‘Blest are those/Whose blood and judgement are so well commingled/That they are not a pipe for fortune's finger/To sound what stop she please.’ – Shakespeare, Hamlet, III, ii, 73
Globally, an estimated 5% of adults will attempt suicide at some point in their life. Reference Nock and Kessler1 Suicidal thinking and self-harm occur with many mental states and include passive neglect, putting oneself in risky situations, and many types of self-harm with varying combinations of intent and threat to life. Inevitably such a spectrum makes determining prevalence rates difficult, but typical figures estimate self-harm occurs in up to about a fifth of adolescents Reference Muehlenkamp, Claes, Havertape and Plener2 and over half of psychiatric in-patients. Reference Perez, Venta, Garnaat and Sharp3
A helpful way of explaining self-harming is to invoke the diathesis-stress model that proposes genetic, developmental and temperamental factors constitute a diathesis disposition; and acute environmental, social and psychological factors are situational stressors. Reference Mann4 Gender, age, ethnicity and religion Reference Moscicki5,Reference Nock, Borges, Bromet, Cha, Kessler and Lee6 early life Reference Maniglio7 and recent adverse events Reference Liu and Miller8 are associated factors in suicidal thinking and acts. Problematically most of the identified risks are common, non-specific, and of themselves reasonably weak predictors of completed suicide. Reference Appleby, Dennehy, Thomas, Faragher and Lewis9–Reference Hughes and Owens13 However, self-harm itself is a strong predictor of future such behaviour it predicts adverse outcomes in adolescents Reference Mars, Heron, Crane, Hawton, Lewis and Macleod14 ; 16% of adults repeat self-harm within a year (2% fatally so) and 7% die by suicide within 9 years. Reference Owens, Horrocks and House15
There is a need to move beyond descriptive epidemiology to understand the mechanisms behind self-harm and suicidal acts. Reference Aleman and Denys16 They are associated with high-risk decision-making, self-criticism, sensitivity to social disapproval and an inability to use memory and reflection about alternative positive outcomes of a stressful situation. Neuropsychological work implicates deficits in impulse control and curtailment of aggression, Reference Chistiakov, Kekelidze and Chekhonin17–Reference Nock, Hwang, Sampson, Kessler, Angermeyer and Beautrais20 executive functioning and emotional and affective regulation. Reference Soloff, Pruitt, Sharma, Radwan, White and Diwadkar21 These mechanisms are found in many mental illnesses and are thus considered as pan-diagnostic explanations for the emergence of suicidal acts and thinking. Reference Mann4
Impulsivity and aggression: cognitive control and restraint
A model of the cognitive control of emotion (MCCE) posits that there are four stages in the production of an emotional response. Reference Ochsner, Silvers and Buhle22 First a stimulus, which could be external to an individual or an internal thought or feeling, is perceived in a given environmental and mental context, leading to a second process of applied attention to this (or an attribute thereof), with some reduction of attention to competing stimuli. A third step is the appraisal of the stimulus with regard to an individual's current needs or goals, and finally varied expressions of emotional behaviour and concomitant change in experience (Fig. 1).
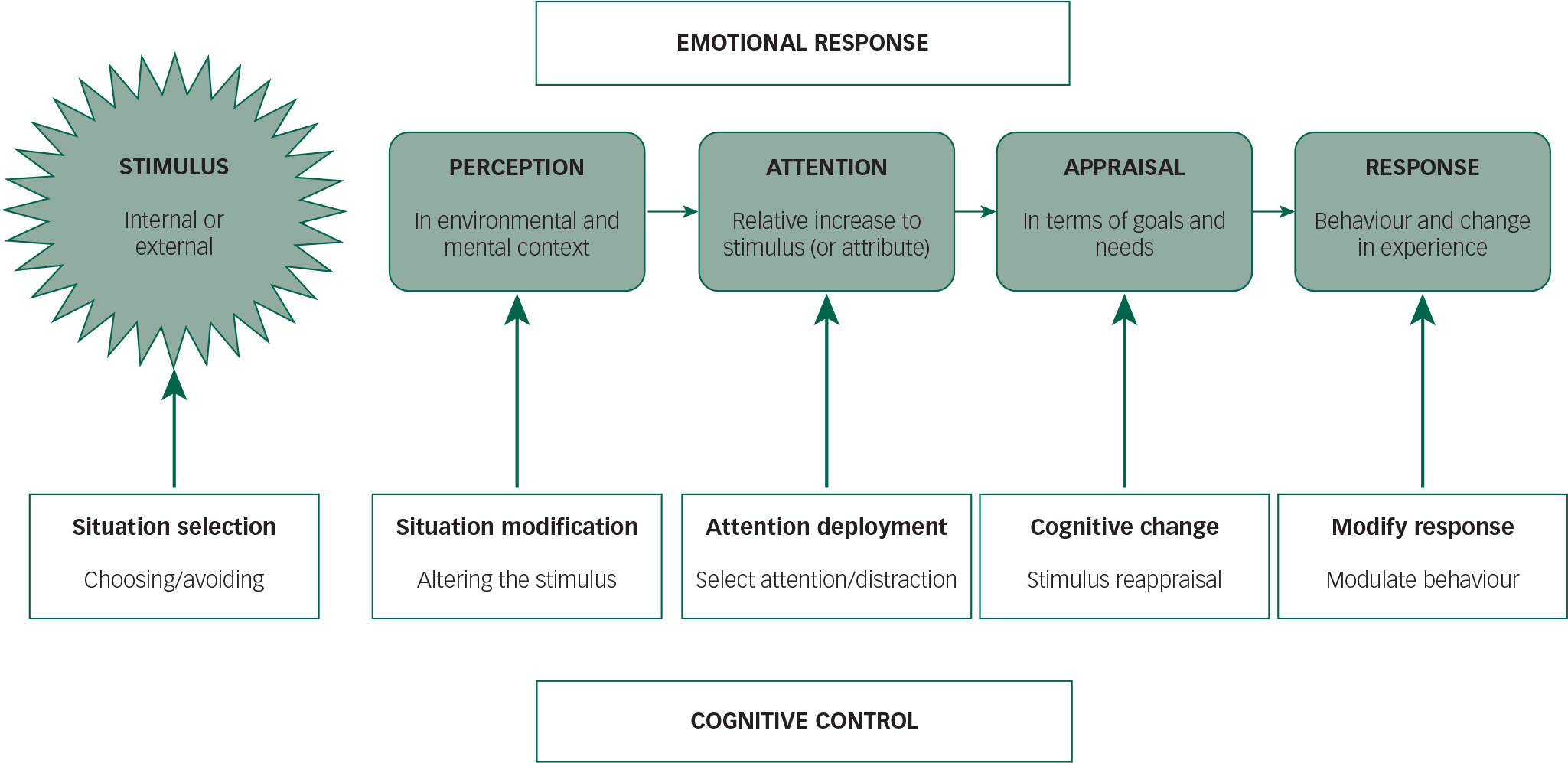
Fig. 1 A model of the cognitive control of emotion, adapted from Ochsner et al. Reference Ochsner, Silvers and Buhle22 The top half of the figure shows the processes of emotional responses in reaction to a stimulus (which can be external or an inner thought or feeling). The bottom half of the figure shows the cognitive strategies that can be utilised to modify such responses.
A critical aspect of such a paradigm is the concept of control of emotions. Several such strategies have been identified: situation selection, namely choosing or avoiding emotionally salient stimuli; situation modification, which attempts to alter a provoking stimulus; attentional deployment, wherein either selective attention or distraction can be utilised to alter stimulus appraisal; cognitive change that reappraises the way one views a stimulus; and response modulation that attempts to change behaviour. Although different approaches can thus be utilised in the control of emotion, these will vary in degree and success between experience type and magnitude, and between individuals in response to similar stimuli.
Neurophysiological research has implicated a frontolimbic network in the MCCE, with phylogenetically more ancient behaviours and emotions produced by subcortical limbic structures, whereas the prefrontal cortex (PFC) exerts vital ‘higher-level’ cognitive and executive control. Dysregulation of the frontolimbic network, with a hypofunctioning ‘top-down’ control from the PFC and an overactive limbic system results in poorer emotional regulation with greater impulsivity and aggression. Reference Coccaro, Sripada, Yanowitch and Phan23 Fitting with an aforementioned pan-diagnostic concept, such abnormalities have been shown in borderline personality disorder, Reference Minzenberg, Fan, New, Tang and Siever24 major depressive disorder Reference Kupfer, Frank and Phillips25 and bipolar affective disorder. Reference Phillips, Ladouceur and Drevets26
Implicated limbic regions include the amygdala, the striatum, the periaqueductal grey matter and the hypothalamus. These are involved in modulating vigilance and threat perception, encoding and learning from affective cues, initiating reactive aggression through fight or flight responses Reference Coccaro, Sripada, Yanowitch and Phan23 and in generating negative emotional states. Reference Amaral27 The insula, which is anatomically deep in the brain, receives strong amygdalar input and raises homeostatic and visceral states to consciousness, particularly in powerful affective states. Reference Critchley, Wiens, Rotshtein, Ohman and Dolan28 All of these regions are richly interconnected, and have strong reciprocal innervation with the PFC.
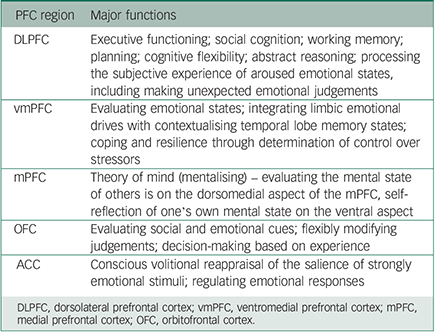
The roles of the PFC are numerous (see Table 1 for a summary). It is critical in cognitive flexibility and the evaluation and contextualisation of emotional states and environmental cues; assaying the mental state of one's self and others; determining the degree of control one has over stressors; and making executive decisions based on such information. Differential activation of these neural circuits is compromised in suicidal thinking across the diagnostic spectrum. This resonates with clinical observations that patients are often unable to reflect, mentalise, take perspective, learn from experience and desist from impulsive acting out in response to an adverse trigger that might be reminiscent of past threats.
Table 1 Prefrontal (PFC) and anterior cingulate (ACC) cortical regions and major functions Reference Coccaro, Sripada, Yanowitch and Phan23,Reference Critchley, Wiens, Rotshtein, Ohman and Dolan28
| PFC region | Major functions |
|---|---|
| DLPFC | Executive functioning; social cognition; working memory; planning; cognitive flexibility; abstract reasoning; processing the subjective experience of aroused emotional states, including making unexpected emotional judgements |
| vmPFC | Evaluating emotional states; integrating limbic emotional drives with contextualising temporal lobe memory states; coping and resilience through determination of control over stressors |
| mPFC | Theory of mind (mentalising) – evaluating the mental state of others is on the dorsomedial aspect of the mPFC, self-reflection of one's own mental state on the ventral aspect |
| OFC | Evaluating social and emotional cues; flexibly modifying judgements; decision-making based on experience |
| ACC | Conscious volitional reappraisal of the salience of strongly emotional stimuli; regulating emotional responses |
DLPFC, dorsolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex.
Available therapies, both psychological and pharmacological, target these processes – with the aim of better controlling any dysfunctional behaviour. This ‘systems neuroscience’ approach offers a conceptual framework for integrating clinical observations, phenomenology, cognitive and neural processing and pharmacological and psychological therapeutic approaches. It also offers clinicians the opportunity to examine more contemporary mechanistic approaches that might lead to new therapies.
rTMS interventions: lessons from clinical data
Transcranial magnetic stimulation (TMS) is a non-invasive and painless neuromodulatory tool that utilises Faraday's law of induction to alter underlying neuronal excitability, via a magnetic coil that can be turned on and off at varying frequencies. Reference Bersani, Minichino, Enticott, Mazzarini, Khan and Antonacci29 So-called ‘slow TMS’ (≤1 Hz) attenuates underlying cortical activity, while ‘fast TMS’ (≥10 Hz) increases it. However, changes vary with underlying cortical activity Reference Tracy, de Sousa de Abreu, Nalesnik, Mao, Lage and Shergill30 and through networks with physiologically connected brain regions. Reference Tracy, O'Daly, Joyce, Michalopoulou, Basit and Dhillon31 Repetitive TMS (rTMS) has been utilised clinically in the treatment of a variety of psychiatric, psychological and neurological disorders, although at this time it is only approved as a treatment for depression by the US Food and Drug Administration (although not by National Institute for Health and Care Excellence (NICE)). 32
Impulsivity
There are several published investigations on the effects of rTMS on suicidal thinking and impulsivity, although such work remains quite preliminary at this time. One recent study applied an intensive regimen of 10 Hz rTMS three times a day to the left dorsolateral prefrontal cortex (DLPFC) for 3 days, in 377 in-patients, achieving rapid improvements in suicidal ideation as measured on the Beck Scale of Suicidal Ideation (SSI). Reference George, Raman, Benedek, Pelic, Grammer and Stokes33 However, there were no differences compared with the sham arm. Another study Reference Hadley, Anderson, Borckardt, Arana, Li and Nahas34 applied daily high-dose (6800-pulse) sessions of 10 Hz rTMS, achieving rapid reductions in both depression symptoms and SSI scores in 5–10 days; this was an uncontrolled case series on just 19 patients, and suicidal ideation was a secondary outcome measurement.
Regarding impulsivity, studies in healthy controls, utilising varying rTMS parameters, have previously found that rTMS of the right DLPFC (3 pulses delivered at 50 Hz, repeated every 200 ms for 40 s), Reference Cho, Ko, Pellecchia, Van Eimeren, Cilia and Strafella35 left DLPFC (1 Hz for 15 min) Reference Figner, Knoch, Johnson, Krosch, Lisanby and Fehr36 or dmPFC (15 10-pulse trains at 10 Hz, with 10 s between trains) Reference Cho, Koshimori, Aminian, Obeso, Rusjan and Lang37 decreases impulsive decision-making as measured on a delay discounting task; specific effects on suicidality have not always been reported, and it remains unclear whether the similar responses effected by stimulation of varying sites is due the a generalisable effect on the PFC, or non-specific effects of any intervention in a hard to treat group of patients.
Borderline personality disorders
It is also of particular interest whether rTMS might offer a novel and more effective therapeutic option for suicidality and impulsivity in traditionally challenging patient populations, such as borderline personality disorder (BPD), although there has been far less work in this group. Barnow and colleagues investigated several different inhibitory and excitatory TMS parameters in 19 unmedicated women with BPD and 19 matched healthy control. They showed an overall reduction in duration of the cortical silent period (CSP) in the BPD group in the right cortex, suggesting a deficit in intracortical inhibitions. Reference Barnow, Völker, Möller, Freyberger, Spitzer and Grabe38 Clinically, Arbabi et al Reference Arbabi, Hafizi, Ansari, Oghabian and Hasani39 described a case report where high-frequency rTMS was applied to the left DLPFC (1500 10 Hz pulses in 30 trains of 5 s each, and a 55-second inter-train intervals) of a 22-year-old woman for ten sessions and they reported a resultant decrease in BPD symptom severity (as measured by the Structured Clinical Interview for DSM Disorders, 2nd edition (SCID-II); there was no change on the Borderline Personality Disorder Severity Index (BPDSI)), depression (measured with the Beck Depression Inventory) and degree of impulsivity (measured with the Barratt Impulsiveness Scale v11). The participant self-reported improvements in sleep, emotional control, self-awareness of behaviour, sociability and motivation for change following TMS. Functional neuroimaging was undertaken before the first and after the last TMS session, and again, 1 month later, with the application of the International Affective Picture System that projected emotionally negative and neutral images during scanning. The neuroimaging results fitted with a frontolimbic dysconnectivity model, showing that rTMS attenuated activation in the amygdala, superior temporal gyrus, superior frontal gyrus, middle frontal gyrus and parahippocampus when exposed to negative imagery; whereas post-rTMS the middle temporal gyrus and post-central gyrus showed increased neural activation to emotional stimuli.
An important feasibility study of rTMS on BPD, with some interesting and positive findings, was reported by Cailhol et al. Reference Cailhol, Roussignol, Klein, Bousquet, Simonetta-Moreau and Schmitt40 They undertook a randomised controlled study of high-frequency rTMS (5-second bursts at 10 Hz, with 25-second between-train intervals, for 20 min per day) on ten patients (nine completed analyses, age range 20–45) with a DSM-IV-TR diagnosis of BPD. The active group (n=5) received two sets of 5 days – separated by a 2-day gap – of daily rTMS to the right-sided DLPFC. Those in the sham group (n=4) received the same protocol but with the coil tilted to 90° so avoiding any delivery to the patient. Two of the active group and one of the sham demonstrated a response of 30% reduction on the BPDSI, Reference Arntz, van den Hoorn, Cornelis, Verheul, van den Bosch and de Bie41 with statistically significant improvements in the affective instability and anger subcomponents of the BPDSI in the real group at 3 months. The active, but not the sham group, also showed statistically significant improvements in the Tower of London test – which assesses planning – at the 3-month follow-up. The rTMS was well tolerated by participants.
Shifting paradigms: moving to modulating neurocognitive processing
There has generally been less direct testing of the impact of neuromodulation on neurocognitive processing that might explicitly inform a neural circuitry model; understandably but problematically, the majority of work looks at clinical outcomes, and existing work that does measure behavioural outcomes is interesting but typically methodologically weak and with ill-defined targeted neuropathophysiology. rTMS has been shown to affect decision-making in paradigms investigating addictive behaviours, at least in the laboratory. Low-frequency (inhibitory) rTMS to the right DLPFC in healthy participants was shown to induce risk-taking on a gambling paradigm, Reference Knoch, Gianotti, Pascual-Leone, Treyer, Regard and Hohmann42 whereas high-frequency (excitatory) rTMS to the left DLPFC can reduce food craving, Reference Uher, Yoganathan, Mogg, Eranti, Treasure and Campbell43 cigarette smoking Reference Eichhammer, Johann, Kharraz, Binder, Pittrow and Wodarz44 and cocaine craving Reference Camprodon, Martinez-Raga, Alonso-Alonso, Shih and Pascual-Leone45 compared with sham TMS. More recently a pilot study by Taylor et al Reference Taylor, Neitzke, Khouri, Borfckardt, Acierno and Tuerk46 showed that rTMS to the left DLPFC promoted resilience in healthy controls during an aversive stimulus model of learned helplessness. These studies indicate that rTMS can modulate behaviour and motivational states by enhancing or attenuating inhibitory prefrontal mechanisms, although one is struck by the generally low participant numbers and lack of attempted later replication. An interesting general omission in the current literature has been the marked lack of concomitant psychological, cognitive remediative or similar intervention in parallel with neuromodulation. Neuromodulation is principled on enhancing brain plasticity, and the potential for this to augment other interventions appears inherently attractive; Keefe et al Reference Keefe, Vinogradov, Medalia, Silverstein, Bell and Dickinson47 proposed the evocative metaphor that neuronal plasticity without parallel intervention was akin to consuming protein without exercising.
Moving forward: from diagnosis to functional neurobiological systems
The clinical problems of suicidal thinking, self-harm and suicide scarcely need reiterating, highlighted by the World Health Organization's estimate 48 of almost a million resultant deaths annually. Despite the effectiveness of existing treatments, both pharmacological and psychological, these problems are significant and persistent, and can be chronic and disabling for many people. What is striking is the juxtaposition between this reality and the scientific literature that has focused relatively less on self-harm, tending to subsume it as an aspect of other mental illnesses rather than viewing it as a problem unto itself, perhaps exemplified by DSM-5 not having a code for suicidal behaviour. The resultant clinical paradigm is that we tend to try to manage suicidality by proxy: treat the background mental illness with the aim that this will indirectly reduce the risks of self-harm, rather than see self-harm as an expression of emotional dysregulation which, in itself, is the cause of comorbid mental illness. Just as schizophrenia, bipolar affective disorder and dementia have neurobiological correlates and causes, so does suicidal thinking and self-harm.
The existing rTMS work on depression, which constitutes a large body of the neuromodulatory literature, focuses more broadly on overall clinical improvement, with the major emphasis on changes in mood symptoms. There has, however, been an almost total neglect of the effects on impulsivity and self-harm. Indeed a very recent consensus statement on rTMS by a group of European experts Reference Lefaucheur, André-Obadia, Antal, Ayache, Baeken and Benninger49 does not even address self-harm as a current or future target, although the earlier mentioned frontolimbic brain circuitry is discussed in terms of mood regulation. There is therefore a need and an opportunity to rectify this gap and move towards translation of cognitive neuroscience activity into therapeutic options.
The model presented here is of a brain phenotype that is abnormally primed to misread threat perception, to have difficulty with emotional regulation, and an increased propensity to greater impulsivity: an individual predisposed to self-harm. Such a model does not take specific aetiological factors into account (or the relative importance of genes and environment), and sidesteps the issues of causality (a hypoactive PFC leading to a hyperactive limbic system or vice versa) and diagnosis; any combination of such factors may be considered to have led to pathological frontolimbic circuitry. Of course it can be reasonably argued that this is a rather simplistic impulsivity-dysfunction model of a very complex psychosocial problem, yet the role of promoting coping and resilience to psychosocial adversity offers an alternative perspective. A potentially important example is the psychological construct of an introjective-anaclitic dichotomy in differentiating the extent to which a particular individual's suicidal ideation and actions are linked to a more internalised self-criticism versus a more general set of expectations about others’ reactions within a social matrix: such factors can be measured, and as earlier described existing neurobiological data tell us they have differential neuroanatomical correlates. rTMS could offer a tool to probe this and other such questions, and the lack of diagnostic chains might therein be regarded as a strength of this model.
It may be, and indeed would seem likely, that different groups or populations might respond differently to such an intervention, and, indeed, we argue that this is an attraction of this proposed model: it presents eminently testable hypotheses to evaluate coping strategies, impulsivity and cognitive models of self-harm, and how rTMS might variously affect them, including in attempting to delineate possible neurocognitive factors more or less amenable to intervention. Undoubtedly many potential difficulties may lie ahead, not least the pragmatics of costings, time and acceptability of rTMS, Reference Tracy and David50 on top of the fact that it remains to be proven that such an intervention would prove effective. Nevertheless, NICE and other large research bodies have asserted the need for more research in specific patient groups, and for trials of different dosages of rTMS. The opportunity cost of testing such a hypothesis would currently appear to outweigh that of inaction.
Acknowledgements
D.K.T., S.S.S. and A.S.D. are supported by the National Institute of Health Research Biomedical Research Centre (BRC) at the South London & Maudsley NHS Foundation Trust and the Institute of Psychiatry, Psychology and Neuroscience, King's College London.






eLetters
No eLetters have been published for this article.