Introduction
Hemodynamic stroke is a type of ischemic stroke secondary to hypoperfusion where blood flow is reduced to a critical level to cause ischemic injury. Reference Klijn and Kappelle1 In patients with steno-occlusive cerebrovascular diseases (SOD), both intracranial and extracranial, post-stenotic reduction in perfusion pressure and flow limitation results in regional hypoperfusion. However, autoregulation-related vasodilation and flow from the collateral circulation can compensate for reduced perfusion pressure. Hence, the risk of hemodynamic stroke in these patients depends on the post-stenotic vasodilatory reserve Reference Tzeng and Ainslie2 and on the availability of recruitable flow through collateral blood vessels. Reference Liebeskind3 The presence of such recruitable flow markedly reduces the risk of stroke Reference Strother, Anderson and Singer4,Reference Liebeskind, Cotsonis and Saver5,Reference Vernieri, Pasqualetti and Matteis6,Reference Liebeskind, Cotsonis and Saver7 and the need for re-vascularization. Reference Sahoo, Suri, Bansal, Devarajan and Sharma8,Reference Lau, Wong, Wong, Mok, Leung and Wong9 However, in contrast to extracranial stenosis, recruitable collateral supply is limited in patients with intracranial stenosis and hence the incidence of hemodynamic stroke is higher. Cerebrovascular reactivity (CVR) is the measure of the change in cerebral blood flow (CBF) in response to a vasodilatory stimulus i.e., the measure of vascular reserve capacity. It measures the change in flow in the parenchymal vessels, i.e., the sum of flows from the feeding artery and collateral blood flow. Reference Willie, Macleod and Shaw10,Reference Rudzinski, Swiat, Tomaszewski and Krejza11 Over the past 10 years, we have developed a noninvasive, reproducible CVR brain stress test Reference Spano, Mandell and Poublanc12 to measure the cerebrovascular reserve in patients with symptomatic steno-occlusive disease (SOD) using a precisely controlled change in end-tidal (end-expiratory) partial pressure of CO2 (PETCO2) as a vasodilatory stimulus and the Blood Oxygen Level Dependent (BOLD) MRI signal as a semiquantitative surrogate of CBF. Reference Mandell, Han and Poublanc13,Reference Sobczyk, Battisti-Charbonney and Poublanc14,Reference Mandell, Han and Poublanc15
In this retrospective study, we examine the sensitivity of the brain stress test in identifying patients at risk for hemodynamic stroke. Secondarily, we examine the relationship between SOD and the pattern of CVR on the one hand, and the incidence of stroke on the other. We were particularly interested in the incidence of stroke after 1 year in a cohort of symptomatic patients who had similar degree of SOD but were discordant as to the presence of normal or impaired CVR. We hypothesized that in patients with similar degrees of stenosis, the CO2 MRI brain stress test can help identify patients with impaired CVR and the incidence of stroke will be different between patients with normal CVR and impaired CVR.
Materials and Methods
Patients
The institutional ethics committee approved the study protocol (UHN REB #13-7168) and all patients provided written informed consent. Consecutive patients with angiographically (CT or MRI) demonstrated intracranial SOD (>70% stenosis or occlusion) who presented with transient ischemic attacks (TIA) or stroke were recruited for CVR testing within 3 months of their presentation. We excluded patients whose symptoms were suspected to be of extra-cranial SOD, cardiac (history of atrial fibrillation, valvular heart disease) and thromboembolic etiology. We screened patients for contraindication to MRI environment or hypercapnia (end-stage chronic obstructive pulmonary disease with CO2 retention, intracranial hypertension).
CVR Measurement
The apparatus and technique for controlling the end-tidal pCO2 (PETCO2) have been described in greater detail elsewhere. Reference Slessarev, Han and Mardimae16,Reference Kretschmann and Weinrich17 In brief, the subjects breathe without restriction while wearing an airtight light plastic mask. Gases are supplied via an automated gas blender running a feed-forward algorithm to target PETCO2 and PETO2 described by Slessarev et al (RespirAct™ Thornhill Medical Inc. Toronto, Canada). Reference Kretschmann and Weinrich17 The user enters the target end-tidal gas values and their durations. The RespirAct™ system then uses the algorithm to calculate the various gas concentrations and flows it will administer to the breathing circuit to attain the target end-tidal gas partial pressures. The gas sequence for CVR measurement consisted of two square wave hypercapnic stimuli (from baseline to baseline plus 10 mmHg) of 90- and 120-s duration, respectively. Iso-oxia (target PETO2 of 100 mmHg) was maintained throughout. PETCO2 and PETO2 were monitored continuously, digitized, and recorded.
Imaging
MR imaging was performed on a 3T whole-body scanner (Signa HDx; GE Healthcare, Milwaukee, Wisconsin) with an 8-channel-phased array head coil for signal reception. Each patient was imaged with an identical CO2-BOLD-CVR protocol preoperatively and postoperatively. Each CVR session included routine clinical acquisitions (sagittal T1-weighted, axial T2-weighted, axial T2-weighted FLAIR, and diffusion-weighted), an axial T1-weighted 3D spoiled gradient-echo acquisition (matrix size, 256 × 256; section thickness, 2.2 mm; intersection gap, 0) for anatomic coregistration, and an axial T2*-weighted single-shot gradient-echo echo-planar BOLD acquisition (flip angle, 85°; TR, 2000 ms; TE, 30 ms; Voxel size 3.75 × 3.75 × 5 mm; intersection gap, 2 mm; the number of frames, 255) during controlled changes in PetCO2.
CVR Maps
MR imaging and PETCO2 data were imported into the software AFNI (National Institutes of Health, Bethesda, Maryland; http://afni.nimh.nih.gov/afni). An AFNI algorithm was used to calculate head motion for each BOLD MR imaging acquisition. Each patient’s whole-brain BOLD MR signal-intensity dataset was temporally shifted to the point of maximum correlation with the patient’s PETCO2 waveform to compensate for the delay of gas analysis resulting from transit time in the long gas sample line. The BOLD MR signal-intensity time waveform then underwent least-squares linear fitting to the PETCO2-time waveform on a voxel-by-voxel basis, and CVR was calculated as the slope % BOLD-MR signal intensity per mm Hg PETCO2. Anatomic images were automatically segmented into gray matter and white matter and transformed into Montreal Neurologic Institute space by using the software SPM8 (Welcome Department of Imaging Neuroscience, Institute of Neurology, University College, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Anatomic images were further segmented into anterior (ACA), middle (MCA) and posterior (PCA) cerebral vascular territories by using masks created from the atlas of Kretschmann and Weinrich Reference Kretschmann and Weinrich17 . Mean gray matter CVR was calculated for each of these segments, for each CVR study. The preoperative and postoperative routine T1- and T2-weighted images were reviewed to identify regions of parenchymal infarction or hemorrhage.
Treatment and Follow-up
Patients were offered revascularization on taking the full clinical and imaging picture into account. Patients with impaired or paradoxical CVR (areas with intracerebral steal) were offered revascularization. Surgical revascularization consisted of extracranial-intracranial (EC-IC) bypass using either a direct superficial temporal artery to middle cerebral artery (STA-MCA) bypass or indirect encephalo-dural–arterial synangiosis (EDAS). EDAS was performed if the STA-MCA bypass was not deemed to be technically achievable. Revascularization was carried out on the recently symptomatic side with impaired CVR. All patients were prescribed standard optimal medical therapy (OMT). Reference Kernan, Ovbiagele and Black18,Reference Kim and Bang19 All patients had both clinical and radiological (structural imaging) follow-ups at 1 year. In addition, patients who had surgical revascularization had postoperative CVR at 3 months and 1 year. Clinical assessment included inquiring about a history of continued or new TIAs, strokes, seizures, or changes in neurological or cognitive function. The occurrence of stroke was diagnosed based on clinical presentation with radiological confirmation.
Performance Metrics and Data Analysis
Outcome Measures
The primary outcome was to identify patients with poor cerebrovascular reserve, a marker of high risk for hemodynamic stroke. Secondary outcomes include improvement in CVR with intervention, a correlation between improvements in CVR and clinical symptoms, and finally the incidence of stroke. We categorized patients as having either normal CVR or impaired CVR on the side ipsilateral to the vascular lesion on presentation. Impaired CVR consists of either reduced CVR or paradoxical or negative CVR. In our previous study from healthy volunteers (n = 30), mean (±SD) CVR in MCA territory was 0.30 ± 0.07 (%BOLD MR signal intensity per mm Hg of PET CO2). Reference Gupta, Chazen and Hartman20 We used 2 SD below normal CVR (0.16%) in the MCA territory as a cut-off threshold for reduced CVR. Improvement in CVR was defined as a > 25% increase in reactivity as compared to preoperative values. In patients with unilateral interventions, CVRs from the vascularized hemisphere were considered, and in patients who had bilateral interventions, the average CVR between the hemispheres was considered.
Data Analysis
Statistical analyses were performed using Graphpad Prism version 8.0, GraphPad Software, San Diego, California USA, and excel (Microsoft Corporation, USA). Demographics data were presented n (%) or mean (±SD) as appropriate. Descriptive statistics were used to measure the incidences of impaired CVR, interventions, response to intervention and the clinical outcome (stroke). A Mann–Whitney test was used to analyze the difference between CVR values between patients who had an intervention to those that did not have an intervention. A Wilcoxon matched-pairs signed rank test (two-sided, α = 0.05) with Bonferroni correction was used for statistical analysis comparing the prerevascularization and postrevascularization CVR values. A Wilcoxon matched-pairs signed rank test (two-sided, α = 0.05) with Bonferroni correction was used for statistical analysis comparing the prerevascularization and postrevascularization CVR values. Fisher’s exact test (2 × 2 contingency table) was used to analyze the incidence of stroke between the normal CVR vs impaired CVR groups and the incidence of ipsilateral stroke with and without intervention in the impaired CVR group.
Results
We recruited 100 patients, with 17 patients excluded (Figure 1). The demographic data are presented in Table 1. The majority (71/83) of patients presented with TIA or ischemic stroke, and a small number (n = 9) of patients presented with hemorrhage which occurred in areas of neovascularization, especially in patients with moyamoya vasculopathy.

Figure 1: Flow chart with patient selection criteria and outcome. n – number, CVR – cerebrovascular reactivity, EC–IC – extracranial–intracranial, EDAS – encephalo-dural–arterial synangiosis, SOD – steno-occlusive disease. TIA – transient ischemic attack.
Table 1: Patient demographics

CVR – Cerebrovascular reactivity; n – number; Yrs – years; SD – Standard deviation; TIA – Transient Ischemic attacks; ICA – Internal carotid artery; MCA – Middle cerebral artery.
There were no reported adverse events during the test. The average (range) resting PETCO2 was 37.4 (27–48) mmHg. Hypercapnia (resting + 10 mmHg) was achieved within 1.4 ± 1.3 mmHg (mean ± SD of) of the target in all patients. Fourteen patients showed normal CVR (0.21% ± 0.04%/mmHg) and impaired CVR (0.09 %± 0.06%/mmHg) was seen in 69 patients, predominantly in the middle and anterior cerebral artery territories. CVR values in MCA and ACA territories on the ipsilateral and contralateral sides are shown in Table 2.
Table 2: Cerebrovascular reactivity values in the ipsilateral and the contralateral middle and anterior cerebral artery territories

Values (%BOLD MR signal intensity/mmHg of PET CO2) are presented as mean ± SD. CVR – cerebrovascular reactivity; MCA – middle cerebral artery; ACA – anterior cerebral artery.
Among the patients who had impaired CVR, 76% (53/69) underwent a revascularization procedure and the reminder (16/69) did not have revascularization (Figure 1). There were no significant differences in the preoperative CVR between the subjects who had an intervention and those who did not (Figure 2) [P = 0.26 for MCA and P = 0.74 for ACA (Mann–Whitney U test)]. In the postsurgery follow-up 86% (46/53) of patients showed improvement in the CVR at 1 year. There were no new symptoms in patients who had revascularization.

Figure 2: Bar graphs showing CVR with no surgical intervention (N = 16) compared to intervention (N = 53) in the middle cerebral artery (MCA) and Anterior cerebral artery (ACA) vascular territories. The CVR Mean (SD) values in the MCA territory were 0.05 (0.07) n = 53 in the intervention group, and 0.07 (0.1) n = 16 in the nonintervention group; the distributions in the two groups did not significantly differ (Mann–Whitney U = 410.5, P = 0.26 two-tailed). The CVR values in the ACA territory were 0.06 (0.08) n = 53 in the intervention group, and 0.08 (0.1) n = 16 in the nonintervention group; the distributions in the two groups did not significantly differ (Mann–Whitney U = 474.5, P = 0.74 two-tailed).
Pre- and post-revascularization CVR values are shown in Figure 3. Unilateral revascularization (n = 35) significantly improved the CVR in the ipsilateral MCA territory (mean ± SD 0.039 ± 0.069) to 0.100 ± 0.062 %BOLD/mm Hg, P = 0.01) (Figure 3). Seven patients showed no significant improvement in CVR after intervention [direct bypass (5), EDAS (2)]; however, they were clinically asymptomatic at 1 year.

Figure 3: Preoperative versus postoperative CVR values in the (A) ipsilateral middle cerebral artery (MCA); (B) contralateral MCA; (C) ipsilateral anterior cerebral artery (ACA), and (D) contralateral ACA territory (N = 35). Bar graphs denote the mean (SEM) error bars. Four asterisks indicate a P < 0.0001, corrected for multiple comparisons.
The incidence of stroke in the cohort at 1 year was 4.8 % (4/83). All the strokes occurred in patients with impaired CVR (4/69) and none in patients with normal CVR despite no revascularization (Figure 4). However, it was not statistically significant (4/69 vs 0/14 p = 1.0, Fisher’s exact test)). Among the patients with impaired CVR, all the strokes happened on the nonrevascularized side; two strokes occurred in patients who had no revascularization and the other two occurred in the contralateral nonrevascularized side in patients with bilateral SOD who had ipsilateral revascularization. (4/16 vs 0/53 p = 0.05, Fisher’s exact test). Note that all the strokes occurred in the area, which showed impaired CVR (Figure 4). Six (6/69, 8.7%) patients with impaired CVR and no revascularization had self-reported worsening of cognitive function at 1 year. Among the patients who had normal CVR, three patients (3/14, 21%) had episodes of TIA’s (ipsilateral to SOD) and one patient (1/14, 7.1%) complained of worsening cognitive function at 1 year. There were no clinical strokes in this group.

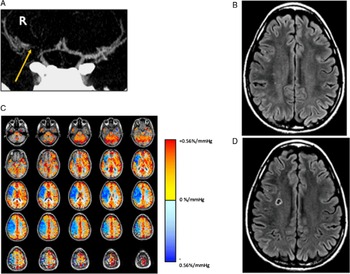
Figure 4: Example of a patient who presented with transient left face and upper and lower extremity paresthesia and weakness. (A) CT angiogram shows severe narrowing of the right middle cerebral artery (yellow arrow). (B) T2-weighted FLAIR image showed no evidence of brain infarct. (C) CVR study shows reduced (paradoxical) reactivity throughout the right MCA territory and preserved reactivity elsewhere. (D) 4-month follow-up MRI demonstrating a new infarct in the right MCA territory corresponding to the region of impaired CVR.
Discussion
Our study confirms the previous findings Reference Spano, Mandell and Poublanc12,Reference Mandell, Han and Poublanc13 that 1) CO2-BOLD MRI CVR can be used as a brain stress test for the assessment of cerebrovascular reserve in patients at risk for hemodynamic stroke. 2) Impaired CVR, a marker of the poor cerebrovascular reserve is associated with a higher incidence of stroke. 3) Normal CVR despite significant stenosis appears to be associated with a low risk of stroke.
In this study, we examined a cohort of symptomatic subjects with similar degrees of severe SOD (>70 % stenosis or occlusion). When subdivided according to a functional hemodynamic assay, the CVR, we found that 17% (14/83) of patients had a normal functional hemodynamic reserve. Their physicians, taking all clinical information into account, advised that they do not need revascularization, but be treated with OMT. At 1 year, none of these patients had a stroke. The remainder of the cohort did have reduced hemodynamic reserve as indicated by a reduction in CVR. The strokes occurred exclusively in this group. The subgroup that did not undergo revascularization had a higher incidence of stroke, though not statistically significant, than those that did. In addition, even in the group who had revascularization, two strokes occurred on the contralateral side with impaired CVR in patients with bilateral disease. We propose that patients, who have normal CVR, have a source of collateral blood flow that can be recruited to compensate for exhausted vasodilatory reserve, in the event of reductions in perfusion pressure or acute increased upstream obstruction, such as thrombosis. This collateral flow may not be as brisk or generous as that recruited by autoregulation in healthy vascular beds, but it appears that it is sufficient to maintain cellular integrity until blood flow readjusts and the neurological function returns, resulting in a TIA, rather than a stroke.
Clinical Implications
Assessing the cerebrovascular reserve capacity is important to determine the risk of hemodynamic stroke. Impaired CVR and stroke risk is not novel finding. In a systematic review and a meta-analysis, Gupta et al reviewed 1061 independent CVR tests in 991 patients and showed a significant positive relationship between impairment of CVR and development of stroke with an OR 3.86 (95% CI, 1.99–7.48). Reference Gupta, Chazen and Hartman20 They also found the association between CVR impairment and risk of stroke conserved across testing modalities (transcranial Doppler, positron emission tomography (PET), Single Photon Emission Computed Tomography (SPECT) and MR imaging techniques) as well as the nature of the vasodilatory stimulus (acetazolamide or variation in inspired CO2 levels). Reference Gupta, Chazen and Hartman20 However, our study showed that normal CVR despite hemodynamically significant SOD has a lower incidence of stroke at 1 year. Reinhard et al have previously shown that the presence of severe SOD was highly specific for the risk of stroke but not sensitive. Reference Reinhard, Schwarzer and Briel21 In line with this finding, our study shows that one needs both the presence of SOD and impaired CVR to be at risk for stroke. First, in our study, a quarter of the patients with good recruitable CVR were at low risk for stroke. It is important to identify these patients because surgical intervention would put them at risk of complications with no compensating reduction of the risk of stroke. Second, the low CVR in the remainder of the patients suggests their vascular lesion was poorly compensated by recruitable collateral flow, putting them at high risk for stroke. This suggests that an optimal clinical algorithm would best first identify those with SOD, and then identify those patients with high and low risk of stroke. Our data do not address whether abnormal CVR marks a high risk of stroke, but abundant studies suggest that this is the case. Our study does suggest that normal CVR would be a marker for a lower risk of stroke.
Randomization of SOD without further regard to identifying a low-risk group will result in an overestimation of the benefit of OMT, as this group will inevitably contain patients with good collateralization and low risk of stroke. It will also result in an underestimation of the benefit of the revascularization arm, as it would include surgical complications in the low-risk group where there will be no additional reduction of risk. Reference Derdeyn, Chimowitz and Lynn22 Interestingly, in patients who underwent revascularization, there is an improvement in CVR but their improvement is seldom to the mean normal range. Hence, these patients may continue to have chronic hypoperfusion or reduced recruitment of collateral flow. Reference Fierstra, Poublanc, Han, Silver, Tymianski and Crawley23
Limitations
Our study has several limitations. Firstly, unlike PET, arterial spin labelling (ASL) and phase-contrast MR angiography, BOLD does not directly measure CBF. The BOLD signal depends on several factors including cerebral metabolic rate, cerebral blood volume, and CBF, and hence, it can only provide an indirect nonquantitative measure of CBF provided other factors remain constant. These factors should always be considered during the interpretation of BOLD-CVR data. However, a previous study comparing ASL-CVR and BOLD-CVR has shown that even in patients with the SOD, the BOLD signal response to hypercapnia predominantly reflects changes in CBF. Reference Mandell, Han and Poublanc15 Similarly, it has been shown recently that BOLD CVR corresponded well to CBF perfusion reserve measurements obtained with (15O-) H2O-PET, especially for detecting hemodynamic failure. Reference Fierstra, van Niftrik and Warnock24 Secondly, this is a prospective observational study looking at a small number of patients considering the low incidence of stroke. However, the CVR benefits from the standardization of the stimulus. The small variability in the stimulus removes this confounder from the data increasing the specificity of the test. Thirdly, there was no randomization with regard to surgical interventions, leaving this as an observational study focusing on the outcome in patients with hemodynamically significant SOD yet normal CVR. The patients with abnormal CVR could not be randomized as they were recommended for revascularization based on previous evidence. There was a higher incidence (though statistically not significant) of stroke in patients with abnormal CVR whether or not they were revascularized. This suggests a strong effect size for abnormal CVR. Another limitation of this study is that patients were followed up for only 1 year, as this is our standard clinical practice. Longer follow-up may provide additional insights, especially in patients with normal CVR with severe SOD. In addition, only patients who had revascularization had follow-up CVR and observed improvement in CVR may partly reflect the natural history of the disease.
Conclusions
Our study confirms the prior findings that impaired CVR, a marker of the poor cerebrovascular reserve is associated with a higher incidence of stroke. Normal CVR despite significant stenosis appears to be associated with a low risk of stroke. This study further establishes that the noninvasive BOLD MRI CVR technique is sensitive and able to identify at-risk patients, and can therefore be more widely used.
Acknowledgements
This work was supported by the Holt-Hornsby and Andreae Vascular Dementia Research Unit in the Joint Department of Medical Imaging at the Toronto Western Hospital and the University Health Network.
Funding
This study was funded in part by a grant from MSH-UHN AMO AFP Innovation fund 2013-15.
Disclosures
RespirActTM is currently a noncommercial research tool assembled and made available by Thornhill Research Inc. (TRI), a spin-off company from the University Health Network, to research institutions to enable CVR studies. JAF is the Chief Scientist and JD is the Senior Scientist at (TRI), and JP, OS, and DJM have contributed to the development of RespirAct™ and have received payments from, or shares in, TRI.
Statement of authorship
LV, JF, and DJM designed the study. LV, CR, and LM analyzed the data and wrote the manuscript. LV, CR, LM, JP, OS, JD, JF, and DJM interpreted the data for the manuscript submission. LV, CR, LM, JP, OS, JD, MT, JF, and DJM contributed to the manuscript revision and reviewed and approved the submitted version.