Breast milk is known as the ideal food to meet the needs of growing newborns( Reference Kramer and Kakuma 1 ). Milk fat plays an important role as a source of energy, as well as in structural and regulatory functions, where the latter depend mainly on the PUFA content( Reference Holman 2 ). PUFA such as linoleic acid (LA, 18 : 2 n-6) and α-linolenic acid (ALA, 18 : 3 n-3) are fatty acids (FA) that cannot be assembled by the mother or the neonate( Reference Scopesi, Ciangherotti and Lantieri 3 ).
Although long-chain PUFA (LCPUFA) such as arachidonic acid (ARA, 20 : 4 n-6), EPA (20 : 5 n-3) and DHA (22 : 6 n-3) can be formed from their respective precursors LA and ALA( Reference Koletzko, Boey and Campoy 4 ), the conversion rates are very low. Most infants cannot synthesise enough LCPUFA from precursor FA( Reference Uauy, Mena and Rojas 5 ). Thus, it is extremely important to provide adequate ARA and DHA in the diet from early infancy. In exclusively breast-fed infants, LCPUFA content in their tissues depends on the content found in their mothers’ milk( Reference Carlson, Rhodes and Rao 6 ). In contrast to the relatively constant proportion of SFA and MUFA in breast milk samples across a large number of countries( Reference Yuhas, Pramuk and Lien 7 ), the level of some PUFA, particularly DHA, is highly variable, with the highest levels in Japanese and the lowest in Canadian and US breast milk samples( Reference Yuhas, Pramuk and Lien 7 ).
Several studies have investigated the association of maternal dietary FA intake with FA composition, including PUFA, in human milk( Reference Antonakou, Skenderi and Chiou 8 – Reference Liu, Li and Yu 10 ). A recent study showed a strong positive correlation during the 1st month postpartum between Greek mothers’ PUFA intake and PUFA, n-3 FA, DHA and LA concentrations in their breast milk, whereas MUFA intake was strongly correlated with PUFA, n-6 FA and LA concentrations( Reference Antonakou, Skenderi and Chiou 8 ). Some studies suggest that DHA dietary intake is positively correlated with breast milk DHA concentrations in Swedish( Reference Xiang, Harbige and Zetterström 9 ) and Chinese( Reference Liu, Li and Yu 10 ) mothers. However, these studies were conducted with small sample sizes and during early-stage lactation.
Koreans have traditionally consumed considerable amounts of fish and seaweeds( Reference Koletzko, Boey and Campoy 4 ), and hence have a relatively high intake of preformed EPA and DHA. To our knowledge, only two studies have measured PUFA levels in Korean milk samples. In milk samples of transitional milk from the mid 1990s( Reference Golfetto, McGready and Ghebremeskel 11 ), higher levels of DHA (0·96 % of total FA) than the approximately 0·3 % typically found in breast milk in Western countries( Reference Brenna, Varamini and Jensen 12 ) was reported, although the levels were similar to that of Japanese women (0·99 % of total FA)( Reference Yuhas, Pramuk and Lien 7 ). However, milk samples of 1–3 months postpartum were collected in late 2000s, and DHA levels were found to be 0·66 % of total FA( Reference Jang, Lee and Park 13 ), a significant decrease from the earlier report( Reference Golfetto, McGready and Ghebremeskel 11 ). South Korea has undergone a tremendous change in dietary habits towards more meat and less fish consumption, and more frequent use of fats and oils during food preparation( Reference Kim, Ko and Kwon 14 , Reference Bae, Kim and Kim 15 ), which could be responsible for the observed changes in LCPUFA levels in breast milk of lactating women. Thus, a new analysis on LCPUFA levels in breast milk is warranted.
Although a sufficient PUFA supply ensures optimum growth and development in infants, no study thus far, to the best of our knowledge, has investigated the association of maternal diet composition and breast milk PUFA content of South Korean mothers. The aims of this study were to determine the fat content and FA composition of breast milk and the association with FA composition of lactating mothers’ diet in South Korea.
Methods
Study subjects
Study subjects were lactating mothers recruited from an online site who agreed to participate in the study. From April 2013 to May 2015, a total of 255 exclusively breast-feeding mothers were recruited from across South Korea. They were from Seoul (n 79), six metropolitan cities (Busan (n 6), Daejeon (n 7), Daegu (n 7), Incheon (n 26), Ulsan (n 1), Gwangju (n 2)) and thirty-four cities from four provinces (Gyeonggi, twenty-four cities (n 113); Chungcheong, five cities (n 8); Gyeongsang, four cities (n 5); Jeonla one city (n 1)).
Five women who delivered at gestational ages<37 weeks ≥43 weeks and three women who delivered babies with low birth weight (<2·5 kg) were excluded. Of the remaining women, six women provided only 1-d dietary record data and were excluded. Therefore, a total of 238 women and their babies were included for the analysis.
Study participants were interviewed by trained interviewers. General information on demographic and socio-economic factors, anthropometry (height, body weight before pregnancy and at the time of data collection) and health-related behaviours (cigarette smoking, alcohol consumption and use of dietary supplements) was collected. Further information on pregnancy outcomes such as gestational age at delivery (weeks), neonatal sex, birth weight (g) and length (cm), as well as age (d) and body weight at sample collection, was obtained from baby record books. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all subjects provided a written informed consent. The study protocols and consent forms were approved by the Institutional Review Boards (0627-201408-HRBR-002-03) at Maeil Innovation Center.
Dietary assessment
Dietary intake data were collected using a food record for 3 consecutive days. Subjects recorded all foods and beverages they consumed during those 3 d. This dietary protocol was completed before and after 1 week of milk collection by the participants at home. The sampling period included 2 weekdays and 1 weekend day. Nutrient intake data were calculated using the computer-aided nutritional analysis program (CAN-Pro 4.0; Korean Nutrition Society), and the average of the 3 d was used to estimate normal dietary intake. A food FA database was constructed using Can-Pro 4.0 database by incorporating values from the United States Department of Agriculture( 16 ) and Korea National Fisheries Research and Development Institute( 17 ).
Breast milk sampling and macronutrient analysis
The breast was cleaned with water, and 150 ml of breast milk was pumped, collected into a sterilised conical tube and sent to the Maeil Human Milk Research Center in an ice-packed container. No specific time of day was defined for expressing breast milk samples. However, the full expression was collected to prevent collection of hindmilk or foremilk. The breast milk samples were stored at −18°C until analysis, which was usually carried out within 1 week.
The frozen milk samples were thawed in a refrigerator at 4°C, heated in a water bath until 37°C and then homogenised before analysis. Fat, lactose and protein concentrations of the breast milk samples were analysed using MilkoScan FT2 (Foss Analytical) as previously described( Reference Chang, Jung and Kim 18 ).
Fatty acids analysis
Breast milk fat was extracted following the modified method of Folch et al. ( Reference Folch, Lees and Sloane Stanley 19 ) with diethyl ether–petroleum ether solvent mixture (1:1, v/v). The content in aliquots of the extract was determined gravimetrically after solvent evaporation. FA methyl esters were prepared by transesterification with boron trifluoride and methanol. Separation and identification of FA were performed using an Agilent 7890 (Agilent Tech.) GC with a flame ionisation detector (FID) (Agilent Tech.). The SP-2560 capillary GC column (100 m×0·25 mm×0·20 µm; Sigma-Aldrich Co.) was used and calibrated against a standard containing thirty-seven FA methyl esters, ranging in chain length from four to twenty-four carbon atoms (Supelco 37 Component Fame Mix; Supelco).
GC-FID analysis was performed under the following instrumental conditions: injection volume of 1 µl and N2 carrier gas flow rate of 1·15 ml/min with a split ratio 50:1 and constant flow control. Injector and detector temperatures were set at 225 and 285°C, respectively. The oven programme was as follows: 120°C for the first 5 min, increased by 3°C/min until 210°C, maintained for 3 min, increased by 1°C/min until 230°C and maintained for 7 min. An aliquot of the supernatant was transferred into an autosampler vial for GC-FID analysis. The FA methyl esters were identified by comparison of their relative retention times with authentic standards, and the mass distribution was calculated electronically by quantification of the peak areas.
Statistical analysis
The data are expressed as means and standard deviations (continuous variables) or as numbers and percentages (categorical variables). Pearson’s correlation test was used to determine the correlation with maternal age and BMI, infant’s age and fat content and FA composition in breast milk. Associations between maternal dietary intakes and breast milk fat content and FA composition were analysed by partial correlation after adjusting for potential confounders such as maternal age, BMI, supplement use and infant’s age. All statistical analyses were performed using SAS 9.3 software (SAS Institute Inc.), and P values<0·05 was considered significant.
Results
General characteristics
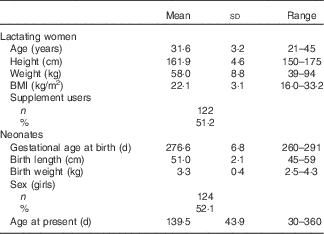
The lactating women were 31·6 (sd 3·2) years old and had a current BMI of 22·1 (sd 3·1) kg/m2 (Table 1). Approximately 51·2 % of the lactating women took dietary supplements, and infant age was 139·5 (sd 43·9) d (range 30–360 d).
Table 1 General characteristics of lactating women and their newborn infants (Mean values and standard deviations; ranges; number of participants and percentages; n 238)
Dietary fatty acid intake of lactating women
The average daily energy and fat intakes of the lactating women were 34 196·7 kJ (8173·2 kcal) and 57·2 g, respectively. The average SFA, MUFA and PUFA intakes were 13·5 g (6·2 % of energy intake), 16·0 g (7·4 % of energy intake) and 11·0 g (5·1 % of energy intake), respectively. n-6 and n-3 FA intakes were 9·9 g (4·5 % of energy intake) and 1·2 g (0·5 % of energy intake), respectively (Table 2).
Table 2 Diet composition of lactating women (Mean values and standard deviations; ranges; n 238)

FA, fatty acids; ARA, arachidonic acid.
Fat and fatty acid profiles of breast milk
The average breast milk fat content was 0.33 (SD 0.14) g/l with ARA, EPA and DHA comprising 0·48 (sd 0·13), 0·15 (sd 0·12) and 0·67 (sd 0·47) % of total FA, respectively (Table 3).
Table 3 Nutrient content and fatty acid composition in breast milk (Mean values and standard deviations; ranges; n 238)
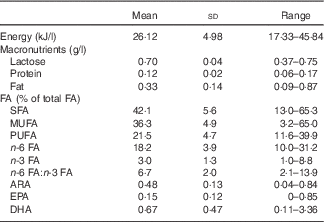
FA, fatty acids; ARA, arachidonic acid.
Fat content in breast milk was negatively correlated with maternal age (r −0·140, P<0·05) and positively correlated with maternal BMI (r 0·213, P<0·01). SFA content in breast milk was positively correlated with infant age (r 0·113, P<0·05) (Table 4). PUFA (22·5 v. 20·5 % of total FA, P=0·0008), n-6 FA (18·9 v. 17·4 % of total FA, P=0·0017) and n-3 FA (3·2 v. 2·8 % of total FA, P=0·0079) were higher in lactating women who used supplements than in those who did not (data not shown).
Table 4 Correlations between maternal age, BMI and infant’s age and fat content and fatty acid composition in breast milk (Pearson’s correlation coefficients; n 238)

ARA, arachidonic acid.
* P<0·05, ** P<0·01.
Correlation between fatty acid levels in the maternal diet and breast milk
Table 5 shows that after adjusting for potential confounders such as maternal age, BMI, supplement use and infant’s age, lactating women’s daily intakes of EPA, DHA, n-3 FA, n-6 FA, SFA and PUFA were positively correlated with the corresponding FA in milk samples. Daily carbohydrate intake was positively correlated with SFA and negatively with MUFA in breast milk. Dietary cholesterol intake was negatively correlated with PUFA in milk.
Table 5 Partial correlation between maternal dietary intakes and fat content and fatty acid (FA) composition in breast milkFootnote † (Partial correlation coefficients; n 238)

ARA, arachidonic acid.
* P<0·05, ** P<0·01, *** P<0·001.
† Adjusted for maternal age, BMI, supplement use and infant’s age.
Discussion
South Korean breast milk PUFA, n-3 FA and DHA were higher than that of western countries as well as most of Asia, although DHA content in particular was lower than that previously reported( Reference Golfetto, McGready and Ghebremeskel 11 ). Lactating mothers’ daily intakes of EPA, DHA, n-3 FA, n-6 FA, SFA and PUFA were positively associated with the corresponding FA in milk samples.
The proportion of total PUFA, n-3 FA and DHA (21·5, 3·0 and 0·67 %, respectively) was higher than that reported in European countries including Spain( Reference de la Presa-Owens, López-Sabater and Rivero-Urgell 20 ), Sweden( Reference Xiang, Harbige and Zetterström 9 ), Germany( Reference Szabó, Boehm and Beermann 21 ), Italy( Reference Grote, Verduci and Scaglioni 22 ) and Greece( Reference Antonakou, Skenderi and Chiou 8 ), as well as northern China( Reference Wan, Wang and Xu 23 ). For DHA, the present study’s results (0·67 % of total FA) are comparable with lactating women in a coastal area of south-eastern China (0·61 %)( Reference Peng, Zhou and Wang 24 ) and the Philippines (0·74 %)( Reference Yuhas, Pramuk and Lien 7 ), but over twice that reported in western countries (approximately 0·3 %)( Reference Brenna, Varamini and Jensen 12 ) and most of Asia, including Nepal (0·23 %)( Reference Glew, Huang and Vander Jagt 25 ) and Bangladesh (0·30 %)( Reference Yakes, Arsenault and Munirul Islam 26 ). Approximately 1 % was reported in Japan( Reference Yuhas, Pramuk and Lien 7 ), which is known as having high fish intake.
The large range of DHA content in breast milk is thought to reflect variations in maternal DHA intake, as populations with high fish intake also have high milk DHA content – for example, comparing women living in an inland (40 mg/d) or coastal area (180 mg/d) of south-eastern China( Reference Peng, Zhou and Wang 24 ). In the present study, Korean lactating women had high DHA intake (140 mg/d) compared with that reported in other countries (e.g. Bangladesh (30 mg/d)( Reference Yakes, Arsenault and Munirul Islam 26 ), Sudan (33 mg/d)( Reference Nyuar, Min and Ghebremeskel 27 ), New Mexico (47 mg/d)( Reference Glew, Wold and Herbein 28 ), Sweden (120 mg/d)( Reference Xiang, Harbige and Zetterström 9 ), Canada (186 mg/d))( Reference Jia, Pakseresht and Wattar 29 ). Korean citizens have traditionally regularly consumed fish and seaweed( Reference Koletzko, Boey and Campoy 4 ), and hence have had a relatively high intake of preformed EPA and DHA. However, in recent years, fish intake in Korea has significantly decreased, whereas meat consumption has increased, with resulting decreased intakes of DHA and EPA and increased ARA intake, particularly among younger women, as documented in the Korean National Health and Nutrition Examination Survey( Reference Kim, Ko and Kwon 14 , Reference Bae, Kim and Kim 15 ) In South Korea, breast milk DHA concentration (0·67 % of total FA) was lower than that reported almost 20 years ago (0·96 % of total FA) in transition milk( Reference Golfetto, McGready and Ghebremeskel 11 ). Thus, DHA content in human milk directly corresponds to maternal dietary DHA intake.
The ARA:DHA ratio in breast milk was 0·96, which is within the recommended range of 0·5–1( Reference Uauy-Dagach, Mena and Hoffman 30 ). The ARA:DHA ratio varies significantly, from 0·51:1 in Japan to 3·16:1 in the USA, as a result of relatively constant ARA levels and highly variable DHA levels( Reference Yuhas, Pramuk and Lien 7 ). The ratio of n-6:n-3 FA in breast milk varies substantially, 4·7–27·8( Reference Jensen 31 – Reference Marín, Sanjurjo and Rodrigo 34 ). Similar to the ARA:DHA ratio, US mothers showed high n-6:n-3 ratios( Reference Jensen 31 ), whereas Japanese mothers show the lowest( Reference Krasevec, Jones and Cabrera-Hernandez 32 ). Breast milk from Korean mothers had lower n-6:n-3 ratio (approximately 6·7) compared with many reported from other countries( Reference Jensen 31 , Reference Aggett, Haschke and Heine 33 , Reference Marín, Sanjurjo and Rodrigo 34 ). Thus, according to current recommendations, the diet of Korean mothers is more balanced regarding n-6 and n-3 PUFA content than that of Western mothers( Reference Aggett, Haschke and Heine 33 , Reference Marín, Sanjurjo and Rodrigo 34 ), particularly US mothers( Reference Jensen 31 ), although not as well as Japanese mothers.
PUFA play an important role in infant growth and development, particularly neurodevelopment( Reference Keim, Daniels and Siega-Riz 35 , Reference Glaser, Lattka and Rzehak 36 ) and visual acuity( Reference Carlson, Ford and Werkman 37 ) in early life. PUFA such as LA and ALA are considered nutritionally essential because they cannot be synthesised de novo from other lipids, carbohydrates and amino acids( Reference Scopesi, Ciangherotti and Lantieri 3 , Reference Koletzko, Boey and Campoy 4 ). This implies that neonatal PUFA uptake is entirely dependent upon supply from an external source. LCPUFA such as ARA and DHA can be transported to the newborn by dietary intake or synthesised in the neonatal liver by chain elongation and desaturation of their respective precursors (LA and ALA)( Reference Scopesi, Ciangherotti and Lantieri 3 ). However, because of the very low conversion rates in most infants( Reference Uauy, Mena and Rojas 5 ), it is very important to provide adequate ARA and DHA through the diet from early infancy. Especially in exclusively breast-fed infants, LCPUFA content in their tissues depends on that in their mother’s milk( Reference Carlson, Rhodes and Rao 6 ). LCPUFA in breast milk may partially reflect FA composition in the maternal diet( Reference Antonakou, Skenderi and Chiou 8 – Reference Liu, Li and Yu 10 ). DHA levels in particular are sensitive to maternal diet( Reference Olafsdottir, Thorsdottir and Wagner 38 ). As sufficient DHA is essential for normal development of visual and/or cognitive function( Reference Uauy, Birch and Birch 39 , Reference Carlson, Werkman and Rhodes 40 ) and ARA for optimal growth( Reference Koletzko and Braun 41 , Reference Hadley, Ryan and Forsyth 42 ), lactating mothers should consume appropriate amounts of ARA and DHA.
In contrast to PUFA, MUFA and SFA concentrations in human milk are relatively constant across many countries( Reference Yuhas, Pramuk and Lien 7 ). In the present study, MUFA and SFA in milk samples were approximately 36·3 and 42·1 % of total FA, respectively, which is comparable with that among lactating women in other countries( Reference Yuhas, Pramuk and Lien 7 ). The mean milk MUFA in the present study was higher than that reported in previous Korean samples( Reference Jang, Lee and Park 13 ) (30·6 v. 36·3 % of total FA), whereas milk SFA was lower (48·0 v. 42·1 % of total FA). The significance of these findings remains to be investigated further.
Lactating women’s daily intakes of EPA, DHA, n-3 FA, n-6 FA, SFA and PUFA were positively associated with the corresponding FA in the milk samples, confirming previous findings( Reference Antonakou, Skenderi and Chiou 8 – Reference Liu, Li and Yu 10 ), although these previous studies have limitations such as small sample numbers or short-term breast-feeding period (until 3 months of lactation) or have been conducted with non-supplements users, which is different from our study where 51·2 % of the lactating women took dietary supplements. Antonakou et al. ( Reference Antonakou, Skenderi and Chiou 8 ) reported that Greek mothers’ PUFA intake during the 1st month postpartum (n 64) was strongly positively correlated with breast milk concentration of PUFA, n-3 FA, DHA and LA, and MUFA intake was strongly correlated with the concentration of PUFA, n-6 FA and LA. DHA dietary intake has been shown to be positively correlated with DHA concentrations of breast milk in Swedish (n 19; 3 months postpartum)( Reference Xiang, Harbige and Zetterström 9 ) and Chinese (n 408; 42 (sd 7) d postpartum) mothers( Reference Liu, Li and Yu 10 ).
Breast milk fat content was negatively correlated with maternal age and positively correlated with maternal BMI. Several studies have shown a positive correlation between maternal BMI status during lactation and breast milk fat content( Reference Grote, Verduci and Scaglioni 22 , Reference Michaelsen, Skafte and Badsberg 43 , Reference Nommsen, Lovelady and Heinig 44 ), whereas studies relating maternal age to fat content in human milk are infrequent and the results are indeterminate. Antonakou et al. ( Reference Antonakou, Skenderi and Chiou 8 ) reported that maternal age was negatively correlated with MUFA and oleic acid values in Greek mothers’ breast milk during the first month. However, these correlations did not remain significant over the whole 6-month study period. A recent study conducted on Italian mothers found that PUFA, LA and n-6 FA were all significantly lower in older than in younger mothers( Reference Grote, Verduci and Scaglioni 22 ). However, all these differences were not significant after correcting for multiple testing. The present study also found a positive correlation between breast milk SFA and infant age (stage of lactation). However, we could not find definitive support for these findings in the literature. Some studies( Reference Antonakou, Skenderi and Chiou 8 , Reference Grote, Verduci and Scaglioni 22 ) show that the SFA level in breast milk did not differ with stage of lactation. Thus, the positive correlation between infant age and breast milk SFA from the present trial should be interpreted with caution.
The limitations of our study should be noted. We did not have data on maternal plasma or erythrocyte FA, which could have substantiated the relationship observed between FA dietary intakes and human milk content. Characterisation of maternal FA desaturase genotype that can affect LCPUFA levels in breast milk might also have improved the reliability of our results. Furthermore, the study outcomes cannot be generalised as representative of the country, because our data were generated from self-selected participants, who are more likely to have healthier dietary habits than the average population as more than half of the participants were taking dietary supplements.
Conclusions
This is the first study in South Korea to examine the association of FA composition of breast milk with dietary intake in exclusively breast-feeding mothers. Breast milk of Korean mothers was found to be richer in DHA, n-3 FA and total PUFA compared with breast milk from mothers in Western countries, as well as some of Asian countries. A significant positive association was found postpartum (30–360 d) between mothers’ daily intakes of EPA, DHA, n-3 FA, n-6 FA, SFA and PUFA and the corresponding FA concentrations in breast milk.
Thus, South Korean women’s dietary characteristics, that is, high intakes of total PUFA, including DHA or n-3 FA, affect their FA milk profile during exclusive lactation. Considering that PUFA are essential and should be supplied in sufficient quantities to guarantee normal visual and/or cognitive development during infancy, it is of great significance to improve South Korean maternal PUFA nutritional status.
Acknowledgements
This study was supported by Brain Korea 21 Plus.
N. C. and J. A. J. designed the study protocols. H. K., S. K., B.-M. J. and H. Y. conducted the study. H. K. and H. Y. analysed the data, and H. K. and N. C. wrote the manuscript. N. C. and J. A. J. were primarily responsible for the final contents. All the authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.








