INTRODUCTION
Meiofaunal organisms are benthic components that play a central role in the seas both as consumers and prey (Leduc & Probert Reference Leduc and Probert2009; Zeppilli et al., Reference Zeppilli, Sarrazin, Leduc, Martinez Arbizu, Fontaneto, Fontanier, Gooday, Kristensen, Ivanenko, Sørensen, Vanreusel, Thébault, Mea, Allio, Andro, Arvigo, Castrec, Danielo, Foulon, Fumeron, Hermabessiere, Hulot, James, Langonne-Augen, Le Bot, Long, Mahabror, Morel, Pantalos, Pouplard, Raimondeau, Rio-Cabello, Seite, Traisnel, Urvoy, Van Der Stegen, Weyand and Fernandes2015). They are also the most diversified and abundant component of the marine benthic domain (Balsamo et al., Reference Balsamo, Albertelli, Ceccherelli, Coccioni, Colangelo, Curini-Galletti, Danovaro, D'Addabbo, Leonardis, Fabiano, Frontalini, Gallo, Gambi, Guidi, Moreno, Pusceddu, Sandulli, Semprucci, Todaro and Tongiorgi2010). An increasing interest in the ecology of the benthic meiofauna began in the 1980s (see Balsamo et al., Reference Balsamo, Semprucci, Frontalini, Coccioni and Cruzado2012 for review), but the small size and the difficult identification of meiofaunal taxa were the main obstacles in their extensive use in biomonitoring (Austen et al., Reference Austen, McEvoy and Warwick1994; Balsamo et al., Reference Balsamo, Albertelli, Ceccherelli, Coccioni, Colangelo, Curini-Galletti, Danovaro, D'Addabbo, Leonardis, Fabiano, Frontalini, Gallo, Gambi, Guidi, Moreno, Pusceddu, Sandulli, Semprucci, Todaro and Tongiorgi2010). Nonetheless, meiofauna appear very useful as bioindicators since the community may contain information that macrofauna, the most used benthic compartment, cannot provide (Mirto & Danovaro, Reference Mirto and Danovaro2004; Gyedu-Ababio & Baird, Reference Gyedu-Ababio and Baird2006; Moreno et al., Reference Moreno, Semprucci, Vezzulli, Balsamo, Fabiano and Albertelli2011; Semprucci et al., Reference Semprucci, Frontalini, Covazzi-Harriague, Coccioni and Balsamo2013). They show widespread distribution, short life and reproductive cycles, direct development, high abundance and biodiversity, and species with specific ecological requirements, all of which are important advantages for biological indicators (e.g. Balsamo et al., Reference Balsamo, Semprucci, Frontalini, Coccioni and Cruzado2012). Among the meiofaunal taxa, phylum Nematoda has been widely and efficiently used in monitoring assessment because they hold all of the above cited advantages of meiofauna as bioindicators (Vanaverbeke et al., Reference Vanaverbeke, Merckx, Degraer and Vincx2011; Balsamo et al., Reference Balsamo, Semprucci, Frontalini, Coccioni and Cruzado2012; Semprucci et al., Reference Semprucci, Losi and Moreno2015).
In the Mediterranean Sea, there is a high variety of wetlands (e.g. lagoon, lake, sebkhas, hill reservoir and dam) that are considered to be among the most biologically diverse and productive ecosystems (Medail & Quezel, Reference Medail and Quezel1999). In particular, coastal lagoons occupy ~13% of the world's coastline (Barnes, Reference Barnes1980). They are generally shallow and isolated, with low water renewal rates, and exhibit spatial and seasonal variations of salinity and temperature that are significantly different from marine habitats (Barnes, Reference Barnes1980). Their setting within the coastal landscape leaves them especially vulnerable to possible physical, ecological and even global climate changes (Abigail et al., Reference Abigail, Atwood, August, Byron, Cobb, Foster, Fry, Gold, Hagos, Heffner, Kellogg, Lellis-Dibble, Opaluch, Oviatt, Pfeiffer-Herbert, Rohr, Smith, Smythe, Swift and Vinhateiro2009). They also act as sinks for organic detritus, fine sediments and pollutants entering the system that can affect benthic organisms (Semprucci et al., Reference Semprucci, Balsamo and Sandulli2016). Furthermore, their role as habitats for specialist species make them of significant value to nature conservation. There are more than 50 Mediterranean lagoons for which some hydrological or ecological data have been published in the scientific literature, but these are only a part of the existing ones (Pérez-Ruzafa et al., Reference Pérez-Ruzafa, Marcos and Pérez-Ruzafa2011).
Lagoon size, degree of communication with the open sea, salinity gradients and trophic status are among the features that best explain the distribution of the benthic communities (Pérez-Ruzafa et al., Reference Pérez-Ruzafa, Marcos and Pérez-Ruzafa2011), but other elements may also be important. Among environmental parameters, sediment grain size (Vanaverbeke et al., Reference Vanaverbeke, Gheskiere, Steyaert and Vincx2002; Semprucci et al., Reference Semprucci, Colantoni, Baldelli, Rocchi and Balsamo2010, Reference Semprucci, Balsamo and Sandulli2016) and quantity and quality of organic matter (OM) are key factors that lead biodiversity and abundance of meiofauna and nematode communities both in coastal and lagoon ecosystems (Pusceddu et al., Reference Pusceddu, Gambi, Manini and Danovaro2007, Reference Pusceddu, Bianchelli, Gambi and Danovaro2011; Ingels et al., Reference Ingels, Kiriakoulakis, Wolff and Vanreusel2009), while salinity has a great importance only in the transitional environments characterized by a relevant salinity gradient (Barnes et al., Reference Barnes, Bamber, Moncrieff, Sheader and Ferrero2008, Semprucci et al., Reference Semprucci, Balsamo and Frontalini2014a). In a Tunisian lagoon system, Mahmoudi et al. (Reference Mahmoudi, Beyrem and Aïssa2002a) reported that salinity, water dissolved oxygen and sediment ammonia content affected nematode diversity, abundance and biomass in the northern sector of Tunisia (Ghar El Melh lagoon), while Essid & Aïssa (Reference Essid and Aïssa2002) documented relevant effects of the OM on nematode abundance and biomass derived from domestic and industrial discharges at the close lagoon of Bizerta. In contrast, heavy metal, organic carbon and hydrocarbon content of sediments appeared the main factors negatively influencing communities in the southern Tunisian sector (i.e. Bou Ghrara lagoon) (Mahmoudi et al., Reference Mahmoudi, Beyrem, Baccar and Aïssa2002b). Accordingly, the complexity of the lagoon systems as habitats makes the study of the possible factors driving faunal distribution crucial. El Bibane is the second largest lagoon in Tunisia, but it is among the lagoons about which ecological information is almost absent (Pérez-Ruzafa et al., Reference Pérez-Ruzafa, Marcos and Pérez-Ruzafa2011). It offers a wide variety of natural habitats for benthos such as meadows of Cymodocea nodosa, Posidonia oceanica and Caulerpa prolifera as well as the longest algal reef of Neogoniolithion notarsii in the Mediterranean, but it is subject to an increasing anthropogenic impact and a possible risk of pollution especially from Libya and the Gulf of Gabes (BRL Ingenierie Idea Consult, 2008). Accordingly, an evaluation of the ecological quality of this lagoon is urgent. Both meiofauna and nematodes have been successfully used for biomonitoring programmes in several Mediterranean lagoons revealing the great importance of their study in such vulnerable ecosystems (e.g. Vitiello & Aïssa, Reference Vitiello and Aïssa1985; Beyrem & Aïssa, Reference Beyrem and Aïssa2000; Mahmoudi et al., Reference Mahmoudi, Beyrem and Aïssa2002a, Reference Mahmoudi, Beyrem, Baccar and Aïssab; Frontalini et al., Reference Frontalini, Semprucci, Armynot du Châtelet, Francescangeli, Margaritelli, Rettori, Spagnoli, Balsamo and Coccioni2014; Semprucci et al., Reference Semprucci, Colantoni, Sbrocca, Baldelli and Balsamo2014b, Reference Semprucci, Balsamo and Sandulli2016). Thus, the present study aims to characterize the meiofauna and free-living nematode communities of this lagoon both from a taxonomic and functional point of view and give the first ecological data that will make possible an assessment of the ecological health of the El Bibane lagoon as well as open up new perspectives for its conservation.
MATERIALS AND METHODS
Study area
The Tunisian lagoon of El Bibane is located to the south-east of the city of Zarzis and north of Ben Gardene, in an arid environment (Figure 1). The lagoon belongs to the Gulf of Gabes and is located on the south-east coast of Tunisia, near the Tunisian–Libyan border. El Bibane lagoon (longitude 11°05′–11°30′E and latitude 33°11′–33°18′N) is the second largest lagoon in Tunisia with 23,000 hectares, which can be increased to 30,000 if we include the Sebkhat Boujmel that adjoins it in its northern part and is connected by a small channel and El Mekhada that leaves transit waters of very high salinity. El Bibane lagoon has an elliptical shape and is directly influenced by the nearby Gulf of Gabes, which is considered among the rare areas with significant continental shelves in the Mediterranean (Medhioub & Perthuisot, Reference Medhioub and Perthuisot1977; Lemoelle et al., Reference Lemoelle, Vidy and Franc1984). It has a surface area of about 230 km2 and a maximum depth of 6 m (Guelorget et al., Reference Guelorget, Frisoni and Perthuisot1982). The lagoon is characterized by an increasing salinity level in the sea inlet and southern margins of the lagoon with a maximum of 50 reached during summer (Medhioub, Reference Medhioub1979; Guelorget et al., Reference Guelorget, Frisoni and Perthuisot1982). The lagoon is rich in benthic habitats and is mainly dominated by meadows of Cymodocea nodosa, Posidonia oceanica and Caulerpa prolifera (Guelorget et al., Reference Guelorget, Frisoni and Perthuisot1982; Pergent & Zaouali, Reference Pergent and Zaouali1992). El Bibane lagoon hosts the longest algal reef of Neogoniolithion notarsii in the Mediterranean (Jelassi et al., Reference Jelassi, Khemaissia, Zimmer, Garbe-Schönberg and Nasri-Ammar2015). It is also known for its valuable economic resources and is actively exploited by traditional fisheries (Guelorget et al., Reference Guelorget, Frisoni and Perthuisot1982) despite suffering a decline in fish productivity in the last 10 years. Significant human impact is not documented in the area with the exception of some organic pollutants of agricultural run-off origin and disturbances related to overfishing and poor fishing practices, including non-selective gear and hazardous state of the fixed gear (Romdhane, Reference Romdhane2002). However, El Bibane lagoon is subject to a possible risk of pollution especially from neighbouring areas (BRL Ingenierie Idea Consult, 2008). The major sea currents or littoral drift have an east–west direction that may make important pollution by the Libyan petrochemical complex. Furthermore, the Gulf of Gabes is reported to be very exposed to pollution through huge industrial activity, and thus may be another pollution source. Large quantities of phosphogypsum (calcium sulphate) from the phosphoric acid and chemical product industry of Gabes are released into the Gulf of Gabes (Rabaoui et al., Reference Rabaoui, Balti, El Zrelli and Tlig-Zouari2013).

Fig. 1. Geographic location of the stations sampled in EL Bibane lagoon during winter 2012.
Furthermore, the terrestrial ecosystems of the lagoon periphery are engaged in steppe and desertification processes.
Sampling strategy
The sediment samples were collected in January 2012. The station depth ranged from 0.8 to 5 m. Station S1 was close to the sea, situated at the entrance of the lagoon; S2 and S4 were the nearest to urban areas with high human activity; S3 was located at the mouth of the Sebkhat Boujmel; S5 is on the south coast of the lagoon, which is known for attracting a significant number of tourists; while stations S6 and S7 are the deepest ones and occupy the centre of the lagoon (Figure 1).
At each sampling station, meiofauna were collected in four replicates using Plexiglas hand-cores (area 10 cm2), preserved in neutralized formalin (5% formaldehyde) and stained with Rose Bengal (0.2 g l−1).
Sediment analyses
A granulometric analysis was carried out on the sediments collected using a vibro-sifter for fractions larger than 63 µm and an X-ray analyser for pelite (silt and clay) fractions (Sedigraph 5200 micrometer). Wentworth scale (Buchanan, Reference Buchanan, Holme and McIntyre1984) was used for the classification of sediment particle sizes. Total organic carbon (TOC) was determined calorimetrically using Coulomat 702.
Meiofaunal and nematode analyses
In the laboratory, sediment samples were washed through 1 mm and 40 µm sieves and the fraction retained by the 40 µm sieve was used to extract meiofaunal organisms by centrifugation using Ludox-HS40 (Mirto & Danovaro, Reference Mirto and Danovaro2004). Then, meiobenthic groups were identified and counted at a higher taxonomic level. Nematodes were counted under a microscope and 100 individuals picked at random in each replicate (Kotta & Boucher, Reference Kotta and Boucher2001). Identification at the genus level was performed using the pictorial keys of Platt & Warwick (Reference Platt and Warwick1983, Reference Platt and Warwick1988) and Warwick et al. (Reference Warwick, Platt and Somerfield1998), NeMys online identification key and literature therein (Guilini et al., Reference Guilini, Bezerra, Deprez, Fonseca, Holovachov, Leduc, Miljutin, Moens, Sharma, Smol, Tchesunov, Mokievsky, Vanaverbeke, Vanreusel and Vincx2016). Shannon's diversity (H’, log2) and richness were calculated to describe the structure of the nematode community. Furthermore, according to the trophic and the life strategies, the nematode functional traits were considered. In detail, nematode genera were classified into four feeding groups to investigate the trophic structure of the community: selective (1A) and non-selective (1B) deposit feeders, epistrate feeders (2A) and predators/omnivores (2B) (Wieser, Reference Wieser1953). The Index of Trophic Diversity (ITD) was calculated following Heip et al. (Reference Heip, Vincx and Vranken1985): ITD = ∑θ2, where θ is the percentage contribution of each feeding type according to Wieser (Reference Wieser1953). ITD values range from 0.25 (highest trophic diversity; i.e. the four trophic groups account for 25% each) to 1.0 (lowest trophic diversity; i.e. one feeding type accounts for 100% of total nematode assemblage). Furthermore, Maturity Index (MI, Bongers, Reference Bongers1990; Bongers et al., Reference Bongers, Alkemade and Yeates1991) was calculated as the weighted average of the individual colonizer-persistent (c-p) values. In detail, Bongers (Reference Bongers1990) distinguished r-strategist species (colonizers or c-p 1), which are more tolerant to environmental variations, and k-strategist species (persisters or c-p 5), which are more sensitive. The contribution of each life-strategy group (c-p 1 to 5) to the total nematode assemblage was then calculated.
Statistical analysis
Cluster analysis derived from Bray–Curtis similarity matrices (square-root transformed) was used to view spatial differences in the meiofaunal and nematode community structures. Furthermore, the formal significance of the differences was tested by means of the Analysis of Similarity (ANOSIM). SIMPER test (cut-off of 50%) was used to determine the contribution of each taxon to the total dissimilarity. All of the multivariate analyses were performed with the PRIMER v6 software (Clarke & Gorley, Reference Clarke and Gorley2006).
Possible differences of the univariate measures (i.e. meiofaunal and nematode abundances, nematode H’, MI, trophic groups and c-p classes) between stations were evaluated using the Analysis of Variance (One-way ANOVA). Prior to analysis, the normality and homoscedasticity assumptions were checked using the Kolmogorov–Smirnov and Levene's tests, respectively. Tukey HSD multiple comparisons test was used to check the significance of the differences in pairwise comparisons between stations (significance level P < 0.05). Principal component analysis (PCA) was carried out on environmental data in order to visualize the trend of the main abiotic variables. The faunal data were projected on the factor-plane as additional variables without contributing to the results of the analysis. This can provide an insight into the possible influence of the environmental variables upon each benthic group (STATISTICA v.8 computer program).
RESULTS
Sedimentological parameters
In the El Bibane lagoon, the sediments of the stations studied revealed a highly variable grain size (Table 1). Gravel amount was higher at S3 (station 22.2%), followed by S5 (19.9%), S4 (19.1%), while the lowest values were detected at S7 station (5.5%). The highest sand values were observed at S5 station (64.5%), followed by S1 (63.5%), S4 (57.3%), while the lowest ones were revealed at S7 (16.6%). Pelite fraction was higher at S7 station (77.9%) followed by S6 (61.1) and S3 (41.8), while the lowest values were detected at S5 (15.6%). TOC ranged from 0.67 ± 0.3 to 5.05 ± 0.8% (dw), the highest values were observed at S7 and S6, while the lowest ones at S2 (Table 1). Among the environmental variables, salinity showed high values at S7 and S6, followed by S3, while the lowest value was detected at S2. Chl-a showed the highest values at S2 and S1, while the lowest ones at S7 and S6.
Table 1. GPS coordinates and environmental parameters measured at each sampling station.
Meiofaunal and nematode community
Meiofaunal richness was higher at S1, S2, S3, S4 and S5 (five taxa); and lower at S6 and S7 (four). Meiofaunal abundance ranged from 158.75 ± 47.8 to 1927 ± 80.03 ind. 10 cm−2 (S7 and S2, respectively). The highest abundance values were found at S2 (Tukey HSD test, P < 0.05), while S6 and S7 stations revealed the lowest differences (Tukey HSD test, P < 0.001).
The dominant taxon was represented by nematodes, which represented on average 87% of the total meiofauna (Figure 2). The next most abundant taxa were copepods (adults and larvae; on average: 6.9%); followed by oligochaetes (2.9%) and polychaetes (2.6%). The contribution of the remaining taxa was less than 1% and therefore they were considered as ‘Others’.

Fig. 2. Abundance of the main meiofaunal taxa in the study area.
ANOSIM analysis showed significant differences of the meiofaunal community between the stations (R = 0.87; P = 0.001) showing the greatest dissimilarities between central lagoon (namely S6 and S7 stations) and coastal stations (S1, S2, S3, S4 and S5). This was also revealed by the cluster analysis (Figure 3A) that highlighted the presence of two main groups: the first represented by S6 and S7 (G1); and the second one by S1, S2, S3, S4 and S5 (G2). SIMPER routine revealed that the differences of meiofaunal community between these stations were mainly due to the higher abundance of nematodes and to the total absence of copepods in G1 than in G2 group.

Fig. 3. Cluster analysis (A) on meiofaunal and (B) on nematode assemblages in each sampling station.
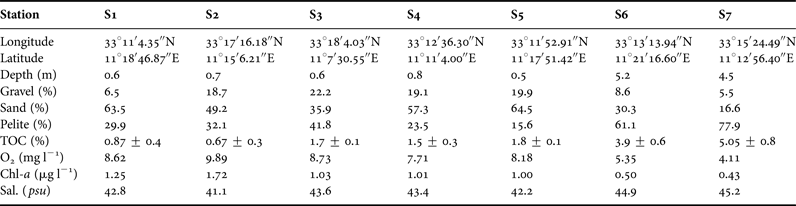
PCA was used to visualize the trends of the communities in relation to the main environmental variables (Figure 4). Two principal components were identified that together explained 91% of the data variance. PC1 explained 79.4% of the variance and was primarily affected by salinity (0.94), TOC (0.92), Chl-a (−0.92), sand (−0.92) followed by pelite (0.87), while PC2 explained 11.6% of the variance and was mainly explained by gravel (−0.47). Projection of the cases on the factor-plane (1 × 2) showed a clear separation of the S6 and S7 stations from the rest (Figure 5). In detail, S6 and S7 stations were distinguished by their higher TOC, salinity and pelite values, while higher gravel percentage mainly distinguished S5 and S4 from the S1, S2 and S3 stations. Among the meiofaunal variables, nematodes, copepods and polychaetes were the main taxa negatively related to the PC1, while oligochaetes were positively related to PC2 (Figure 4).

Fig. 4. Principal component analysis (PCA) plot based on the abiotic (active) and biotic (supplementary) variables. AN, abundance of nematodes; AO, abundance of oligochaetes; AC, abundance of copepods; AP, abundance of polychaetes; TOC, total organic carbon; Sd, Sand; Plt, Pelite; Grl, Gravel; Chl-a, chlorophyll ‘a’.
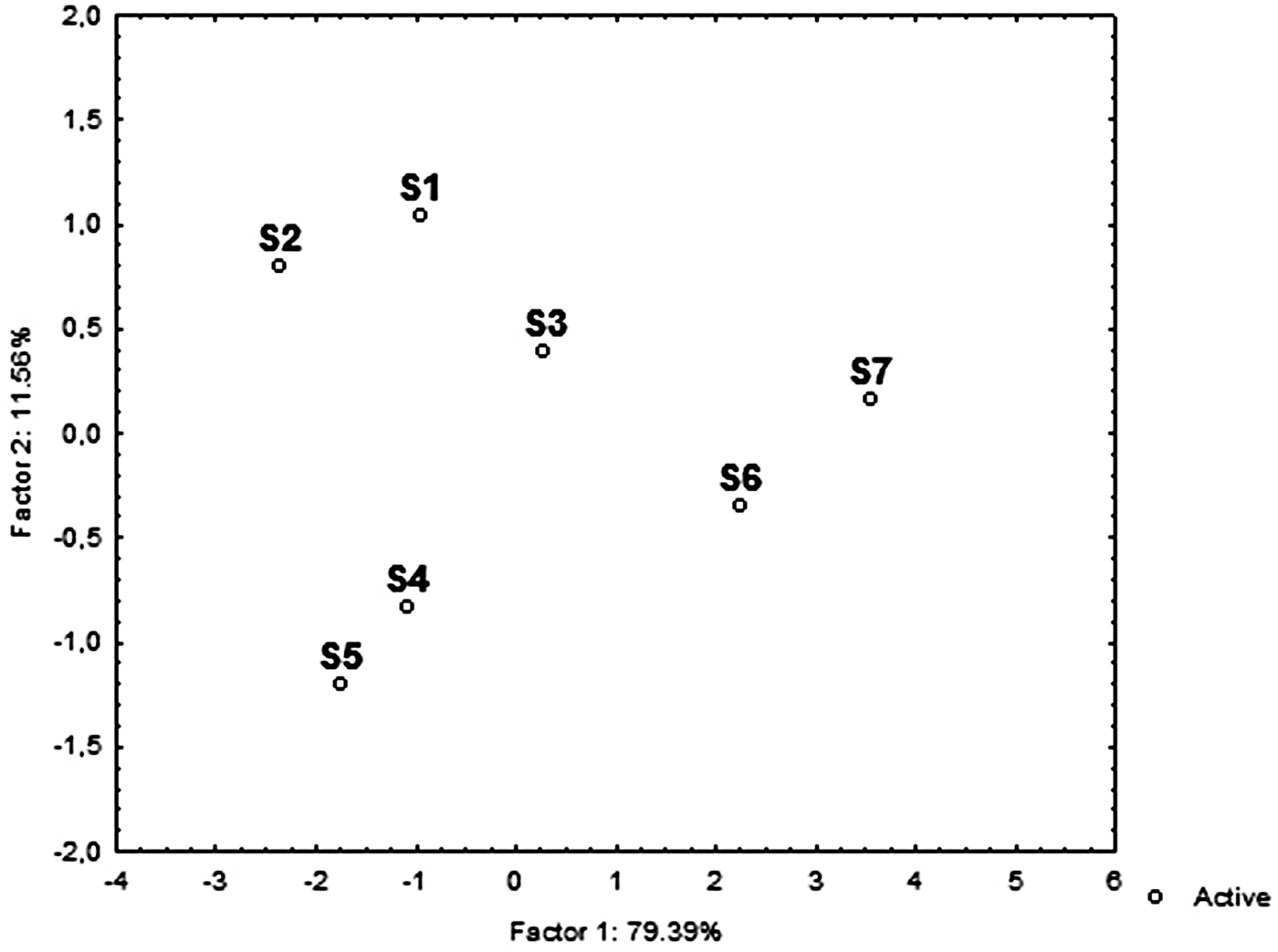
Fig. 5. PCA ordination diagram of study area based on the main environmental variables.
The nematode community was made up of a total of 62 genera belonging to 22 families. The nematode richness was higher at S3 and S5 stations (39 and 29 genera, respectively), than at S1 (27), S2 (17), S4 (22), S6 (10) and S7 (8) (Figure 6). Xyalidae family was represented by nine genera, followed by Chromadoridae (seven genera), Cyatholaimidae (six) and Oncholaimidae (five). Xyalidae was also the most abundant family, representing 14% of the total nematode fauna, followed by Chromadoridae (12%), Cyatholaimidae (10%) and Oncholaimidae (8%). The highest nematode abundance was found at S2 (Tukey HSD test, P < 0.05), while the lowest was at S6 and S7 (P < 0.001).

Fig. 6. Number of nematode genera (S) and Shannon diversity index (H’) at each sampling site.
H’ ranged from 2.3 ± 0.22 to 4.8 ± 0.14 and was significantly different between stations (ANOVA, P < 0.001). S3 was characterized by a significantly high H’ (Tukey's test, P < 0.01), while S6 and S7 showed the lowest diversity (Tukey's test, P < 0.01) (Figure 6).
ANOSIM detected significant differences in the nematode community between the stations (R = 0.98; P = 0.001) showing the two main groups documented by meiofaunal community. However, G2 was further subdivided into two sub-groups: G2a (S3) and G2b (S1, S2, S4 and S5) (Figure 3B).
The genera that mainly distinguished (being more abundant) G1 (S6 and S7) were: Metoncholaimus, Metalinhomoeus, Paracomesoma, Viscosia, Daptonema and Theristus (SIMPER 50%), while genera that were more abundant in G2 were mainly Trichotheristus, Paramonohystera, Marylynnia, Synonchiella and Terschellingia. Furthermore, the genera that mainly distinguished G2a vs G2b were Metoncholaimus, Daptonema, Bathylaimus all more abundant at G2a and Terschellingia, Ammotheristus, Prochromadorella, Dorylaimopsis, Promonhystera, Scaptrella, Sabatieria that were more abundant at G2b (SIMPER 50%).
On average the dominant trophic group was represented by 1B at all stations, followed by 2B, 2A and 1A (Figure 7). All the trophic groups had significantly different results in the station comparisons with the only exception being 1B. In particular, 1A was significantly higher at S3 (ANOVA, P < 0.001, Tukey's test, P < 0.05), 2A was lower at S6 and S7 (ANOVA, P < 0.001, Tukey's test, P < 0.05 and P < 0.001), while 2B was lower at S3 and higher at S7 (ANOVA, P < 0.001, Tukey's test, P < 0.01). ITD ranged from 0.28 ± 0.01 (S3) to 0.45 ± 0.02 (S7). S7 showed significantly higher ITD values (Tukey's test, P < 0.05) compared with the other stations, while S3 had lower values especially if compared with S4, S5 and S7 (Figure 7).
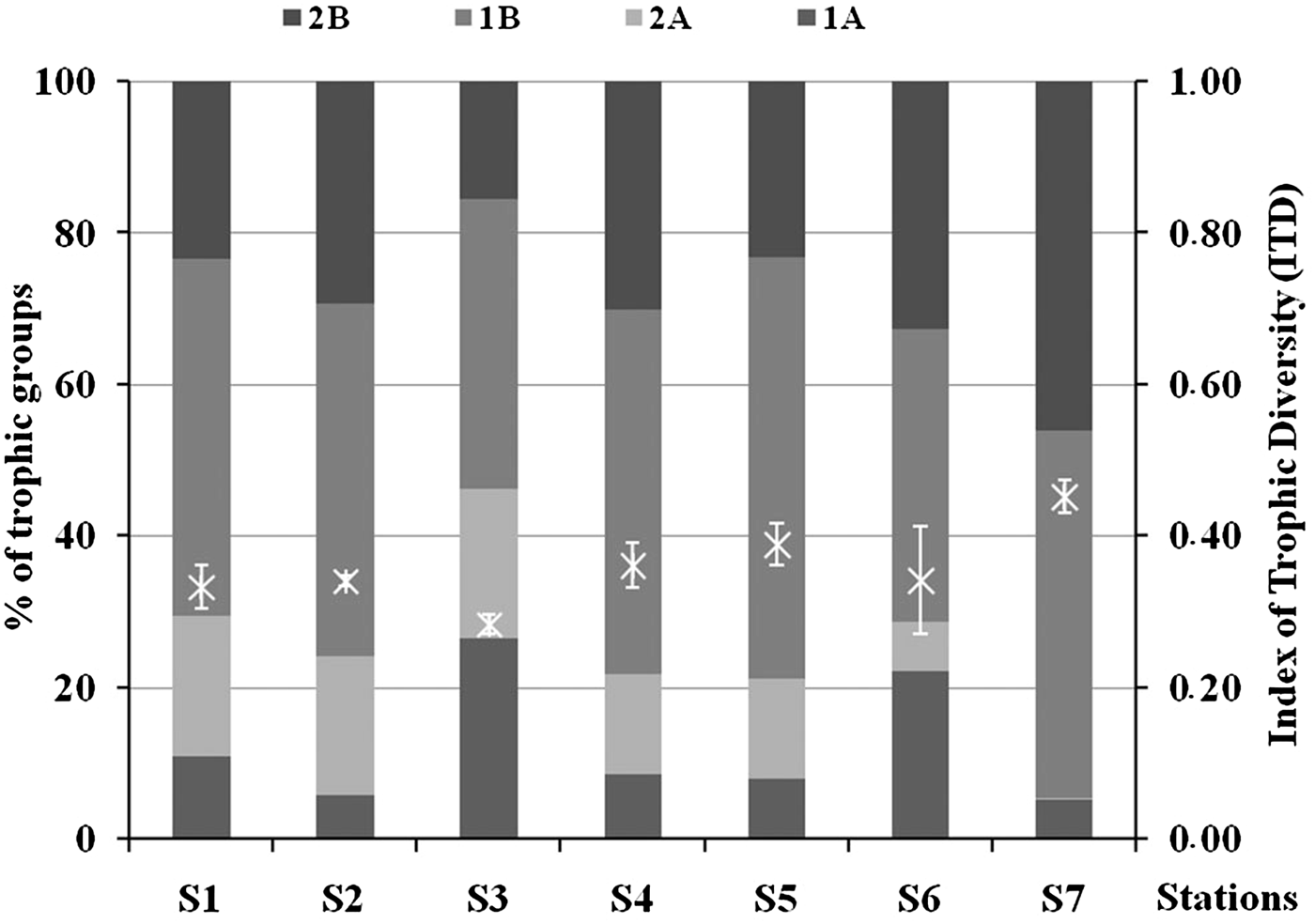
Fig. 7. Percentage of contribution of the different trophic groups and Index of Trophic Diversity (ITD ± standard deviation) in each sampling station. 1A – selective deposit feeders; 1B – non-selective deposit feeders; 2A –epigrowth feeders; 2B – omnivores/predators.
Maturity index (MI) ranged between 2.33 ± 0.06 (S6) and 2.66 ± 0.07 (S4) due to the clear dominance of c-p 2 and c-p 3 genera (Figure 8). MI showed significant differences between stations (ANOVA, P < 0.001). In detail, it was significantly lower at S3, S5 and S6 than S1, S2, S4 and S7 stations (Tukey's test, P < 0.05). ANOVA revealed a significant difference also of the c-p classes: c-p 1, c-p 2, c-p 4 (ANOVA, P < 0.001) and c-p 3 (ANOVA, P < 0.05). C-p 1 was particularly abundant at S3 (Tukey's test, P < 0.001), while c-p 2 was significantly higher at S5 and S6 (Tukey's test, P < 0.01). C-p 3 revealed significantly higher abundances at S2 and lower at S5 (Tukey's test, P < 0.01), while S7 and S4 stations showed the highest amount of c-p 4 (Tukey's test, P < 0.05) and lowest at S6.
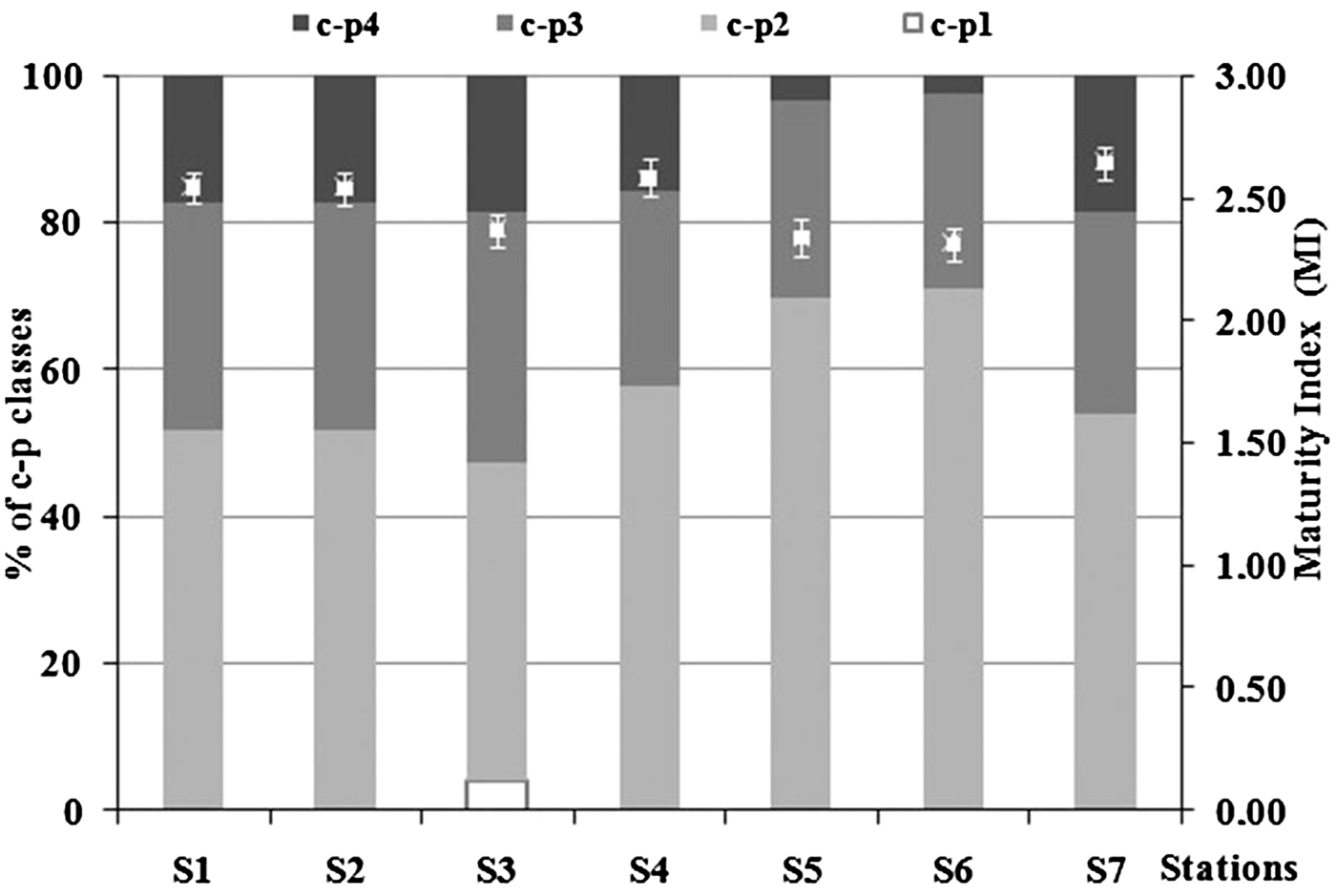
Fig. 8. Percentage of contribution of the different c-p classes and Maturity Index (MI ± standard deviation) in each sampling station.
PCA showed that the main nematode variables related to the PC1 were: Trichotheristus (−0.94), Theristus (0.91), Metalinhomoeus (0.75), H’ (−0.74), Bathylaimus (−0.72) and Marylynnia (−0.66) and ITD (0.40). Instead, Paracomesoma (0.50), Enoploides (−0.45), Terschellingia (0.41) and MI (0.30) were mainly related to PC2 (Figure 9).
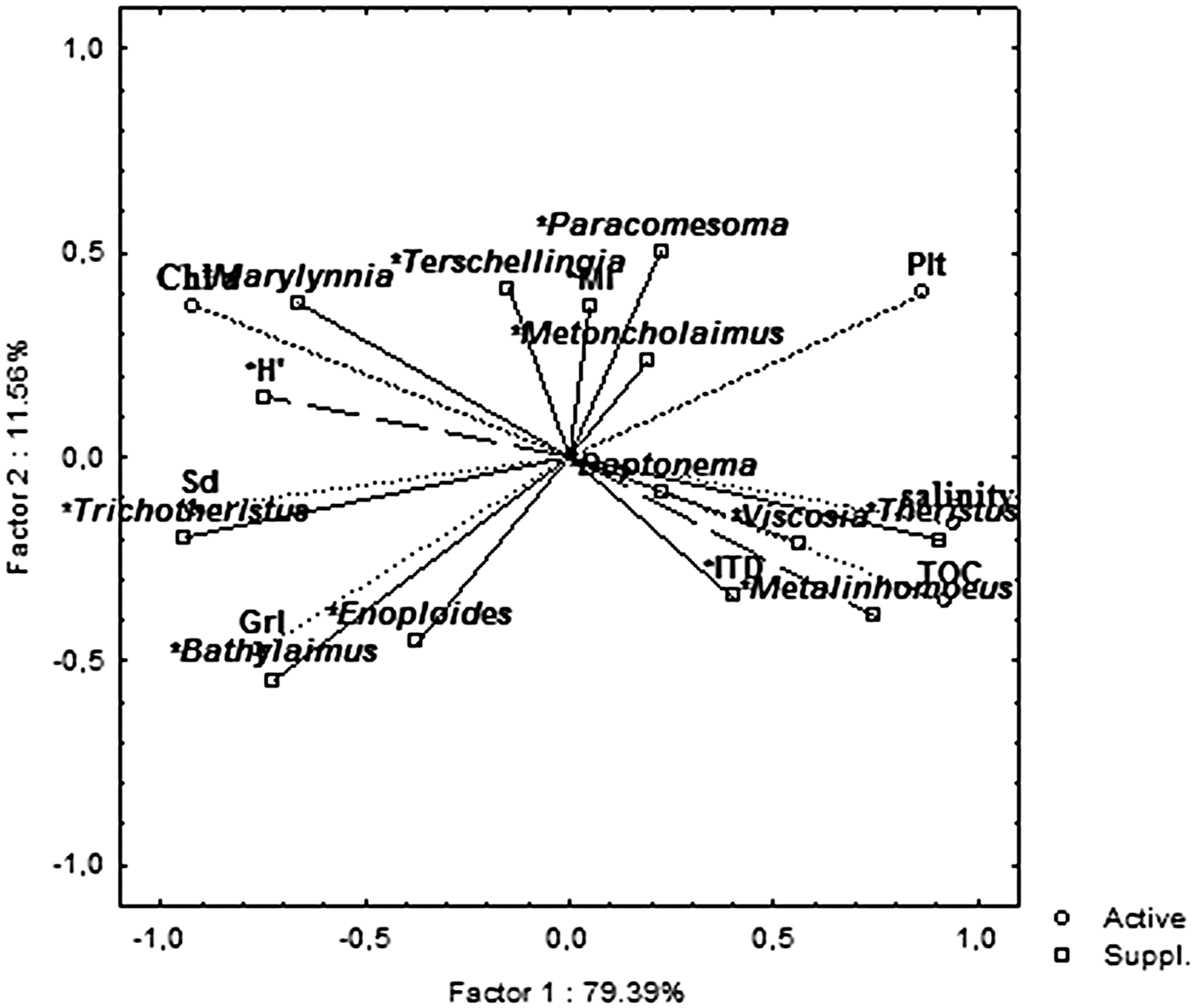
Fig. 9. Principal component analysis (PCA) plot based on the abiotic (active) and nematode (supplementary) variables. TOC, total organic carbon; Sd, Sand; Plt, Pelite; Grl, Gravel; Chl a, chlorophyll ‘a’.
DISCUSSION
El Bibane is a Tunisian lagoon with a high conservation value, but pollution from industrial and municipal waste waters, pesticides and chemical fertilizers, through soil erosion, water run-off and sea currents need to be mentioned (Barhoumi et al., Reference Barhoumi, Jouili, Elbarhoumi, Derouiche, El Megdiche, Bouabdallah, Touil and Driss2016). In particular, because of the significant exchanges with the sea, the waters of the lagoon are subject to a risk of contamination by pollutants from industrial sites located mainly in Libyan petrochemical complexes, but also at the Gulf of Gabes (Barhoumi et al., Reference Barhoumi, Jouili, Elbarhoumi, Derouiche, El Megdiche, Bouabdallah, Touil and Driss2016). As such, the estimation of the benthic biodiversity of this area is urgent as is an evaluation of the ecological quality of the sediments.
Environmental parameters revealed a clear zonation of the lagoon with a subdivision of the marginal stations from those located in the central area. The factors that led this first zonation were mainly salinity, TOC, Chl-a, followed by sand and pelite. In particular, salinity is higher in the central sector according to the salinity trends documented in El Bibane lagoon during the winter period (Lemoalle, Reference Lemoalle1986). In accordance with the same author a low Chl-a and O2 percentages were found at S6 and S7 stations, probably related to the low water transparency of this area of the lagoon. As reported by Medhioub & Perthuisot (Reference Medhioub and Perthuisot1981), fine sediments and TOC derived from the sea were mainly deposited in the central sector where a decrease of the hydrodynamic energy may be inferred. This may also confirm the influence of tidal currents coming from sea that along with the fine component of the sediments and OM could deposit pollutants. The association between mud and OM is frequently reported in literature (Pilarczyk & Zeidler, Reference Pilarczyk and Zeidler1996; Semprucci et al., Reference Semprucci, Balsamo and Frontalini2014a, Reference Semprucci, Balsamo and Sandulli2016) as well as the adsorptive properties of pollutants by clay and silt particles (Bernardello et al., Reference Bernardello, Secco, Pellizzato, Chinellato, Sfriso and Pavoni2006). A further subdivision of the marginal stations in northern and southern zones, mainly due to the coarser-grained sediments detectable in the southern lagoon area and probably due to exceptional flood events of the Fessi River, was observed (Affouri et al., Reference Affouri, Dezileau and Kallel2016).
Meiofaunal abundance was comparable to studies carried out in Tunisian lagoons (e.g. Vitiello & Aïssa, Reference Vitiello and Aïssa1985; Mahmoudi et al., Reference Mahmoudi, Beyrem, Baccar and Aïssa2002b; Mahmoudi, Reference Mahmoudi2003) as well as in other transitional environments located in the eastern Mediterranean area (Fabbrocini et al., Reference Fabbrocini, Guarino, Scirocco, Franchi and D'Adamo2005; Cibic et al., Reference Cibic, Blasutto and Bettoso2009; Semprucci et al., Reference Semprucci, Balsamo and Sandulli2016). Nematodes were the dominant taxon in the El Bibane lagoon in agreement with the meiofaunal structure found worldwide in brackish-water systems (e.g. Guerrini et al., Reference Guerrini, Colangelo and Ceccherelli1998; Cibic et al., Reference Cibic, Franzo, Celussi, Fabbro and Del Negro2012; Boufahja et al., Reference Boufahja, Vitiello and Aissa2014; Semprucci et al., Reference Semprucci, Balsamo and Frontalini2014a, Reference Semprucci, Balsamo and Sandulli2016). Contrary to the general trends reported in literature (Danovaro et al., Reference Danovaro, Gambi, Mirto, Sandulli, Cecchierelli, Gambi and Dappiano2004; Semprucci et al., Reference Semprucci, Colantoni, Baldelli, Rocchi and Balsamo2010; Fonseca et al., Reference Fonseca, Maria, Kandratavicius, Venekey, Gheller and Gallucci2014), the stations characterized by the finest sediments (S6 and S7) showed the lowest meiofaunal abundances. Thus, although OM deposited in the sediments potentially has nutritional value for benthic consumers (Neira et al., Reference Neira, Sellanes, Soto, Gutiérrez and Gallardo2001), all the meiofaunal major taxa appeared negatively affected by it. This effect on meiofauna was reported by other authors (Schratzberger & Warwick, Reference Schratzberger and Warwick1998; Essid & Aïssa, Reference Essid and Aïssa2002; Mahmoudi et al., Reference Mahmoudi, Beyrem and Aïssa2002a, Reference Mahmoudi, Beyrem, Baccar and Aïssab) and could be due to the higher sulphide concentrations that can negatively affect benthic communities (Sutherland et al., Reference Sutherland, Levings, Petersen, Poon and Piercey2007) or could confirm a possible accumulation of pollutants in the central area of El Bibane lagoon. At the nearby lagoon of Bizerta, Essid & Aïssa (Reference Essid and Aïssa2002) documented relevant effects of the OM on nematode abundance and biomass derived from domestic and industrial discharges. In particular, nematodes (with the exception of deposit feeders) appeared strongly affected by the creation of an organic-mineral complex between smectite and organic compounds and they reacted through a migration to the deeper layers. Mahmoudi et al. (Reference Mahmoudi, Beyrem, Baccar and Aïssa2002b) also observed a negative correlation between nematode abundance, biomass and diversity and TOC at the Bou Ghrara lagoon.
Nematode richness was higher than that revealed in other Mediterranean transitional areas (Guerrini et al., Reference Guerrini, Colangelo and Ceccherelli1998; Fabbrocini et al., Reference Fabbrocini, Guarino, Scirocco, Franchi and D'Adamo2005; Semprucci et al., Reference Semprucci, Balsamo and Frontalini2014a) and comparable to that documented in the European systems (e.g. Hendelberg & Jensen Reference Hendelberg and Jensen1993; Barnes et al., Reference Barnes, Bamber, Moncrieff, Sheader and Ferrero2008; Ferrero et al., Reference Ferrero, Debenham and Lambshead2008). From a taxonomic point of view, the nematode community appeared to be represented by typical brackish-water nematode genera (Remane, Reference Remane1933; Gerlach, Reference Gerlach1954; Ferrero et al., Reference Ferrero, Debenham and Lambshead2008) and showed a clear zonation that perfectly mirrored the habitat features. This was evident analysing the results of both cluster and PCA analyses. In particular, a shift of typical muddy to coarse sand communities was detectable observing the PCA plot from central stations (S6 and S7, mainly dominated by Metalinhomoeus, Theristus, Viscosia) to the northern ones (S3, S1 and S2, by Daptonema, Metoncholaimus, Paracomesoma, Terschellingia, Marylynnia) up to the southern lagoon sector (S4 and S5, by Trichotheristus, Bathylaimus and Enoploides). The majority of the genera named above are typical of fine-grained sediments, while Trichotheristus may be regarded as highly related to sand and Bathylaimus and Enoploides to gravel. A strict relation between coarse sands and these two latter genera is, in fact, reported by several authors (e.g. Gheskiere et al., Reference Gheskiere, Vincx, Urban-Malinga, Rossano and Scapini2005; Fonseca et al., Reference Fonseca, Maria, Kandratavicius, Venekey, Gheller and Gallucci2014).
Shannon diversity was particularly lower at the S6 and S7 according to the known negative relation between biodiversity and grain-size decreasing (Vanaverbeke et al., Reference Vanaverbeke, Gheskiere, Steyaert and Vincx2002; Steyaert et al., Reference Steyaert, Vanaverbeke, Vanreusel, Barranguet, Lucas and Vincx2003; Semprucci et al., Reference Semprucci, Colantoni, Baldelli, Rocchi and Balsamo2010). However, a possible synergistic impact of OM and pollutants cannot be excluded (Essid & Aïssa, Reference Essid and Aïssa2002; Mahmoudi et al., Reference Mahmoudi, Beyrem, Baccar and Aïssa2002b). ITD values also showed in this area a dominance of trophic guilds, a phenomenon observed in stressed habitats (Essid, Reference Essid2008; Schratzberger et al., Reference Schratzberger, Lampadariou, Somerfield, Vandepitte and Vanden Berghe2009; Netto & Valgas, Reference Netto and Valgas2010) and mainly due to the dominance of 1B (i.e. Metalinhomoeus and Theristus) and 2B (i.e. Viscosia). Non-selective deposit feeders are a common and dominant trophic group in muddy sediments rich in organic detritus (Essid & Aïssa, Reference Essid and Aïssa2002; Michiels & Traunspurger, Reference Michiels and Traunspurger2004; Adão et al., Reference Adão, Alves, Patrício, Neto, Costa and Marques2009; Sandulli et al., Reference Sandulli, Semprucci and Balsamo2014), while 2B were generally more related to coarse sediments (Netto et al., Reference Netto, Warwick and Attrill1999). Their greater abundance in the fine sediments of the lagoon was mainly due to Viscosia genus (Oncholaimidae) that is a facultative predator able to exploit a wide range of food resources (Moens & Vincx, Reference Moens and Vincx1997). All the genera detected at the central area are well recognized as opportunistic species (Millward & Grant, Reference Millward and Grant1995; Gyedu-Ababio et al., Reference Gyedu-Ababio, Furstenberg, Baird and Vanreusel1999; Beyrem et al., Reference Beyrem, Boufahja, Hedfi, Essid, Aïssa and Mahmoudi2011; Gyedu-Ababio, Reference Gyedu-Ababio2011; Sandulli et al., Reference Sandulli, Semprucci and Balsamo2014; Semprucci et al., Reference Semprucci, Colantoni, Sbrocca, Baldelli and Balsamo2014b) and all appeared highly correlated to TOC. When MI was analysed, it showed good ecological quality at S7 in contrast to H’ and ITD. The high MI value was mainly due to Oncholaimidae genera such as Viscosia and Metoncholaimus. These genera are c-p 3 and c-p 4 according to Bongers et al. (Reference Bongers, Alkemade and Yeates1991) (i.e. intermediate colonizer and sensitive genera, respectively), but are also described as opportunistic genera under organic enrichment in several studies (Warwick, Reference Warwick1993; Danovaro et al., Reference Danovaro, Fabiano and Vincx1995; Beyrem et al., Reference Beyrem, Boufahja, Hedfi, Essid, Aïssa and Mahmoudi2011; Ürkmez et al., Reference Ürkmez, Sezgin and Bat2014). It is noteworthy that MI does not show a clear effect of the anthropogenic activity in the transitional environments of Cienfuegos Bay (Caribbean Sea) and Varano Lake (Mediterranean Sea) (Armenteros et al., Reference Armenteros, Ruiz-Abierno, Fernández-Garce, Pérez-García, Díaz-Asencio, Vincx and Decraemer2009; Semprucci et al., Reference Semprucci, Balsamo and Frontalini2014a). This could be related to the ‘Estuarine Quality Paradox’ (Elliot & Quintino, Reference Elliot and Quintino2007) that may be generalized to all the transitional environments. This hypothesis states that the community features under human stress may coincide with those under natural stress as a consequence of the high fluctuations of the physico-chemical parameters of the habitats and that species living in such environments adapt to these fluctuations, becoming tolerant to further changes (Adão et al., Reference Adão, Alves, Patrício, Neto, Costa and Marques2009). Thus, the particularly high ability of the communities of these habitat types to withstand environmental stress could make MI and c-p classes unsuitable for the detection of human stress, these being exclusively based on nematode life strategies.
According to Viaroli et al. (Reference Viaroli, Bartoli, Giordani, Magni and Welsh2004) and Nixon (Reference Nixon1995), the ecological quality (EcoQ) status of El Bibane lagoon may be classified as alerting only at S7. In agreement with Moreno et al. (Reference Moreno, Semprucci, Vezzulli, Balsamo, Fabiano and Albertelli2011) and Semprucci et al. (Reference Semprucci, Balsamo and Frontalini2014a, Reference Semprucci, Colantoni, Sbrocca, Baldelli and Balsamob), Shannon diversity revealed the worst conditions at S7 and S6 (poor and moderate EcoQ), while the best was at S3 (high EcoQ). Instead, MI highlighted the worst conditions at S3, S5, S6 and the best ones at S4 and S7. C-p classes revealed poor EcoQ at S5 and S6, while the other stations showed moderate EcoQ.
The present results, showing the high complexity, vulnerability and environmental value of El Bibane lagoon, highlight the need for periodical monitoring of the benthic communities of the lagoon to detect possible alterations of the EcoQ status of El Bibane sediments and develop sustainable management strategies.
ACKNOWLEDGEMENTS
The authors are grateful to all the fishermen of El Bibane lagoon who facilitated the sampling during the field surveys. We also thank Professor Younes Jedoui and Dr Maher Gzam from the Higher Institute of Water Sciences and Techniques, University of Gabes, for their help either in sampling campaigns or in providing some laboratory facilities. The authors thank Dr Badreddine Sallemi (Bizerte University) for helping us with the English language.












