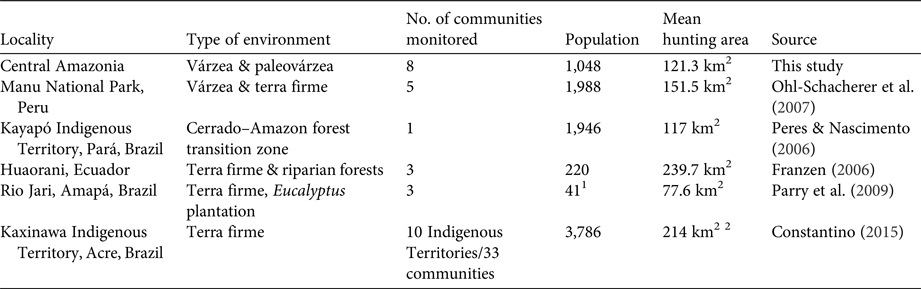
1 Number of families
2 Mean area per Indigenous Territory
The frequency of the harvesting sites, distributed in distance classes from the home community, was unimodal in the várzea environment but bimodal in the paleovárzea environment. In the latter, harvesting sites > 20 km from the home community were used frequently for hunting primates (Fig. 2).

Fig. 2 Distribution of the total number of primates harvested during 2003–2013 in várzea and paleovárzea environments by communities in Amanã and Mamirauá Sustainable Development Reserves in Central Amazonia (Fig. 1), by distance of harvesting sites from the hunters’ home communities.
When the frequency distribution of distance to harvesting sites in the paleovárzea was analysed in terms of primate species it was observed that A. juara was hunted more severely in more distant places (in a monomodal distribution), whereas C. ouakary was hunted predominantly close to the home communities (also a monomodal distribution). Harvesting sites of S. macrocephalus were distributed along the hunting trails, with a bimodal distribution. Although in the paleovárzea harvesting sites located > 20 km from home communities were important for the hunting of A. juara and Sapajus macrocephalus (Fig. 3), in the várzea the harvesting of A. juara (the only species hunted heavily there) occurred mainly at short distances from the communities (monomodal distribution).

Fig. 3 Distribution of the three main primate species harvested during 2003–2013 by communities in Amanã and Mamirauá Sustainable Development Reserves in Central Amazonia (Fig. 1), by distance of harvesting sites from the hunters’ home communities.
A majority of the harvesting sites in the paleovárzea (58) were located in areas of igapó or at the edges of water bodies, and only 13 sites are located further away, in the forests. The most intensely hunted areas were primarily in the vicinity of the settlements and along the margins of the water bodies (Fig. 1). No systematic relationship was found between the number of harvesting sites used by a community and the size of its population (Rs = 0.25, P > 0.05) or the number of years of monitoring (Rs = 0.48, P > 0.05).
Discussion
The difference in the size of hunting areas between the two environments studied is probably attributable to the density of primates. Várzea environments, which are highly productive, usually support higher primate densities than paleovárzea environments (Peres, Reference Peres1997; Queiroz, Reference Queiroz2005b), and thus hunters can locate target species within a relatively small area. However, fishing was the main economic activity and source of animal protein for the várzea dwellers (Lopes et al., Reference Lopes, Valsecchi, Vieira, Amaral and Costa2012; Valsecchi et al., Reference Valsecchi, El Bizri and Figueira2014; Morcatty & Valsecchi, Reference Morcatty and Valsecchi2015), which may explain the lower number of primates hunted in this environment, where the diet is based on aquatic animals such as fish, chelonians and alligators (Lopes et al., Reference Lopes, Valsecchi, Vieira, Amaral and Costa2012; Morcatty & Valsecchi, Reference Morcatty and Valsecchi2015).
The sizes of hunting grounds recorded were similar to those recorded in previous studies for all game species (Table 3). Most studies of hunting, including in the Amazon, have found that hunters rarely walk more than 10 km from their home community to their harvesting sites (de Thoisy et al., Reference de Thoisy, Renoux and Julliot2005; Smith, Reference Smith2005, Reference Smith2008; Peres & Nascimento, Reference Peres and Nascimento2006; Parry et al., Reference Parry, Barlow and Peres2009, Read et al., Reference Read, Fragoso, Silvius and Luzar2010; Constantino, Reference Constantino2015). However, few of these studies have discriminated between movements occurring within the forest, along country roads and hunting trails, and movement along rivers and other water bodies, in canoes. Franzen (Reference Franzen2006), however, evaluated this aspect of hunting patterns among the Huaorani people in Ecuador, recording mean distances of 2.9, 9.9 and 14.5 km per hunt in three study communities, which were corrected to 2.8, 4.2 and 3.2 km per hunt, respectively, when only the forest environments were considered. In Central Amazonia most movements occur in canoes or small boats on rivers, channels or lakes (Fig. 1). When we analysed the hunting sites that were accessed by walking through the forest, the mean distance moved from the home community was 11.59 km in the paleovárzea and 3.24 km in the várzea. However, when the distance travelled by water was discounted, considering only the distance walked through the forest, the mean distances decreased to 2.06 km in the paleovárzea and 0.48 km in the várzea. The rivers of the Amazon, besides being important transit routes, are also used during subsistence activities, such as fishing and hunting, or for access to areas of family gardens (Fig. 1).
In Panama 100% of primate harvesting occurred within 7 km of the study communities, and 78% within 4 km (Smith, Reference Smith2008). In Acre 95.9% of all game hunting by the Kaxinawa people in terre firme forest occurred within 5 km of the village, and at least 65% was within 2.5 km (Constantino, Reference Constantino2015). In our study hunters travelled up to 40 km in the paleovárzea and 21 km in the várzea. During the 11 years of monitoring at least 30% of all captures of primates were recorded within 10 km of the hunter's home community. More distant harvesting sites were recorded for the communities that hunted in the paleovárzea, partly as a result of the specific history and cultural practices of the communities of Boa Esperança and Nova Jerusalém. Both settlements were previously located far from their present location. However, hunters still use those old sites (Alencar, Reference Alencar2007) and visit them for various activities, including hunting. We observed a relationship between spatial use for hunting activities and the distribution of water bodies, similar to that observed elsewhere (e.g. Panama, Smith, Reference Smith2008; Peru, Ohl-Schacherer et al., Reference Ohl-Schacherer, Shepard, Kaplan, Peres, Levi and Yu2007).
Harvesting of howler monkeys tended to occur in the vicinity of the communities in the várzea but at greater distances from the communities in the paleovárzea. This was expected, given the species’ folivorous diet and relatively small home range size (Palacios & Rodriguez, Reference Palacios and Rodriguez2001; Jung et al., Reference Jung, Mourthe, Grelle, Strier and Boubli2015), and its occurrence at high densities in the várzea environment of Mamirauá (Queiroz, Reference Queiroz1995). In contrast, the frugivorous Cacajao ouakary occupies relatively large home ranges (Boubli, Reference Boubli1999), yet it was harvested in large numbers close to the communities in the paleovárzea. This may be because all of the communities monitored are surrounded by strips of igapó, which is the main habitat used by the C. ouakary during the dry season because of the abundance of unripe fruit (Barnett & Brandon-Jones, Reference Barnett and Brandon-Jones1997; Barnett et al., Reference Barnett, Defler, Oliveira, Queiroz, Bezerra, Veiga, Barnett, Ferrari and Norconk2013).
Given the total population of the communities monitored (1,048 inhabitants), relatively few (148) sites were used to harvest primates during the 11-year study period. Franzen (Reference Franzen2006) recorded 101 harvesting sites (for all hunted species) during only 4 months of monitoring, in a study of 220 Huorani in Ecuador. In an 8-month study of the Buglé and Ngobe communities in Panama (725 residents), Smith (Reference Smith2008) recorded a total of 1,269 harvesting sites for all hunted species. It is possible hunting was the most important activity in these communities in terms of both subsistence and income generation, whereas in the communities of Mamirauá and Amanã hunting was of secondary importance and was a source of bushmeat but not of income.
Although many species of primates found in the study area are not among the preferred game species of the local communities (Valsecchi & Amaral, Reference Valsecchi and Amaral2009; Lopes et al., Reference Lopes, Valsecchi, Vieira, Amaral and Costa2012), primates are a preferred dietary resource for some families (authors, pers. obs.), and at least one primate species is among the most hunted game species in the region. Alouatta juara was the most hunted primate in the várzeas of Mamirauá and the second most hunted primate in the paleovárzea of Amanã. These findings reinforce the need for continuous monitoring of hunting areas, as well as analysis of their spatial variation over time, to understand the extent of the impacts of hunting pressure on local primate populations and to ensure the long-term food security of local communities.
The stability in the size of the areas used for hunting primates, the number of sites used and the distances travelled to hunting sites suggests that primate hunting in the region is not causing a significant reduction in natural capital, or else the removal of this biological resource is balanced by the replacement rate. The source–sink model described by Novaro et al. (Reference Novaro, Redford and Bodmer2000) probably applies here, mainly in the paleovárzea areas, which are interconnected with continuous non-inhabited forests, serving as sources for the inhabited areas. The pattern in the spatial–temporal distribution of hunting during the 11-year study period corroborates models that point to possible sustainability of this activity. The model based on the differentiated productivity of the hunted species (Robinson & Redford, Reference Robinson and Redford1991) would apply to the two environments under analysis, considering the spatial distribution of hunting (Figs 2 & 3) and the areas where there is a higher intensity of hunting (Fig. 1), showing that all monitored communities have in their proximity large areas where there is little or no primate hunting.
The spatial patterns of primate hunting in the study communities are probably similar to those found in other communities in the same or similar environments in Central Amazonia. Residents of communities in várzea areas are primarily fishers, for whom hunting is a secondary source of animal protein (Lima, Reference Lima2005; Lopes et al., Reference Lopes, Valsecchi, Vieira, Amaral and Costa2012). For communities in paleovárzea environments, prey populations are probably being supported by prey movements between source and sink areas (hunting areas).
We have shown that spatial analysis of hunting activities can provide important insights into hunting, as well as providing indicators of hunting sustainability in a given area. The spatial patterns of hunting may be used to inform the development of guidelines for the use of the fauna in a given area, with the participation of local inhabitants, as well as for the conservation of game species, especially those under the greatest pressure from hunting. The involvement of local communities in management decision making will ensure greater legitimacy and can broaden the recognition of regulations for the use of biological resources.
Acknowledgements
We thank the Fauna Use Monitoring System monitors and all the hunters who kindly helped in our research. We also thank the Mamirauá Sustainable Development Institute and the Ministry of Science and Technology for logistical and financial support for this research.
Biographical sketches
Priscila Pereira works in the fields of scientific research and conservation, with a particular focus on hunting and the use of hunting grounds. João Valsecchi works mainly in the field of wildlife conservation, with a focus on wildlife hunting, ecology and conservation of mammals. Helder Queiroz works on the ecology of flooded forests and the conservation of Amazon biodiversity, with particular emphasis on in-situ conservation in protected areas of the Amazon.








