Introduction
A common cause of out-of-hospital cardiac arrest (OHCA) requiring cardiopulmonary resuscitation (CPR) is thrombus formation resulting in coronary artery occlusion or pulmonary embolism.Reference Deakin, Nolan and Soar 1 In order to save the lives of those affected, the cause needs to be identified as soon as possible in order to start the targeted therapy. This process begins with the advanced cardiac life support (ACLS). Thrombolysis in the field can be considered in certain situations. The European Resuscitation Council (ERC) 2010 guidelines do not recommend this in all cases of cardiac arrest, however thrombolytics can be considered when pulmonary embolism is highly suspected or proven in order to improve survival and neurological outcome of patients.Reference Deakin, Nolan and Soar 1 In addition to thrombolysis, an active mechanical guide wire fragmentation of the thrombus with a flexible endovascular wire is effective in improving pulmonary perfusion and clinical outcome.Reference Murphy, Mulvihill and Mulcahy 2
In OHCA, optimal CPR is vital for the survival of the patients.Reference Larsen, Eisenberg and Cummins 3 Effective chest compressions with a depth of at least 38 mm and maintenance of a high rate are most relevant for the quality of CPR.Reference Stiell, Brown and Christenson 4 This may sometimes be difficult to implement in all environments with manual chest compressions. Using automated chest compression devices may help to ensure a continuous good performance of chest compressions and perfusion throughout the treatment, transport, and movement of patients. It can be used during further diagnositic testing such as computed tomography (CT).Reference Deakin, Nolan and Soar 1 , Reference Wirth, Körner and Treitl 5 Early CT in these situations has demonstrated improved survival rates.Reference Huber-Wagner, Lefering and Qvick 6
Case Report
A 26-year-old female presented at a general practitioner’s surgery with dyspnoea. Whilst obtaining history the patient arrested. CPR was initiated immediately by the presiding staff and emergency medical services (EMS) activated at min 0 (Table 1). The First Responder Unit was dispatched and arrived at min 5. Manual CPR was continued and an automated external defibrillator (AED Pro, Zoll, Chelmsford, USA) was applied. There was no shock recommendation given by the device.
Table 1 Timeline of events

Mechanical CPR (mCPR) was continued by the automated chest compression device LUCAS® (Jolife, Lund, Sweden). A physician-staffed ambulance reached the scene shortly after, at min 7. The patient remained pulseless. The initial electrocardiography (ECG) showed a systole and intermittent bradycardia with a rate of less than 30 beats/min, resulting in a pulseless electrical activity (PEA). The patient was endotracheally intubated by the physician and 3 mg adrenaline (epinephrine) were given via the tube. Intravenous access was obtained via a foot vein and 1 mg adrenaline was administered every 5 min, as well as a dose of 80 IU vasopressin.
Pulmonary embolism was the highly suspected diagnosis given the history of dyspnoea prior to collapse, knee brace after surgery, and intake of oral contraceptives. Rescue-in-field thrombolysis was initiated therefore by the EMS physician with 50 mg alteplase (Actilyse Boehringer Ingelheim GmbH, Ingelheim am Rhein, Germany). In addition, 5000 IU unfractionated heparin were given.
Under ongoing mCPR, the patient was transported to our shock trauma unit with integrated CT and arrived at min 21. The correct position of the tube was confirmed by ETCO2 of 20 mm Hg; SpO2 was 68 mm Hg with FiO2 of 1.0.
A change of the automated chest compression device to the AutoPulse® device (Zoll Medical GmbH, Chelmsford, MA, USA) was necessary as LUCAS devices do not fit into the gantry of our CT due to their height. At min 25 the multidetector CT imaging was initiated in a 64-slice CT scanner (Light Speed VCT, GE Healthcare, Waukesha, Wisconsin, USA). A scout scan was performed while running the AutoPulse device (Figure 1). In order to do this, we administered a contrast bolus IV, followed by two minutes of automated chest compressions for distribution. The mCPR device was put on hold shortly, whilst the whole body CT was performed. Contrast-enhanced CT of the chest showed a central pulmonary embolism of both sides (Figure 2).

Figure 1 Scout scan with running AutoPulse® device.
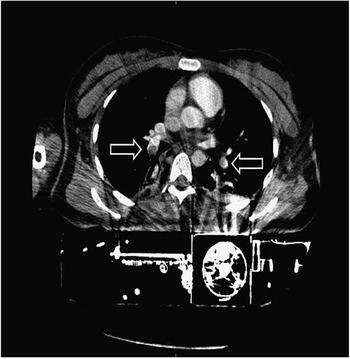
Figure 2 Contrast enhanced chest CT showing central pulmonary embolism of both sides (arrows).
Systemic thrombolysis was continued. At min 58 the ECG showed a systole with intermittent bradycardic episodes at a rate of 30/min and a blood pressure of 85/50 mm Hg, still under vasopressor support.
In the angiography suite, the thrombus was fragmented on both sides using a Terumo® endovascular flexible wire (Terumo Europe N.V. Interleuvenlaan 40B-3001 Leuven, Belgium) via a jugular vein access at min 68.
Immediately after the procedure the blood pressure rose to 103/55 mm Hg and the vasopressor support was reduced.
During intensive care unit (ICU) treatment the patient suffered from several complications. She developed systemic inflammatory response syndrome (SIRS) and disseminated intravascular coagulation (DIC) with multiple organ dysfunction syndrome, including renal failure. On day 2, due to an acute abdominal compartment syndrome, a decompression laparotomy and double-loop ileostomy were performed. On day 9, a hemicolectomy of the right colon and a single-loop transversostomy was necessary due to segmental colon ischemia. Part of the small intestine was resected and an ileostomy accomplished.
The patient recovered with no neurological deficit and was discharged from hospital at day 40. At day 112 the ileostomy and the colostomy could both be closed and a normal colonic passage was reconstituted.
Discussion
As stated in the ERC 2010 guidelines, ongoing CPR is not a contraindication for fibrinolysis.Reference Deakin, Nolan and Soar 1 Although not all cases of cardiac arrest should undergo fibrinolysis in the field, the ERC 2010 guidelines suggest it should be considered when pulmonary embolism is highly suspected in the field and proven in hospital.Reference Deakin, Nolan and Soar 1 The TROICA-studyReference Spöhr, Arntz and Bluhmki 7 was designed to assess safety and efficiency of prehospital thrombolytic therapy with tenecteplase versus placebo in cardiac arrest, and is one of the largest trials, with 1,050 enrolled patients. The results did not show an improvement in 30-day survival for all patients, but cases with pulmonary embolism did benefit from early thrombolysis.Reference Bottiger, Arntz and Chamberlain 8
In a smaller case series and retrospective analysis of patients with pulmonary embolism undergoing CPR,Reference Scholz, Hilmer and Schuster 9 the patient outcomes support thrombolytic therapy when pulmonary embolism is highly suspected. Our patient had known risk factors, including a history of knee surgery with immobilisation, as well as oral contraceptive use. Most importantly, her main symptom prior to cardiac arrest was dyspnoea. The pulmonary embolism was proven later in our shock trauma unit by early contrast-enhanced CT. In addition, we performed an active fragmentation of the embolism (which studies suggest may improve circulation and recovery of patientsReference Murphy, Mulvihill and Mulcahy 2 ).
During manual CPR there can be negative effects of rescuer fatigue, resulting in ineffective chest compressions.Reference Abella, Sandbo and Vassilatos 10 In an uncontrolled clinical setting there may even be frequent interruptions of the compressions.Reference Deakin, Nolan and Soar 1 , Reference Wik, Kramer-Johansen and Myklebust 11 For our patient, CPR was performed from the beginning with mCPR devices at a constant rate and depth. The ERC 2010 guidelines state that during an ambulance transfer and movement of patients, mCPR devices may help to maintain a good quality of CPR.Reference Deakin, Nolan and Soar 1 With mCPR, there is no interruption of the compressions in the process of further examination in the hospital, as shown in an initial case series.Reference Wirth, Körner and Treitl 5
The PROCAT study examined the benefit of an early imaging algorithm in an ICU database of OHCA with return of spontaneous circulation (ROSC). Coronary angiography was performed unless the patient had neurological or respiratory signs, in which case a chest CT was done. In this manner nearly two-thirds of the cases were diagnosed, leading to targeted therapy.Reference Chelly, Mongardon and Dumas 12 The feasibility of early imaging with focused CT in resuscitation (FACTR) has been discussed in other reports.Reference Kanz, Huber-Wagner and Kay 13 , Reference Moriwaki, Tahara and Kosuge 14
During cardiac arrest there is no circulation for contrast-enhanced CT imaging. The use of mCPR devices when performing a CT seems to be valuable.Reference Wirth, Körner and Treitl 5 Those devices are able to provide a blood flow and therefore also the distribution of the contrast solution when used during CT image acquisition. The ERC 2010 guidelines state that mCPR in cardiac arrest may be used effectively whilst undergoing CTs.Reference Deakin, Nolan and Soar 1 In an initial study, the CT image quality was conclusive. The main lesions could be identified in all patient cases that led to clinical key decisions and focused treatment.Reference Wirth, Körner and Treitl 5 Therefore, the authors concluded that the use of mCPR devices is feasible during image acquisition.Reference Wirth, Körner and Treitl 5
Although several trials have been made, it cannot be proven that humans benefit from mechanical chest compressions.Reference Abella 15 Also, targeted post-ROSC CT has not been adopted in the American Heart Association ACLS 2010 guidelines or by the International Liaison Committee on Resuscitation (ILCOR).
Conclusion
This case report shows an interesting approach to out-of-hospital cardiac arrest (OHCR) caused by pulmonary embolism in both diagnoses and treatment. Contrast-enhanced CT imaging with ongoing automated chest compressions is feasible within 30 minutes after admission.
Competing Interests: None declared.





