Introduction
The Falkland Islands (Las Malvinas) are located off the southeast coast of South America in the southwest Atlantic Ocean, ~260 nautical miles east of Argentina. The archipelago, made up of two larger islands and over 700 smaller ones, sits on the Patagonian Shelf, which includes Burdwood Bank to the south (Figure 1). The majority of the territorial sea reaches <100 m in depth but the surrounding exclusive economic zone encompasses waters that extend below 4000 m. Biogeographically, the Islands sit within the Magellan biogeographic region (as defined by Koubbi et al., Reference Koubbi, De Broyer, Griffiths, Raymond, d'Udekem d'Acoz, Van de Putte, Danis, Grant, Gutt, Held, Hosie, Huettman, Post, Ropert-Coudert, Stoddart, Swadling, Wadley, De Broyer, Koubbi, Griffiths, Raymond, Udekem d'Acoz, Van de Putte, Danis, David, Grant, Gutt, Held, Hosie, Huettman, Post and Ropert-Coudert2014), sharing many species with southern Argentina, Chile, and Patagonia. Nevertheless, Darbyshire (Reference Darbyshire2018) demonstrated an affinity of the Falkland Islands polychaete fauna with those of both South Georgia and Antarctica.

Figure 1. Map of Falkland Islands waters indicating the localities of each taxon record reported in this paper. Map inset shows the position of the Falkland Islands in the southwest Atlantic. Additional stations where Kinbergonuphis dorsalis was recorded by Monro (1930, 1936: D, WS), Hartmann–Schröder (1962: HS), Averincev (1972: A) and HERO783 are also indicated as well as those records from Hartman's (Reference Hartman1967) stn 350 (Elt).
Falkland Islands polychaetes have been relatively poorly investigated with most specimens being recorded and published in the early 1900s by Pratt (Reference Pratt1898, Reference Pratt1901), Pixell (Reference Pixell1913), Ramsay (Reference Ramsay1914), Fauvel (Reference Fauvel1916), and Monro (Reference Monro1930, Reference Monro1936). Between 2008 and 2012, multiple environmental baseline surveys were undertaken around the Islands with respect to oil exploration and these results have been compiled and published by Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020). Most of the baseline work was undertaken in deep waters to the north, east, and south of the Islands in the North and East Falkland Basins (~450–1800 m depths) providing a far more comprehensive record of the polychaete fauna in the region's deeper waters. More recently, in 2011, 2012, and 2015, extensive surveys were undertaken around the Islands which were a mix of intertidal and some limited shallow water sampling, further revealing previously unrecognized diversity of polychaetes from those shallow habitats (Darbyshire, Reference Darbyshire2018). The increased interest in oil exploration within the Falkland Islands area makes it more important than ever that accurate knowledge of the fauna should be available.
The families Eunicidae Berthold, 1827 and Onuphidae Kinberg, 1865 belong to the Order Eunicida Dales, Reference Dales1962 along with five other families (Budaeva and Zanol, Reference Budaeva, Zanol, Purschke, Westheide and Böggemann2021) that all possess a ventral muscular pharynx with mineralized or sclerotized jaws (Tzetlin and Purschke, Reference Tzetlin and Purschke2005). The two families are the only extant ones in the order to possess eulabidognath maxillae (Paxton, Reference Paxton2009) and, together with the additional synapomorphies of five prostomial appendages, peristomial cirri, and subacicular hooks in median and posterior parapodia (Struck et al., Reference Struck, Purschke and Halanych2006, Reference Struck, Golombek, Weigert, Franke, Westheide, Purschke, Bleidorn and Halanych2015; Tilic et al., Reference Tilic, Bartolomaeus and Rouse2016), recent genetic data supported the combination of the two families to form the superfamily Eunicoidea Orensanz Reference Orensanz1990 (Tilic et al., Reference Tilic, Stiller, Campos, Pleijel and Rouse2022). Eunicoidea species can be found from intertidal zones to the deep sea and play an important role in benthic communities including as both prey and predator species, by acting as a stabilizing force on the sediment through burrows and tube building and, in some cases, by enhancing surrounding biodiversity through epiphytic growth on tubes (Elgetany et al., Reference Elgetany, El-Ghobashy, Ghoneim and Struck2018; Budaeva, Reference Budaeva, Purschke, Westheide and Böggemann2021; Zanol and Budaeva, Reference Zanol, Budaeva, Purschke, Westheide and Böggemann2021).
Onuphidae consists of 22 valid genera containing over 300 species (Budaeva et al., Reference Budaeva, Schepetov, Zanol, Neretina and Willassen2016) found from intertidal to abyssal depths, and the family has been described as one of the most successful deep-water families of polychaetes (Arias and Paxton, Reference Arias and Paxton2022). For the Magellan region specifically, Orensanz (Reference Orensanz1974b) listed eight species of Onuphidae as present. Around the Falkland Islands, Monro (Reference Monro1930, Reference Monro1936), Hartman (Reference Hartman1967), Averincev (Reference Averincev1972), and Fauchald (Reference Fauchald1982c) all recorded species of Onuphidae, although none of these were from less than 100 m. Many of these identifications were later changed or synonymized with other species by Orensanz (Reference Orensanz1990), who conducted a detailed review of Antarctic and Subantarctic Eunicemorpha. The latter publication also reduced his original 1974(b) list of Magellan species from eight to six (plus one doubtful species). In a review of species in the Falkland Islands region, Darbyshire (Reference Darbyshire2018) listed only two species as having previously been recorded, however, that review failed to take into account the many re-identifications made by Orensanz (Reference Orensanz1990) of specimens from the region. Since then, offshore exploration surveys in the area have additionally provided much greater knowledge of the deep-water species. Table 1 lists the different taxa recorded from Falkland Island waters and provides details on how their identification has changed from that first reported up until now.
Table 1. Eunicoidea species reported from the Falkland Islands region with details of who reported them, if records were subsequently re-assigned to a different name or taxon and who by, and the current assignation of that record

Eunicidae is a large family containing 11 genera and more than 400 species (Zanol and Budaeva, Reference Zanol, Budaeva, Purschke, Westheide and Böggemann2021). Species occur in habitats from the intertidal to the deep sea, although around the Falkland Islands only one species of Leodice (as Eunice) and one species of Marphysa have previously been recorded from the region (Fauvel, Reference Fauvel1916; Monro, Reference Monro1930; Darbyshire, Reference Darbyshire2018), each of those from very different habitats and depths (Table 1). A single species of Marphysa was reported by Fauvel in 1916, from specimens collected and sent to him by Rupert Vallentin from the intertidal region of West Falkland. Fauvel identified the species under the name Marphysa corallina (Kinberg, Reference Kinberg1865), a species originally described from Hawaii, however, this identification was amended by Orensanz (Reference Orensanz1990) to Marphysa aenea (Blanchard in Gay, Reference Blanchard and Gay1849), first described from the Pacific coast of Chile. This is the only species of Eunicidae currently reported from the intertidal and shallow regions of the Islands. Further offshore, Leodice pennata (Müller, Reference Müller1776) was recorded by Monro (Reference Monro1930, as Eunice pennata) at 115 m depth and more recently it was also recorded from deeper water (1321–1842 m; Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020).
In total, the current publication provides a review of ten taxa of Eunicoidea from the region: eight taxa (from six genera) of Onuphidae and two taxa (from two genera) of Eunicidae. Only five of the taxa can confidently be identified at species level at this time (Table 1). Figure 1 illustrates where each of the taxa was recorded around the Falkland Islands region. Kinbergonuphis sp. is described and figured and characters not quite conforming to the original description of K. dorsalis detailed. Hyalinoecia falklandica sp. nov. is newly described from specimens previously identified as Hyalinoecia artifex (Verrill, Reference Verrill1881), Hyalinoecia stricta Moore, Reference Moore1911 and Hyalinoecia tubicola (Müller, Reference Müller1776) and the relationship with those species discussed. Finally, the previous identification of Marphysa aenea is shown to be erroneous and a description and discussion of the as-yet undetermined species provided.
Materials and Methods
Intertidal and shallow water specimens (Darbyshire, Reference Darbyshire2018) from the Falkland Islands were collected by digging, sieving sediments through a 0.5 mm sieve, opening rock crevices, turning rocks, and sampling algal crusts. Specimens collected by Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) were obtained using a 0.25 m2 USNEL box core or Van Veen grab, with samples sieved through a 0.5 mm mesh. Specimens were fixed in 4% formaldehyde in seawater (Darbyshire, Reference Darbyshire2018; Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) and, in some cases, a sample of tissue was also preserved in 100% ethanol (Darbyshire, Reference Darbyshire2018). Where possible, those specimens collected by Darbyshire (Reference Darbyshire2018) were relaxed in a 7% magnesium chloride solution prior to fixing. All specimens were transferred to 70–80% ethanol solution for long-term storage post-fixation.
Morphological examinations and measurements were made using a Nikon Eclipse E400 binocular microscope and a Nikon Labophot-2 compound microscope, and drawings were produced using camera lucida attachments on each microscope. Microscope photographs were compiled and stacked using a Leica Wild microscope and Helicon Focus™ software. Specimens used for scanning electron microscopy (SEM) were prepared using a Quorum K850 critical point drier and Agar sputter coater (AGB7341) with subsequent imaging undertaken on a JEOL Neoscope JCM-7000 benchtop SEM.
Lengths are provided as L10 (length at chaetiger 10), W10 (width at chaetiger 10 excluding parapodia), and TL (total length). Terminology relating to Onuphidae follows that of Budaeva (Reference Budaeva, Purschke, Westheide and Böggemann2021) and for Eunicidae that of Zanol and Budaeva (Reference Zanol, Budaeva, Purschke, Westheide and Böggemann2021). Specific terminology of prostomial appendages follows that of Zanol et al. (Reference Zanol, Da Silva and Hutchings2017) and for the pectinate chaetae of Marphysa, the classification proposed by Molina-Acevedo and Carrera-Parra (Reference Molina-Acevedo and Carrera-Parra2015, Reference Molina-Acevedo and Carrera-Parra2017) for the blade and teeth and that of Zanol et al. (Reference Zanol, Da Silva and Hutchings2016) for the shaft are followed.
Specimens of Kinbergonuphis sp. and Marphysa sp. are accessioned in the zoological collections of Amgueddfa Cymru-Museum Wales (NMW.Z). The holotype and paratypes of Kinbergonuphis dorsalis were borrowed from the Zoological Museum Hamburg (ZMH) for examination along with comparative material, also identified as K. dorsalis, collected by Hartmann-Schröder (Reference Hartmann-Schröder1962) and the HERO cruise (1983). A specimen identified by Fauvel (Reference Fauvel1916) as Marphysa corallina as well as Monro's specimens of Kinbergonuphis dorsalis (as Onuphis quadricuspis and Onuphis dorsalis) and Eunice pennata were borrowed from or examined at the Natural History Museum, London (NHMUK) along with those specimens of Onuphidae and Eunicidae recorded by Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020). Specimens collected by the USNS Eltanin, identified by Hartman and/or Orensanz, were borrowed from the Smithsonian National Museum of Natural History (USNM) including the now-designated holotype, paratypes, and non-type material of Hyalinoecia falklandica sp. nov. Museum accession numbers of specimens examined, with number of specimens in parentheses after, are provided in the Materials Examined section for each species. All locality details for examined specimens are provided in a supplementary spreadsheet (S1).
DNA extraction and amplification
A 524 bp region of the 16S large subunit mitochondrial ribosomal DNA was sequenced for three specimens of Kinbergonuphis sp. using the Palumbi (Reference Palumbi, Hillis, Moritz and Mable1996) primers 16SarL and 16SbrH. DNA was extracted using a Qiagen DNeasy kit and amplified using GE Healthcare Illustra PuReTaq PCR beads with 1–2 μl of template and 0.25 μl of each primer (10 mM). Each reaction was made up to 25 μl using ultra-pure water and cycling conditions (Eppendorf Mastercycler) were as follows: 94°C for 150 s, 35 cycles of 94°C for 45 s, 51°C for 45 s, 72°C for 45 s, and finally 72°C for 10 min. Products were cleaned using a Sigma–Aldrich GenElute PCR clean up kit, quantified on agarose gels, and sequenced by DNA Sequencing and Services, Dundee University. Sequences were edited and compiled in ApE v.2.0.38 and sequences submitted to GenBank. Multiple unsuccessful attempts were also made to sequence the COI ‘barcoding’ gene using both the universal cytochrome oxidase subunit I (COI) primers (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) and a combination of the forward primer ACOIAF (Colgan et al., Reference Colgan, Hutchings and Brown2001) and reverse primer COIEU-R (Zanol et al., Reference Zanol, Halanych, Struck and Fauchald2010).
Results
SYSTEMATICS
Order EUNICIDA
Superfamily EUNICOIDEA Orensanz, Reference Orensanz1990
Family ONUPHIDAE Kinberg, Reference Kinberg1865
Subfamily ONUPHINAE Kinberg, Reference Kinberg1865
Genus Kinbergonuphis Fauchald, Reference Fauchald1982a
Type species Onuphis tenuis Hansen, Reference Hansen1882
Diagnosis (modified from Budaeva, Reference Budaeva, Purschke, Westheide and Böggemann2021)
Small to medium-sized worms with most species less than 10 cm long. Prostomium distally incised or extended with oval or ovoid frontal lips. Antennae with short to moderately long antennophores with 3–10 rings and long to moderately long styles reaching chaetigers 5–25. Median antenna shorter or equal to lateral antennae. Palpostyles longer than palpophores. Nuchal organs are straight with narrow middorsal separation. Anterior 3–7 pairs of parapodia slightly modified, not enlarged. Ventral cirri subulate on first 2–7 chaetigers. Branchiae usually present, from chaetiger 6, rarely before or after, single or pectinate with up to seven filaments. Pseudocompound falcigers on anterior parapodia unidentate to tridentate with short hoods. Large median hooks are present in transitional parapodia in some species. Pectinate chaetae oblique or transverse with up to 20 denticles. Bidentate hooded subacicular hooks from chaetigers 12–32, 2–3 per parapodium. Maxillae (Mx) V present; MxVI absent. Tubes are thin with inner mucous or parchment-like layer with outer layer of mud or sand grains.
Remarks
The above diagnosis has been modified to reflect that, although the majority of the nearly 40 species of Kinbergonuphis possess oblique pectinate chaetae, at least 10 have been described as transverse (straight) and the number of denticles ranges from 8 to 20.
Kinbergonuphis dorsalis (Ehlers, Reference Ehlers1897)
Figure 1, 2A–G; Table 1; S1
Diopatra dorsalis Ehlers, Reference Ehlers1897: 71–74, pl. 5: figs. 108–118.
Onuphis quadricuspis sensu Monro, Reference Monro1930: 131–132, fig. 49. Not M. Sars, Reference Sars1872.
Onuphis dorsalis. — Monro, Reference Monro1936: 151–152.—
Hartmann-Schröder, Reference Hartmann-Schröder1962: 114–117, figs. 115–119.—Averincev, Reference Averincev1972: 174, pl. 33, figs. 1–8 (in part).
Kinbergonuphis dorsalis.—Fauchald, Reference Fauchald1982a: 18–19, fig. 7a–h.—Orensanz, Reference Orensanz1990: 24–30, pl.3: a–h.
Type Locality
Strait of Magellan, Punta Arenas, Chile; intertidal.
Diagnosis
Ventral cirri on chaetigers 1–6. Branchiae from chaetiger 6, single filament; second filament usually present, third filament occasionally present. First five chaetigers with pseudocompound falcigers; unidentate on chaetiger 1, bidentate and/or tridentate on chaetigers 2–5. Two subacicular hooks from chaetigers 11 to 17 onward. Pectinate chaetae flat, slightly oblique, up to 18 denticles, 1–4 per parapodium.
Type Material
ZMH P-4806 (holotype); ZMH V-4808 (7 paratypes); ZMH V-4807 (1 paratype).
Additional Material Examined
as Onuphis quadricuspis: NHMUK 1930.10.8.1364–1365 (3); NHMUK 1930.10.8.1771–1772 (2); as Onuphis dorsalis: NHMUK 1936.2.8.2215–2217 (3); NHMUK 1936.2.8.2249–2250 (2); NHMUK 1936.2.8.2253–2255 (3); NHMUK 1936.2.8.2218–2227 (10); NHMUK 1936.2.8.2227–2232 (5); NHMUK 1936.2.8.2251–2252 (2); NHMUK 1936.2.8.2233–2236 (4); NHMUK 1936.2.8.2237–2247 (11); NHMUK 1936.2.8.2248 (1); as Kinbergonuphis dorsalis: ZMH P-14292 (2); ZMH P-18496 (4).
Description
Holotype complete with 163 chaetigers, L10 = 6.0 mm, W10 = 2.1 mm, TL = 69 mm. Four complete paratypes with 45–49 and 114 chaetigers, L10 = 1.35–4.3 mm, W10 = 0.65–1.5 mm, TL = 4.5–40 mm; four incomplete paratypes (juvenile) with 24–41 chaetigers. Colour of preserved specimens cream, no pigmentation present (present in original description); anterior body with iridescent cuticle, especially on palpo- and antennophores. Description based on holotype.
Prostomium with rounded anterior margin, very weakly incised; frontal and upper lips ovoid. Palps reaching chaetiger 1, lateral antennae reaching chaetiger 3, median antenna reaching chaetiger 2 (Figure 2A, B). Antennae and palps smooth with gradually tapering styles; palpo- and antennophores with three basal rings plus one long distal ring. Eyes not observed (present in original description). Peristomium half as long as first chaetiger. Peristomial cirri slender, slightly longer than peristomium, inserted distally on peristomium in line with lateral antennae.

Figure 2. Kinbergonuphis dorsalis: Holotype ZMH P-4806 (A) lateral view; (B) close-up lateral view; Paratype ZMH P-4807 (C) unidentate pseudocompound falciger, chaetiger 1; (D) tridentate pseudocompound falciger, chaetiger 3; (E) bidentate, pseudocompound falciger, chaetiger 4; (F) pectinate chaeta, chaetiger 90; (G) subacicular hook, chaetiger 91; dc, dorsal cirrus; gp, glandular pad; pol, postchaetal lobe; prl, prechaetal lobe; vc, ventral cirrus. Scale bars: A, 5 mm; B, 1 mm; C–E, 50 μm; F–G, 20 μm.
First three pairs of parapodia modified, enlarged, and directed anteriorly. Parapodia of chaetiger 4 slightly enlarged, directed anterolaterally. Parapodia of chaetigers 5–6 directed posteriorly, from parapodia of chaetiger 7 onwards with no modification (Figure 2A, B). Prechaetal lobes ovate on chaetigers 1–5, reduced considerably on chaetiger 6. Postchaetal lobes long, triangular (Figure 2A, B), reducing in size, becoming more rounded by chaetiger 13, reduced to a low mound thereafter. Dorsal cirri present throughout, subulate and large on chaetigers 1–10; from chaetiger 11 onwards, dorsal cirri unchanged but appearing substantially less robust. Ventral cirri subulate on first four chaetigers, slightly reduced on chaetiger 5, in transitory form on chaetiger 6, replaced by ventral glandular pads from chaetiger 7 (Figure 2A, B). Branchiae present on chaetigers 6–132; two branchial filaments between chaetigers 10 and 56, single filament on all other chaetigers (Figure 2A, B).
First five pairs of parapodia with pseudocompound falcigers: unidentate (Figure 2C) on chaetigers 1–3, bidentate (Figure 2E) on chaetiger 3, tridentate with elongate, apical tip (Figure 2D) on chaetiger 4, tridentate with blunt, apical tooth on chaetiger 5. Simple falcigers absent. Limbate chaetae present on all chaetigers. Presence of pectinate chaetae unclear due to breakage but definitely present from at least chaetiger 19 to end of body, only 1 per parapodium apparent, slightly oblique with up to 18 denticles (Figure 2F). Aciculae present, two per parapodium on chaetigers 1–8, three from chaetiger 9. Bidentate subacicular hooks (Figure 2G) present from chaetiger 15, two per parapodium.
Pygidium with anus terminal; two pairs pygidial cirri ventral to anus, dorsal pair approximately three times longer than ventral pair.
Variation
All paratypes have subulate ventral cirri on parapodia of chaetigers 1–4 with transitory form on chaetiger 5 and glandular pads from chaetiger 6. Large paratype with single branchial filaments except on chaetigers 38–39 where rudimentary second branchial filaments are present. All juveniles except one were abranchiate; specimens with branchiae (46 chaetigers long, complete) possessed a few, single filaments only.
Large paratype with pseudocompound falcigers present on first four pairs of parapodia: unidentate on chaetigers 1–3, bidentate on chaetigers 3–4, tridentate with elongate, apical tip on chaetiger 3–4. Juveniles with pseudocompound falcigers present on first three or four pairs of parapodia and one short-bladed, bidentate compound falciger on a variable number of parapodia between chaetigers 4 and 11. Subacicular hooks start on chaetiger 16 on large paratype and chaetigers 11 or 12 on juveniles.
The three Falkland Islands specimens from stn 51 (Monro, Reference Monro1930; Figure 1) are all posteriorly incomplete with 36–44 chaetigers, L10 = 4.7–7.5 mm, W10 = 1.5–1.7 mm, and TL = 20–28.5 mm. Eyes were not observed but present on all other specimens collected by Monro in 1930 and 1936 although sometimes hard to see. Some pigmentation is present on the largest specimen from stn 51 from chaetiger 4, forming transverse bands over the posterior half of each segment. Branchiae start from chaetiger 6 with a single filament followed by two filaments from chaetigers 7 or 10 and three from chaetiger 22, 24 or 28. Many anterior parapodia missing but unidentate pseudocompound falcigers are present on first four pairs of parapodia, bidentate and tridentate pseudocompound falcigers on parapodia of chaetigers 3–5. Bidentate subacicular hooks from chaetigers 15 or 16, two per parapodium. The only whole specimen collected by Monro was a small animal from station WS212 (Monro, Reference Monro1936; Figure 1). This specimen consisted of 110 chaetigers, with maximally two branchial filaments (on chaetigers 19–29) and subacicular hooks from chaetiger 14. Pseudocompound falcigers were present on chaetigers 1–4 only.
Hartmann-Schröder's 1962 specimens (ZMH P-14292) consisted of one anterior fragment, four median fragments and one posterior fragment (one median fragment matches up with the anterior to form the specimen of 110 chaetigers described by Hartmann-Schröder) along with one complete juvenile (16.5 mm long, 67 chaetigers). In contrast with the original description, all specimens were uniformly dark brown with no pigmentation pattern and a glossy, iridiscent cuticle in the anterior part of the body. Branchiae start from chaetiger 6, as single filaments to chaetiger 9, two filaments from chaetiger 10, and three from chaetiger 19; postchaetal lobes are clear to chaetiger 15. Unidentate pseudocompound falcigers were present on first five pairs of parapodia, tridentate pseudocompound falcigers present on chaetiger 4. The juvenile specimen has branchiae from chaetiger 6 to 40, single filaments only. Unidentate pseudocompound falcigers present on first three pairs of parapodia, bidentate pseudocompound falcigers on chaetigers 2 and 4 and tridentate pseudocompound falcigers on chaetigers 3–4. Two bidentate subacicular hooks from chaetiger 12. Pectinate chaetae are slightly oblique, 1–2 per parapodium, with up to 15 denticles. No eyes were observed although Hartmann–Schröder reported eyespots on the juvenile specimen.
Four specimens from the HERO cruise (stn783 A-B, Figure 1; ZMH P-18496), two adults and two juveniles, were also examined. The two adults are 61.3 mm long with 147 chaetigers (complete) and 31.7 mm long with 67 chaetigers (posteriorly incomplete). The complete specimen, which appeared to be at an earlier ontogenetic stage, had branchiae with a single filament on chaetigers 6–7 and two filaments from chaetiger 8. The incomplete specimen has two branchial filaments on chaetiger 6 and three filaments from chaetiger 7. Pseudocompound falcigers, unidentate or bidentate, present on first four pairs of parapodia, no tridentate pseudocompound falcigers present although several falcigers were broken. Subacicular hooks from chaetiger 15 or 17 and postchaetal lobes no longer prominent after chaetigers 14 or 17. The juvenile specimens are 5.7 mm long with 40 chaetigers and 6.2 mm long with 35 chaetigers, both complete. Branchiae absent on both juveniles. Pseudocompound falcigers present on first three or four pairs of parapodia (unidentate on chaetiger 1, bidentate on chaetiger 1–3 or 4) followed by 2–3 short-bladed, compound bidentate falcigers from chaetiger 4 to 12 with subacicular hooks from chaetiger 12. Pectinate chaetae slightly oblique, up to 4 with 10–15 denticles present from at least chaetiger 3.
Remarks
Kinbergonuphis dorsalis is distinguished from all but one other Kinbergonuphis species through the combination of having subulate ventral cirri to chaetiger 5 (transitionary form chaetiger 6), branchiae from chaetiger 6, and pseudocompound falcigers unidentate, bidentate, and tridentate. Only Kinbergonuphis orensanzi (Fauchald, Reference Fauchald1982b) shares this combination of characters, but that species has large hooks present from chaetigers 3 to 6 that are absent in K. dorsalis. Around the Falkland Islands, both K. dorsalis and Kinbergonuphis oligobranchiata (Orensanz, Reference Orensanz1974a) have been recorded (Figure 1). Kinbergonuphis dorsalis is recorded from just north of Falkland Sound (115 m: Monro, Reference Monro1930) as well as to the south, west, and further north of the Islands (127–930 m: Monro, Reference Monro1936; Averincev, Reference Averincev1972), and K. oligobranchiata from the east, south, and far north of the Island zone (512–1517 m: Averincev, Reference Averincev1972; Orensanz, Reference Orensanz1990; Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020). Kinbergonuphis dorsalis is easily distinguished through the ventral cirri (cirriform on chaetigers 1–4 only in K. oligobranchiata but present on chaetigers 1–5 in K. dorsalis) and the pseudocompound falcigers, which are only tridentate in K. oligobranchiata but uni-, bi-, and tridentate in K. dorsalis.
Since the original description of K. dorsalis, several other authors have provided detailed descriptions of the species from nearby regions. Augener (Reference Augener1931) reviewed and published details of Ehlers' type material as part of a description of his own specimens from Antarctica (published as Onuphis dorsalis but now dismissible as that species due to branchiae starting on chaetigers 11 or 12). Monro (Reference Monro1930, Reference Monro1936) provided descriptions of specimens that he identified first as Onuphis quadricuspis and then later Onuphis dorsalis from the Falkland Islands region, as well as others from off the Argentinean coast (Figure 1). Hartmann-Schröder (Reference Hartmann-Schröder1962) collected fresh material from further north in Argentina (Figure 1), but all collected specimens were incomplete and, abnormally, are a solid, dark brown-black colour (preserved material described as ‘reddish-brown’ in the original description) across the whole body with strong iridescence. Her description, but not specimens, was later reviewed by Orensanz (Reference Orensanz1974a) along with a description of additional specimens from Argentina. Averincev (Reference Averincev1972) reported several records of O. dorsalis from samples taken around the region as part of the Soviet Antarctic expeditions. Fauchald (Reference Fauchald1982a), then transferred the species to Kinbergonuphis as a new combination but only reviewed the holotype in the work. Orensanz (Reference Orensanz1990) then reviewed the species and all records in a more comprehensive study, along with observations on additional specimens from the region, although he did not directly observe the type specimens or those collected from the Magellan region by Monro (Reference Monro1930, Reference Monro1936), Hartmann-Schröder (Reference Hartmann-Schröder1962) or Averincev (Reference Averincev1972).
In Ehlers' (Reference Ehlers1897) original description, K. dorsalis was described as having brown pigmentation on anterior chaetigers and eyes present. Our observations of the holotype found all pigmentation to be absent, which is assumed to have faded as an artefact of the extended preservation period. The absence of eyes, which are generally very small in those species of Kinbergonuphis that have them, is possibly due to the same reason. Ehlers' description of the eyespots was that they were positioned close to the base of the ‘middle antennae’ although it is not clear whether he was talking about the median antenna or the lateral antennae which he also referred to as ‘middle’. Later descriptions by Hartmann-Schröder (Reference Hartmann-Schröder1962), Averincev (Reference Averincev1972) and Orensanz (Reference Orensanz1974a), on fresh non-type material described eyes as being absent. Hartmann-Schröder's description (1962) did detail small eyespots on her juvenile specimen ‘between the paired antennae’ although we could not see these, either because they had faded over time or due to the very dark pigmentation present on the specimens. Eyespots were present, although very faded in some cases, on most of Monro's 1936 publication specimens, but not on those from 1930. Fauchald's (Reference Fauchald1982a) review of the K. dorsalis holotype did not mention whether eyes were present or absent.
There are also discrepancies between the different descriptions of the chaetal complement of the species by different authors. Ehlers (Reference Ehlers1897) describes both unidentate and bidentate pseudocompound falcigers as present on chaetigers 1–5 although he doesn't specify the arrangement specific to chaetiger number. Tridentate pseudocompound falcigers are not described or figured as present. Our observations confirm all three types of pseudocompound falciger to be present on chaetigers 1–5 of the holotype and chaetigers 1–4 of the paratypes. Hartmann-Schröder (Reference Hartmann-Schröder1962) described her specimens with 1–3 teeth present on the pseudocompound falcigers of the first five chaetigers and, again, our observations confirmed there to be uni-, bi-, and tridentate pseudocompound falcigers present in both of her specimens. Averincev (Reference Averincev1972) reported pseudocompound falcigers on chaetigers 1–4 comprising unidentate (chaetigers 1–2), bidentate (chaetiger 3), and tridentate (chaetiger 4) forms while Orensanz (Reference Orensanz1974a) found unidentate pseudocompound falcigers present on chaetigers 1–5 and both bi- and tridentate pseudocompound falcigers present from chaetigers 2 to 5. Fauchald (Reference Fauchald1982a) on the other hand, stated that all falcigers in the studied holotype were unidentate except on chaetiger 5 where they were tridentate only, however this is shown here to be incorrect.
Another discrepancy in the descriptions concerns the number and the appearance of subacicular hooks. Ehlers (Reference Ehlers1897) description suggests that 5–6 subacicular hooks start ‘with the appearance of the gills’, decreasing in number to one or two posteriorly. Our observations, however, found only two hooks from chaetiger 15 to the end of the body (slightly earlier on smaller paratypes) with no other type of hook occurring between the end of the pseudocompound falcigers and the start of the subacicular hooks. On Monro's specimens they originate on chaetigers 14–16 and on chaetiger 12 or 17 on Hartmann-Schröder's specimens. Other literature reports the origin variably as chaetiger 20 (Averincev, Reference Averincev1972), chaetiger 12–16 (Orensanz, Reference Orensanz1974a), and chaetiger 14 (Fauchald, Reference Fauchald1982a). As with many other species, the start of the subacicular hooks has been demonstrated to show ontogenetic variation (Orensanz, Reference Orensanz1990) and so must be treated with caution when comparing specimens and descriptions.
Similarly, pectinate chaetae were also variably described as either starting on chaetiger 16 (Hartmann-Schröder, Reference Hartmann-Schröder1962), chaetiger 5 (Averincev, Reference Averincev1972), or chaetiger 3 (Orensanz, Reference Orensanz1974a) or the start was not mentioned (Ehlers, Reference Ehlers1897; Fauchald, Reference Fauchald1982a) and with either 13 denticles (Hartmann-Schröder, Reference Hartmann-Schröder1962), 14–17 denticles (Averincev, Reference Averincev1972), or 18 denticles (Fauchald, Reference Fauchald1982a). On the juvenile specimen of Hartmann-Schröder, the first pectinate chaeta is actually present on chaetiger 5. Ehlers (Reference Ehlers1897) illustrated the pectinate chaetae as transverse and this was reiterated by Fauchald (Reference Fauchald1982a), however, Hartmann-Schröder (Reference Hartmann-Schröder1962) illustrated her specimens as having oblique pectinate chaetae as did Orensanz (Reference Orensanz1974a, Reference Orensanz1990). Examination of the type material confirmed the pectinate chaetae to be slightly oblique. The low angle of the denticles means that at some angles the chaetae can appear transverse so observation of the chaetae on several parapodia is recommended and could explain the discrepancy in the descriptions. Pectinate chaetae are very fragile and easily lost which makes determining the first chaetiger of their appearance difficult. However, an accurate description of whether pectinate chaetae are oblique or transverse, and the number of their denticles can aid in species description.
Orensanz (Reference Orensanz1990) described K. dorsalis as having branchiae with up to 3 (usually 2) filaments, unidentate pseudocompound falcigers on chaetigers 1–4, pseudocompound falcigers with 1–3 teeth on chaetigers 3–4, tridentate or bidentate pseudocompound falcigers on chaetiger 5, subacicular hooks starting from chaetigers 14–16, and palpo- and antennophores with 3 basal rings. Juveniles (25 chaetigers) were found to possess short-bladed falcigers on chaetigers 1–12 that then transitioned to subacicular hooks. The latter character was confirmed in observations on two juveniles from the HERO cruise (783A-B) in the ZMH collection (P-18496), which were absent in larger specimens from the same sample, as well as Ehlers' juvenile paratypes. Hartmann-Schröder's juvenile specimen, with 67 chaetigers, did not possess such falcigers.
Although Fauchald (Reference Fauchald1982a) found the descriptive differences to be minor, the wide-ranging distribution and depths reported, combined with the differences in descriptions such as the dark pigmentation of Hartmann-Schröder's specimens and the different distribution of pseudocompound falcigers (no pseudocompound falcigers in chaetiger 5 and bidentate falcigers present in chaetiger 1) and more numerous pectinate chaetae in the HERO specimens, may point to a complex of species being involved that needs further investigation. Of the other K. dorsalis specimens detailed, those from the HERO cruise, from just north of Rio Gallegos, were collected closest to the original type locality at Punta Arenas. However, they also differ from the type specimens in several characters. A more comprehensive review of the species using a greater number of animals from closer to the type locality, in combination with molecular data, is desirable to help describe the degree of morphological variation present and the status of the species.
Distribution
Falkland Islands (Figure 1): north of Falkland Sound in 115 m (Monro, Reference Monro1930) as well as to the south, west, and north of the wider region in 127–915 m (Monro, Reference Monro1936; Averincev, Reference Averincev1972). Wider distribution: from intertidal habitats to 930 m depth (Orensanz, Reference Orensanz1990), mainly in the Magellanic region of southern South America and off Argentina to as far north as the La Plata river. Wesenberg-Lund (Reference Wesenberg-Lund1962) also reported one record of the species from the Pacific coast of Chile in Golfo Corcovado in 8 m.
Kinbergonuphis sp.
Figures 1, 3A–D, 4A–I; Table 1, 2; S1
Kinbergonuphis sp. Darbyshire, Reference Darbyshire2018: 38.

Figure 3. Kinbergonuphis sp.: NMW.Z.2011.039.0232 (A) dorsal view, live specimen; (B) ventral view, live specimen; (C) subtidal population, Kidney Island, fine–medium sand 4.6 m; (D) intertidal population, South Harbour, fine sand. Scale bar: A, B 1 mm.
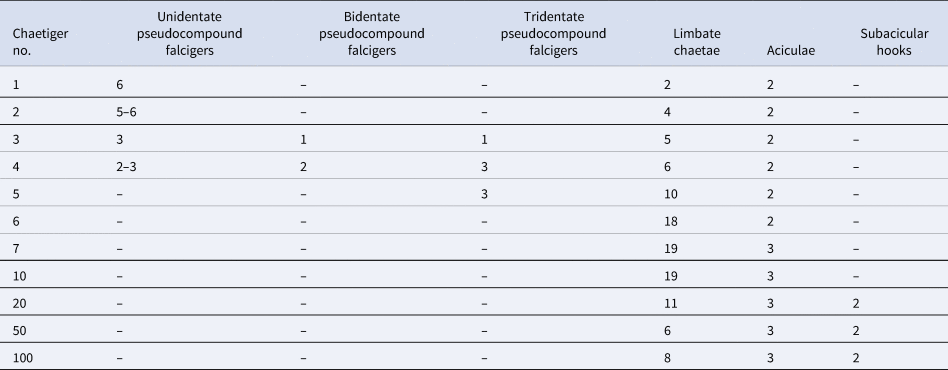
Table 2. Chaetal count and complement for Kinbergonuphis sp. (NMW.Z.2012.082.0207) for anterior chaetigers and selected chaetigers after
Diagnosis
Eyes present. Ventral cirri on chaetigers 1–6. Branchiae from chaetiger 6, single filament, second filament occasional, third filament rare. First five chaetigers with pseudocompound falcigers: unidentate on chaetiger 1–2, bidentate on chaetiger 3–4, tridentate on chaetigers 3–5. Two bidentate subacicular hooks from chaetigers 11–17 onward. Pectinate chaetae flat, slightly oblique, up to 18 denticles, 1 per parapodium.
Material Examined
East Falkland: NMW.Z.2011.039.0215–0217 (3); NMW.Z.2011.039.0230–0232 (4); NMW.Z.2011.039.0227–229 (106); NMW.Z.2011.039.0224–0226 (98); NMW.Z.2011.039.0218–0219 (9); NMW.Z.2011.039.0220 (1); NMW.Z.2011.039.0221,0223 (2); NMW.Z.2011.039.0222 (1); NMW.Z.2012.082.0218 (1); NMW.Z.2012.082.0150 (3); NMW.Z.2012.082.0151, 0152, 0160 (13); NMW.Z.2012.082.0153 (1); NMW.Z.2012.082.0154 (1); NMW.Z.2012.082.0155–0157, 0163 (10); NMW.Z.2012.082.0158–9, 0161–2 (8). West Falkland: NMW.Z.2012.082.0165–0166 (3); NMW.Z.2012.082.0167 (3); NMW.Z.2012.082.0164 (3); NMW.Z.2012.082.0170–201, 205 (126); NMW.Z.2012.082.0168–169 (3); NMW.Z.2012.082.0202-203, 0206 (16); NMW.Z.2012.082.0208 (3); NMW.Z.2012.082. 0204, 0209, 0210 (14); NMW.Z.2012.082.0211 (2); NMW.Z.2012.082.0212 (2); NMW.Z.2012.082.0215 (2); NMW.Z.2012.082.0214 (4); NMW.Z.2012.082.0213 (4); NMW.Z.2012.082.0207 (9); NMW.Z.2012.082.0216 (1); NMW.Z.2012.082.0217 (1); NMW.Z.2015.002.0006 (2); NMW.Z. 2015.002.0007–0008 (9).
Description
Description based on ‘best’ (complete, well-preserved, representing all characters clearly; NMW.Z.2012.082.0170) specimen of 176 chaetigers (L10 = 7.5 mm, W10 = 2.5 mm, TL = 101 mm long) with additional images from selected specimens that best demonstrate particular characters (NMW.Z.2012.082.0158, 0163, 0186, 0204). Variation shown by remaining specimens and juveniles detailed in later section.
Live animals cream with pale orange-brown pigmentation, preserved specimens cream with reddish-brown pigmentation (Figure 3A, B). Prostomium pale with darker oval patch on posterior boundary of peristomium. Palpo- and antennophores slightly pigmented as well as bases of styles; palps with no pigmentation. Peristomium with light pigmentation across entirety. Chaetigers 1–12 with very light, dorsal horizontal bands in centre of each segment; parapodia with pigmented area on anterodorsal margin (chaetigers 1–10), bases of dorsal cirri (chaetigers 1–5), and bases of branchial filaments (chaetigers 6–13). Pigmentation reducing in intensity on posterior body, absent from chaetiger 18 onwards. Cuticle iridescent, particularly on palpo- and antennophores and lateral margins of anterior segments.
Prostomium with rounded anterior margin, weakly incised; frontal and upper lips ovoid. Palps reaching chaetiger 1, lateral antennae reaching chaetiger 4, median antenna reaching chaetiger 3 (Figure 3A). Antennae and palps smooth with gradually tapering styles; palpo- and antennophores with three basal rings plus one long distal ring. One pair of small, black eyes on outer posterolateral side of bases of lateral antennae. Peristomium ⅔ as long as first chaetiger. Peristomial cirri slender, slightly longer than peristomium, inserted distally on peristomium in line with lateral antennae.
First three pairs of parapodia modified, enlarged, directed anteriorly. Parapodia of chaetiger 4 slightly enlarged, directed anterolaterally (Figure 3A, 4A). Parapodia of chaetigers 6–8 directed posteriorly; from parapodia of chaetiger 9 onwards with no modification. Prechaetal lobes broadly ovate on chaetiger 1, ovate on chaetigers 2–5, reduced from chaetiger 6 onward not extending beyond prechaetal fold. Postchaetal lobes long, triangular (Figure 3A), reducing in size from chaetiger 6–12, thereafter as a low mound. Ventral cirri subulate on first four chaetigers, slightly reduced on chaetiger 5, in transitory form chaetiger 6 (Figure 3B, 4A), replaced by ventral glandular pads from chaetiger 7. Single branchial filament on chaetigers 6–135 (Figure 3A, 4A).

Figure 4. Kinbergonuphis sp.: NMW.Z.2012.082.0158 (A) lateral view; NMW.Z.2012.082.0163 (B) unidentate and bidentate pseudocompound falcigers, chaetiger 3; (C) tridentate pseudocompound falciger, chaetiger 3; NMW.Z.2012.082.0158 (D) pectinate chaeta, chaetiger 51; (E) subacicular hooks, chaetiger 14; NMW.Z.2012.082.0186 (F) maxillae; (G) mandibles; NMW.Z.2012.082.0204. (H) embryos attached to inside of tube; (I) multicell stage embryo. Scale bars: A, I, 1 mm; B, E, 20 μm; C–D, 10 μm; F–G, 0.5 mm; H, 5 mm.
First five pairs of parapodia with pseudocompound falcigers: unidentate (Figure 4B) on chaetigers 1–4, bidentate (Figure 3B) on chaetigers 3–4, tridentate with elongate, apical tooth on chaetigers 3–4 (Figure 4C), tridentate falcigers with blunt, apical tooth on chaetiger 5. Simple falcigers and large median hooks absent. Limbate chaetae (Figure 4B) present on all chaetigers, increasing in number to first branchial chaetigers then decreasing posteriorly. Pectinate chaetae (Figure 4D) present from chaetiger 4 or 5 to end of body, up to 4 per parapodium; slightly oblique with up to 16 denticles. Aciculae slender and curved, usually three per parapodia except on parapodia of first six chaetigers which have two. Bidentate subacicular hooks (Figure 4E) present from chaetiger 15, two per parapodium. Chaetal count and distribution provided in Table 2.
Maxillary apparatus (Figure 4F) and mandibles (Figure 4G) pale. Maxillary formula = 1 + 1, 6 + 7, 7 + 0, 9 + 11, 1 + 1. Maxillary carriers more than half as long as maxilla I (Figure 4F).
Pygidium with anus terminal. Two pairs pygidial cirri ventral to anus, dorsal pair ~5 times longer than ventral pair.
Embryos found attached to inner wall of the tube of some specimens (Figure 4H); dark yellow in colour, 1–1.25 mm diameter, most at 4-cell stage of division or slightly later (Figure 4I).
GenBank accession numbers (16S): ON787615, OQ592145, OQ592146
Variation
Complete specimens with 37–190 chaetigers, L10 = 2.6–7.3 mm, W10 = 0.8–2.5 mm, TL = 18–116 mm. The start of the subacicular hooks varied with body size and ranged from chaetigers 11 to 17. Subulate ventral cirri were present on the first four pairs of parapodia only in smaller animals, with an intermediate/developing cirrus present on parapodia of chaetiger 5 and ventral glandular pads from chaetiger 6. The ‘developing’ ventral cirrus was still subulate unlike the transitory, rounded cirrus present on parapodia of chaetiger 6 of larger specimens. A clearly subulate ventral cirrus on parapodia of chaetiger 5 developed in animals at around 80–100 chaetigers.
Juvenile specimens (approximately 60 chaetigers or less) occasionally presented bidentate pseudocompound falcigers on chaetiger 2 (most specimens possessed only unidentate pseudocompound falcigers on the first two chaetigers), the median antenna could be longer than the lateral antennae (in larger animals the lateral antennae were always longer than the median), and an additional pair of eyespots were present on the anterior edge of the prostomium between the frontal palps and the lateral antennae. Of 13 juveniles (less than 60 chaetigers) examined, six possessed a single short-bladed, compound bidentate falciger on some or all of the parapodia of chaetigers 5–10 with subacicular hooks present on parapodia from chaetiger 11; four out of those six were abranchiate, all other juveniles showed developing branchiae to some extent.
Out of 468 specimens examined, both complete and incomplete, 61 specimens possessed two branchial filaments on at least one chaetiger. The second branchial filament first occurred from chaetiger 8 to 44, with over 50% of animals first developing one between chaetigers 21 and 29. The smallest animal examined (of 7 complete specimens), on which two branchial filaments were found, was 45 mm long with 108 chaetigers, with the second filament starting on chaetiger 24; the largest was 116 mm long with 193 chaetigers, with the second filament first occurring on chaetiger 30. The earliest occurrence of the second filament, in a complete specimen, occurred on chaetiger 13 out of 178 (59 mm body length). There was no apparent relationship between length or number of chaetigers and where the second filament first occurred. After the additional filament first develops, presence is irregular and it may only occur on that single parapodium. The additional filament is frequently absent from subsequent individual parapodia or one or more segments but may, equally, occur consistently for a variable number of subsequent chaetigers. A third branchial filament was only identified in two separate parapodia (chaetigers 19 and 38) of a single posteriorly incomplete specimen (34 mm long with 46 chaetigers; NMW.Z.2015.002.0007).
Eyes were present on most specimens, varying from clear to very faint. Level of pigmentation was highly variable with a few animals showing very dark pigmentation similar to that of Hartmann-Schröder's (Reference Hartmann-Schröder1962) specimens of K. dorsalis (see above) with unclear pattern discernable through the pigment. Other specimens exhibited little or no pigmentation at all while the majority were of an intermediate level.
Remarks
Kinbergonuphis sp. demonstrates minor differences from K. dorsalis described above. All larger specimens examined of K. dorsalis possessed at least two branchial filaments, most had three filaments over a number of segments. The majority of the specimens reported or examined of K. dorsalis were incomplete, however, of those few available that were complete, the smallest was of 110 chaetigers and already had ten pairs of branchiae with two filaments (Monro, Reference Monro1936, NHMUK1936.2.8.2216). Those specimens examined, from Ehlers, Monro, Hartmann-Schröder, and the HERO cruise, demonstrated a consistent presence of the second branchial filament once developed, without skipping parapodia or segments before reverting back to a single filament. In Kinbergonuphis sp., however, the presence of an additional branchial filament is uncommon, present in only 61 of the 468 studied specimens, there seems to be no direct relationship to specimen size and second branchial filaments are first present in more posterior chaetigers than in K. dorsalis. A third branchial filament was only noted once – in two separate parapodia of one animal. Grimes et al. (Reference Grimes, Paiva, Petersen and Schulze2020) in studies on Hermodice carunculata (Pallas, Reference Pallas1766), found that animals would increase the number of branchial filaments in response to increased hypoxic conditions. When considered as a potential explanation for the discrepancy in the numbers of branchial filaments observed here between Kinbergonuphis sp. from the Falkland Islands and specimens of K. dorsalis examined from previous studies, this is not thought to be a significant factor. The specimens of Kinbergonuphis sp were collected from a range of sites around the Falkland Islands coastline that demonstrated differing sediment types including both anoxic sediments and cleaner sands with no apparent correlation between any habitat and the presence of additional branchial filaments. The type specimens of Ehlers were from intertidal sites as were those of Hartmann-Schröder (Reference Hartmann-Schröder1962), while those from the HERO cruise were from only 30 m depth, all comparable to the habitats sampled here. Monro's specimens (Reference Monro1930, Reference Monro1936) were from deeper waters (110 m) but showed no significant difference in branchial development to the intertidal specimens.
Additionally, Kinbergonuphis sp. specimens are longer than those of K. dorsalis. For equivalent sizes, Kinbergonuphis sp. specimens are longer than those few whole specimens of K. dorsalis that have been available to observe. The holotype of K. dorsalis is only 69 mm long with 163 chaetigers whereas specimens of Kinbergonuphis sp. are 80–100 mm in length for a similar number of chaetigers. Similarly, the specimens of K. dorsalis collected closest to the Falkland Islands, those of Monro (Reference Monro1930) from just north of Falkland Sound in 115 m depth, are posteriorly incomplete with three branchial filaments but show a smaller body size than specimens of Kinbergonuphis sp. that have the same relative number of chaetigers. Body size can, however, be affected by factors relating to preservation, including relaxation before fixing, and may not be a reliable character for comparison.
Amplification of the COI and 16S mitochondrial genes was attempted, however only 16S was successful. The three sequences retrieved were identical, representing a single haplotype. The only species of Kinbergonuphis with sequences currently available is Kinbergonuphis pulchra (Fauchald, Reference Fauchald1980), which does not occur in the region and is distinguishable from both K. dorsalis and Kinbergonuphis sp. through multiple characters. No molecular analyses are provided here as no meaningful comparisons could be made due to the lack of sequences from congeners. The sequences have been submitted to GenBank, and the accession numbers are provided here for future use.
As mentioned previously, a review of K. dorsalis, from the actual type locality region, along with molecular data, would help clarify the morphological variability truly present and whether some of these specimens from further afield, that demonstrate inconsistencies with the types, are worthy of greater note. Until that time, the specimens reported here from the Falkland Islands are identified to genus only to highlight their differences from K. dorsalis.
Habitat
Intertidal and shallow water (less than 10 m) in fine to medium sandy sediments; often found in dense colonies both widely dispersed on the seabed (Figure 3C) or more discretely intertidally (Figure 3D).
Distribution
Recorded around the Falkland Islands archipelago in shallow and intertidal waters (0–10 m) (Figure 1).
Kinbergonuphis oligobranchiata (Orensanz, Reference Orensanz1974a)
Figure 1, 5A–H; Table 1; S1
Onuphis oligobranchiata Orensanz, Reference Orensanz1974a: 93–94, pl.6.
Kinbergonuphis oligobranchiata . – Fauchald, Reference Fauchald1982a: 26–27, fig. 6c, table 6. – Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020: 66.
Anchinothria cf. pycnobranchiata. – Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020: 66 (in part).
Type Locality
Western Atlantic, off Argentina, Buenos Aires Province; −38.76667, −54.88333; 900 m.
Diagnosis
Eyes absent. Ventral cirri on chaetigers 1–4. Branchiae from chaetiger 6, single filament; second filament rare. First four chaetigers with pseudocompound falcigers: bidentate on chaetigers 1–4, tridentate on chaetigers 3–4. Two bidentate subacicular hooks from chaetigers 11–16. Pectinate chaetae flat, oblique, up to 15 denticles, 1–2 per parapodium.
Material Examined
as Kinbergounuphis oligobranchiata: USNM 97947 (1); USNM 97948 (5); NHMUK 2018.23537 (1); as Anchinothria cf. pycnobranchiata: NHMUK 2018.23591 (1).
Description
Description based on USNM specimens, details of Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) specimens provided in Variation section.
Specimens all posteriorly incomplete with 13–33 chaetigers, L10 = 3–4.1 mm, W10 = 0.3–1.1 mm, TL = 3.8–9.8 mm. Body colour pale cream in alcohol, no pigmentation apparent. Prostomium with rounded anterior margin, weakly incised; frontal and upper lips ovoid. Palps reaching chaetiger 2, lateral antennae reaching chaetiger 6, median antennae reaching chaetiger 8 (Figure 5B–D). Antennae and palps with smooth, gradually tapering styles; palpo- and antennophores with two basal rings and one long distal ring. Eyes absent. Peristomium ⅔ as long as first chaetiger. Peristomial cirri slender, slightly longer than peristomium, inserted distally on peristomium in line with lateral antennae (Figure 5B).

Figure 5. Kinbergonuphis oligobranchiata: USNM 97948 (A) tube with specimen fragment; (B) dorsal view; (C) ventral view; (D) lateral view; (E) bidentate falciger, chaetiger 2; NHMUK 2018.23537 (F) tridentate falcigers, chaetiger 3; USNM 97948 (G) pectinate chaeta, chaetiger 24; (H) subacicular hook, chaetiger 24. Scale bars: A, 5 mm; B–D, 1 mm; E–F, 50 μm; G–H, 20 μm.
Parapodia of chaetiger 1 modified, enlarged and directed anteriorly (Figure 5B–D). Parapodia of chaetiger 2–5 directed anterolaterally. Postchaetal lobes long, subulate, reducing in size from chaetiger 6–12, equal to or shorter than acicular lobes from chaetiger 13 onward (Figure 5B–D). Dorsal cirri long, subulate with slight ventral expansion at base; shorter than postchaetal lobes on chaetigers 1–5, longer thereafter. Ventral cirri subulate on first four chaetigers, replaced by glandular pad from chaetiger 5 (Figure 5B). Single branchial filament from chaetiger 6 (Figure B, D), second branchial filament on one specimen from chaetiger 13.
First two pairs of parapodia with bidentate and tridentate pseudocompound falcigers, with hoods (Figure 5E–F); tridentate pseudocompound falcigers on chaetigers 3–4. Smallest specimen (USNM 97947) with pseudocompound falcigers on first two pairs of parapodia only. Unidentate falcigers absent. Pectinate chaetae oblique, 1–2 per parapodium, from chaetiger 5, with up to 15 denticles (Figure 5G). Bidentate subacicular hooks (Figure 5H) from chaetiger 11 or 14 onward.
Maxillary apparatus not seen. Tube soft, membranous, translucent (Figure 5A).
Variation
Although Kinbergonuphis oligobranchiata was reported from two of the Falkland Islands offshore exploration stations (Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020), only one of those specimens was available for investigation which was in poor condition and showed evidence of previous dehydration. Some observations could be made however and variations from that detailed above are as follows: specimen posteriorly incomplete with 56 chaetigers, L10 = 4.53 mm, W10 = 0.8 mm, TL = 20.8 mm. No second branchial filament was observed. First four pairs of parapodia with bidentate (possibly subtridentate, observations difficult due to preservation) and tridentate pseudocompound falcigers, with hoods, in contrast to tridentate pseudocompound falcigers only on chaetigers 3–4 of the USNM specimens. Bidentate subacicular hooks present from chaetiger 15 onward, slightly later than observed on the USNM specimens.
Another specimen, previously identified as Anchinothria cf pycnobranchiata, was also found to be K. oligobranchiata. Morphology was consistent with the other specimens detailed above with the following additions: posteriorly incomplete with 48 chaetigers, L10 = 3.9 mm, TL = 14.5 mm, W10 = 0.7 mm; stained pink, no pigmentation or eyes observed. Lateral and median antennae reaching chaetiger 6. Branchiae present on chaetigers 9–34, single filament. First four pairs of parapodia with pseudocompound falcigers, up to 4 per parapodium, all tridentate except for one bidentate pseudocompound falciger on chaetiger 2. Pectinate chaetae mostly broken, flat, oblique with up to 15 denticles. Two bidentate subacicular hooks present from chaetiger 16 to end of body.
Remarks
Kinbergonuphis oligobranchiata had not been described when Hartman published her 1967 volume, but there is no listing of onuphid specimens from stn 339 in the publication and only a mention of 7 unidentified onuphids from stn 557. Orensanz (Reference Orensanz1990), however, examined the specimens, identified them as K. oligobranchiata and included them in his review and on the distribution maps. He did not, however, reference them in the distribution he provided for the species which was given only as ‘off Argentina’.
The description of specimens presented by Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) on the Marine Flora and Fauna of the Falkland Islands website (https://falklands.myspecies.info) is simple and lacks details, thus the present description attempts to remedy that given the condition of the available specimen. Contrary to the description provided, the specimen investigated was found to only possess single branchial filaments from chaetiger 6 (none bifurcate) although the other characters observed matched the limited detail provided. However, as there were clearly more specimens collected originally it is assumed that this description probably refers to those unavailable specimens. The studied specimen agrees in morphology with the specimens listed by Hartman (Reference Hartman1967). The specimen mis-identified as Anchinothria cf. pycnobranchiata showed characters consistent with the other specimens examined and all specimens were collected from the same region to the east of the Falkland Islands.
The original description of the species (Orensanz, Reference Orensanz1974a) was limited and appeared to be based on poorly preserved juvenile specimens (Fauchald, Reference Fauchald1982a), however, Orensanz (Reference Orensanz1990) provided more detail and demonstrated the characters subject to ontogenetic variation to be based on adult specimens. The specimens observed here all fall within the variation range of those characters detailed in the updated description. Pectinate chaetae are described by Orensanz (Reference Orensanz1974a, Reference Orensanz1990) as oblique although Fauchald (Reference Fauchald1982a) described them as transverse, apparently from the same specimens. Pectinate chaetae on the specimens examined from the Falkland Islands were clearly oblique.
Kinbergonuphis oligobranchiata can be distinguished from K. dorsalis and Kinbergonuphis sp. by having ventral cirri on the first four chaetigers, instead of five, and by having bidentate and tridentate falcigers on the first four chaetigers, as opposed to uni-, bi-, and tridentate falcigers on the first four or five chaetigers.
Distribution
Falkland Islands (Figure 1): East of the Falkland Islands, East Falklands Basin and south of Beauchêne Island in 512–1517 m (Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020). Wider distribution: off Argentina 142–900 m (Orensanz, Reference Orensanz1990).
Genus Onuphis
Type species Onuphis eremita Audouin and Milne Edwards, Reference Audouin and Milne Edwards1833
Diagnosis (from Budaeva 2021)
Small- to medium-sized worms up to 30 cm long with about 200 chaetigers. Prostomium often anteriorly extended; with ovoid or oval frontal lips. Antennae and palps with 10–25 rings and short to moderately long styles reaching chaetiger 5–25. Median antenna shorter than lateral antennae. Palpostyles shorter than palpophores. Nuchal organs straight with narrow to wide middorsal separation. Peristomial cirri present. Anterior 2–5 pairs of parapodia modified but not enlarged. Ventral cirri subulate on anterior 4–6 chaetigers. Branchiae rarely absent, usually present from chaetiger 1, rarely from chaetiger 3–6; single or pectinate with up to 12 filaments. Pseudocompound falcigers on modified parapodia usually tridentate (rarely only bidentate, sometimes bi- to multidentate) with short, pointed hoods. Pectinate chaetae flat. Paired bidentate hooded subacicular hooks from chaetiger 10–12. Maxillae V present; MxVI absent. Tubes cylindrical, with thin mucous or tough parchment-like inner layer covered with sediment particles.
Onuphis pseudoiridescens Averincev (Reference Averincev1972)
Figure 1, 6A–I; Table 1; S1
Onuphis (Nothria) pseudoiridescens Averincev, Reference Averincev1972: 176, pl.32, figs 1–9.
Onuphis iridescens. — Monro, Reference Monro1936: 150–151.
Nothria ?iridescens. — Hartman, Reference Hartman1967: 91.
?Paronuphis antarctica. — Hartman, Reference Hartman1967: 96–97 (in part).
Onuphis heterodentata Fauchald, Reference Fauchald1982c: 241–243, fig.2, table 2.
Onuphis lithobiformis Fauchald, Reference Fauchald1982c: 243–245, fig.3.
Onuphis pseudoiridescens. — Orensanz, Reference Orensanz1990: 20–23, pl. 1a–i, fig. 8. — Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020: 66.
Type Locality
Western Atlantic: off Argentina, Uruguay & Falkland Islands; 202–659 m
Diagnosis
Palpostyles shorter than palpophores. Eyes absent. Ventral cirri on chaetigers 1–5. Branchiae from chaetiger 1, single filament. Tridentate pseudocompound falcigers present on chaetigers 1–4 with short, pointed hoods. Two bidentate subacicular hooks from chaetigers 11–15. Pectinate chaetae flat, oblique, 13–18 denticles, 1–3 per parapodium.
Material Examined
as ?Paronuphis antarctica: USNM 58439 (20); as Onuphis pseudoiridescens: NHMUK 2018.24031, 2018.19091–19100 (5); NHMUK 2018.23563 (1).
Description
Six specimens from Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) were available for examination, the following description is based on and encompasses all of them. Details in parentheses relate to specimens from Hartman (Reference Hartman1967) although most are in poor condition with many structures missing or degraded, absence of additional data indicates that it matches that already detailed.
Specimens posteriorly incomplete, 42–99 (37–62) chaetigers; L10 = 2.7–5.9 (5.3–8.0) mm, W10 = 0.7–1.6 (0.9–1.1) mm, TL = 12–40.4 (14.9–32.0) mm. Prostomium anteriorly extended (Figure 6B); frontal and upper lips oval (Figure 6A, B). Palpophores with 11–15 (12) rings and one slightly longer ring, palpostyles shorter than palpophores (Figure 6A, B) reaching to chaetiger 1. Lateral antennae with 10–14 (13) rings and one slightly longer ring, antennal styles reaching to chaetiger 5–10 (5–7), longer than median antenna (Figure 6A–C). Median antenna with 5–7 (7) rings and one slightly longer ring, antennal styles reaching to chaetiger 3–6 (5–6). Eyes absent. Peristomium half as long as first chaetiger. Peristomial cirri very slender, nearly twice as long as peristomium, inserted distally on peristomium in line with lateral antennae.

Figure 6. Onuphis pseudoiridescens: NHMUK 2018.24031 (A) dorsal view; (B) ventral view; (C) lateral view; (D) tridentate pseudocompound falciger, chaetiger 1; (E) tridentate pseudocompound falciger, chaetiger 2; (F) tridentate pseudocompound falciger, chaetiger 3; (G) tridentate pseudocompound falciger, chaetiger 4; (H) pectinate chaeta, chaetiger 39; (I) subacicular hook, chaetiger 40. Scale bars: A–C, 1 mm; D–G, 50 μm; H, 20 μm; I, 25 μm.
First four parapodia with tridentate, pseudocompound falcigers, up to 4 (5) in each parapodium, with short, pointed hoods (Figure 6D–G). Limbate chaetae from chaetiger 1, up to 12 in anterior chaetigers; pectinate chaetae flat, oblique, with 13–15 (12–18) denticles (Figure 6H), up to 3 per parapodium, start unclear but present from at least chaetiger 6 (5). Bidentate subacicular hooks (Figure 6I) from chaetiger 11 or 14 (10–15). Tube thin, soft, covered in silt particles.
Remarks
Monro (Reference Monro1936) reported five specimens of this species (as Onuphis iridescens (Johnson, Reference Johnson1901)) from a single station (WS212, Figure 1) north of the Falkland Islands (242–249 m). Hartman (Reference Hartman1967) identified 22 specimens from samples taken in 567–595 m directly south of the Islands as Nothria? iridescens that were later re-described as two new species (Onuphis (Nothria) heterodentata and Onuphis (Nothria) lithobiformis) by Fauchald (Reference Fauchald1982c). Orensanz (Reference Orensanz1990) later synonymized both O. heterodentata Fauchald, Reference Fauchald1982c and O. lithobiformis Fauchald, Reference Fauchald1982c with O. pseudoiridescens and also attributed Monro's records (Reference Monro1930, Reference Monro1936) to the species. In his remarks, Orensanz (Reference Orensanz1990) found that O. pseudoiridescens and O. iridescens were indistinguishable morphologically but inhabited separate geographic regions, with O. pseudoiridescens present in southwest Atlantic localities and O. iridescens present in the northeast Pacific. No other species of Onuphis have been reported from the Falkland Islands region.
The record of ?Paradiopatra antarctica (Monro,Reference Monro1930), published by Hartman (Reference Hartman1967) as ?Paronuphis antarctica, is here reassigned to O. pseudoiridescens. Orensanz (Reference Orensanz1990) reassigned all of Hartman's (Reference Hartman1967) records of Paronuphis antarctica (Monro, Reference Monro1930), en masse, to Notonuphis antarctica (Monro, Reference Monro1930) including those from the single station near the Falkland Islands from which she recorded the species (Table 1). However, Hartman's specimens from station 558, east of the Falkland Islands, were only tentatively identified as that species and were not commented on further nor were they examined by or commented on by Orensanz. Orensanz (Reference Orensanz1990) described the distribution of P. antarctica as endemic to the South Shetland and South Orkney Islands and the adjacent southern Scotia Sea, omitting the Falkland Islands from both the text and the map provided. In 2011, Budaeva and Fauchald made Notonuphis a junior synonym to Paradiopatra and included Hartman's record in their distribution map for the species, an outlying point to an otherwise limited Antarctic distribution. New examination of the specimen lot found the specimens to be in a very poor condition with many structures difficult to discern due to significant degradation or loss. However, the majority of the specimens were determined to be Onuphis pseudoiridescens although an additional juvenile Nothria anoculata was also identified (see later). The removal of the record from Paradiopatra antarctica, leaves the species with a more discrete distribution around the northwest Antarctic peninsula and South Shetland Islands (Budaeva and Fauchald, Reference Budaeva and Fauchald2011).
Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) found O. pseudoiridescens to be one of the most common taxa in samples from the SeaLion field exploration area to the north of the region (450–463 m) and also recorded it from the Toroa site to the southeast in 615 m. Details of actual abundance are not provided on the Marine Flora and Fauna of the Falkland Islands website (https://falklands.myspecies.info) or in their publication and only six specimens were available to examine so a full accounting of potential variation cannot be given. Branchiae are described as normally starting from chaetiger 1, but Orensanz (Reference Orensanz1990) did find that they could start as far back as chaetiger 4. In one small SeaLion specimen, branchiae did not start until chaetiger 5 but this does not seem a significant variation. Variation in the presence of ventral cirri and the start of the subacicular hooks was almost identical to that reported by Orensanz (Reference Orensanz1990). Although the species is reported from depths as shallow as 21 m (Orensanz, Reference Orensanz1990), all records from around the Falkland Islands are from depths greater than 200 m but fall within the currently accepted depth range.
Distribution
Falkland Islands (Figure 1): north, south, and east of the Islands in 212–845 m (Monro, Reference Monro1936; Hartman, Reference Hartman1967; Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020). Wider distribution: southern South America, southern Chile, Strait of Magellan and off Argentine Patagonia, 21–861 m (Orensanz, Reference Orensanz1990).
SUBFAMILY Hyalinoeciinae Paxton, Reference Paxton1986a
Genus Anchinothria Paxton, Reference Paxton1986a
Type species Diopatra pourtalesii Ehlers, Reference Ehlers1879)
Diagnosis (from Budaeva, Reference Budaeva, Purschke, Westheide and Böggemann2021
Body short, up to 100 segments. Median antenna longer than lateral antennae. Palpo- and antennophores short, consisting of 2–5 rings. Nuchal grooves straight. Peristomial cirri present. Anterior 2–3 pairs of parapodia enlarged, directed anteroventrally with bi- to trilobed prechaetal lobes. Ventral cirri subulate on anterior 2–3 chaetigers. Branchiae present or absent, single or dichotomously branched with up to 10 filaments. Uni- or bidentate simple or pseudocompound falcigers on first 2–4 pairs of anterior parapodia, in one species on first seven pairs of parapodia. Pectinate chaetae wide with rolled margins, so-called ‘scoop-shaped’, from chaetigers 2–3, in one species from chaetiger 14. Subacicular hooks from chaetigers 4–16. Maxillae V present; Mx VI absent. Tubes dorsoventrally flattened with parchment-like inner layer covered with mud and often incrusted with scattered large elongated foraminiferans, glass sponge spicules, or echinoid spines attached along longitudinal margins.
Anchinothria sp.
Figure 1, 7A–F; Table 1; S1
Leptoecia vivipara. – Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020: 66 (in part).
Diagnosis
Eyes absent. Ventral cirri on chaetigers 1–2. Branchiae absent. Bidentate pseudocompound falcigers present on chaetigers 1–2. Two bidentate subacicular hooks from chaetigers 9–10. Pectinate chaetae scoop-shaped, oblique, 8–14 denticles, 1–2 per parapodium.
Material Examined
as Leptoecia vivipara: NHMUK 2018.23504 (1).
Description
One specimen complete (Figure 7A) with 49 chaetigers; L10 = 1.7 mm, W10 = 0.5 mm, TL = 7.6 mm. Preserved colour white; pigmentation absent. Prostomium with rounded anterior margin, weakly incised; frontal and upper lips oval. Palps short, to chaetiger 2; lateral antennae long, thin, to chaetiger 9 (Figure 7A–C); median antenna long, thin, to chaetiger 6; palpo- and antennophores with three short rings and one longer ring. Eyes absent. Peristomium half as long as first chaetiger. Peristomial cirri slender, degraded, inserted distally on peristomium just lateral to lateral antennae.

Figure 7. Anchinothria sp.: NHMUK 2018.23504 (A) whole specimen, lateral view; (B) dorsal view; (C) ventral view; (D) bidentate, simple and pseudocompound falcigers, chaetiger 1; (E) pectinate chaeta, chaetiger 40; (F) subacicular hook, chaetier 24. Scale bars: A–C, 1 mm; D–F, 20 μm.
First two pairs of parapodia enlarged, directed anteriorly. Ventral cirri subulate, present chaetigers 1–2 (Figure 7C). Branchiae absent. First two pairs of parapodia with bidentate, pseudocompound falcigers (Figure 7D), up to 4 per parapodium; one simple, bidentate falciger present in one parapodium of chaetiger 1 (Figure 7D). Pectinate chaetae from chaetiger 3, 1–2 per parapodium, scoop-shaped, oblique with 8–14 denticles (Figure 7E). Two bidentate, subacicular hooks (Figure 7F) from at least chaetiger 9 or 10 to end of body. Maxillae not observed.
Pygidium with two pairs of anal cirri, both ventrally inserted, one pair short, one pair long, thin. Tube not present.
Remarks
Of the two specimens originally identified by Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) as Anchinothria cf. pycnobranchiata one specimen proved to be Nothria anoculata Orensanz, Reference Orensanz1974a (see later) and the second specimen was re-identified as Kinbergonuphis oligobranchiata (see earlier). However, a small specimen was examined that had been mis-identified as Leptoecia vivipara and is here re-assigned to Anchinothria sp.
The description of the Falkland Islands specimens on the Marine Flora and Fauna of the Falkland Islands website appears to be based on the mis-identified N. anoculata specimen leading to the discrepancies noted there from the original description of Anchinothria pycnobranchiata. Those include branchiae present (actually absent), pseudocompound falcigers present beyond chaetiger 2 (first two pairs of parapodia only) and subacicular hooks from chaetiger 6 (actually chaetiger 9 or 10). Orensanz (Reference Orensanz1990) agreed with Pettibone (Reference Pettibone, Weber, Beaufort and Stock1970) on presence or absence of branchiae not being a diagnostic character due to variability even within specimens from the same sample. Anchinothria pycnobranchiata is reported as reaching 70–75 mm in length for 70–80 chaetigers (Orensanz, Reference Orensanz1990), and the specimen described here is less than 8 mm long with only 49 chaetigers. The lack of falcigers on chaetiger 3 could well be due to the small size and likely juvenile condition of the specimen. Similarly, the presence of one simple falciger amongst the other pseudocompound falcigers also suggests that pseudocompound is a juvenile trait with development just starting toward a simple form. Orensanz (Reference Orensanz1990) also discusses the first appearance of the subacicular hooks, stating that they actually appear from chaetiger 4, but are frequently broken in earlier chaetigers due to their slender stature until they become more robust in later segments.
Two specimens were also recorded from a single station (350, see Figure 1) just south of the region by Hartman (Reference Hartman1967) as Nothria abranchiata (reassigned by Orensanz, Reference Orensanz1990 to A. pycnobranchiata) and are mentioned here due to the proximity of the locality. No other publications that have examined specimens from the region (Monro, Reference Monro1930, Reference Monro1936; Hartman, Reference Hartman1953; Hartmann-Schröder, Reference Hartmann-Schröder1983) recorded the species. The type locality for A. pycnobranchiata is the eastern Pacific, off Chile (−34.11667, −73.93333) at a depth of 4069 m, far deeper than the specimen described here was recorded from (1782 m). The distant type locality and much deeper type locality depth, along with the morphological variation between this specimen and the known details for A. pycnobranchiata, give cause to provide an identification to genus only for this specimen.
Distribution
Falkland Islands (Figure 1): East Falklands Basin (southern end, north of Burdwood Bank) in 1782 m (Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020).
Genus Leptoecia Chamberlin, Reference Chamberlin1919
Type species Leptoecia abyssorum Chamberlin, Reference Chamberlin1919
Diagnosis (from Budaeva, Reference Budaeva, Purschke, Westheide and Böggemann2021)
Small-sized worms 10–40 mm long with up to approximately 80 chaetigers. Prostomium rounded, conical, or pointed with reduced or absent frontal lips. Antennae with short 1–4 ringed antennophores and long styles. Nuchal organs slightly curved with wide middorsal separation, may be absent in some species. Peristomial cirri absent. Anterior 1–2 pairs of parapodia modified, first pair prolonged with auricular prechaetal lobe and digitiform postchaetal lobe. Ventral cirri subulate on first two chaetigers. Branchiae absent. Simple or pseudocompound uni- to bidentate falcigers with short blunt hoods on 1–2 pairs of anterior parapodia. Pectinate chaetae flat with about ten denticles. Paired bidentate hooded subacicular hooks from chaetigers 12 to 50. Maxillae V and VI absent. Tubes secreted, quill-like, circular in cross-section or flattened dorsoventrally, with two longitudinal ribs.
Leptoecia sp.
Figure 1, 8A–H; Table 1; S1
Leptoecia vivipara. – Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020: 66 (in part).
Diagnosis
Prostomium pointed; peristomial cirri absent. Eyes absent. Ventral cirri on chaetigers 1–2. Branchiae absent. Pseudocompound falcigers present on chaetigers 1–2: unidentate on chaetiger 1, bidentate on chaetiger 2. Bidentate subacicular hooks from chaetiger 4, 1 anteriorly, 2 from chaetiger 16. Pectinate chaetae flat, transverse, up to 12 denticles, up to 5 per parapodium in anterior chaetigers, reduced posteriorly.
Material Examined
as Leptoecia vivipara: NHMUK 2018.23503 (1).
Description
Single, complete specimen (Figure 8A–D) of 28 chaetigers, L10 = 7.1 mm, W10 = 0.25 mm, TL = 6.4 mm. Prostomium pointed (Figure 8B–D), peristomial cirri absent (Figure 8B), frontal lips absent (Figure 8D). Antennae with short, single-ringed antennophores and long, slender styles. Eyes absent. Peristomium ⅓ as long as first chaetiger; peristomial cirri absent.

Figure 8. Leptoecia sp.: NHMUK 2018.23503 (A) whole specimen with tube, dorsal view; (B) dorsal view; (C) ventral view; (D) lateral view; (E) unidentate, pseudocompound falcigfer, chaetiger 1; (F) bidentate, pseudocompound falciger, chaetiger 2; (G) pectinate chaeta, chaetiger 3; (H) subacicular hook, chaetiger 18. Scale bars: A, 1 mm; B–D, 0.5 mm; E–H, 20 μm.
First two pairs of parapodia modified, directed anteriorly; first pair enlarged, elongated. Dorsal cirri reduced to nodule from chaetiger 9 onwards but present to end of body. Ventral cirri subulate on chaetigers 1–2 (Figure 8D); branchiae absent.
First pair of parapodia with 2–3 unidentate pseudocompound falcigers (Figure 8E) with short, blunt hoods. Second pair of parapodia with four, bidentate pseudocompound falcigers (Figure 8F) and flat, transverse pectinate chaetae with up to 12 denticles (Figure 8G). Up to five pectinate chaetae in parapodia of chaetiger 3, reduced in number thereafter. Limbate chaetae present from chaetiger 3, up to three in each parapodium. Subacicular hooks (Figure 8H) from chaetiger 4: one from chaetiger 4–15, two from chaetiger 16. Tube cylindrical, smooth, straight, translucent (Figure 8A).
Remarks
Of the three specimens previously identified as Leptoecia vivipara by Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020), one was reassigned to A. pycnobranchiata (see earlier), one to the new species of Hyalinoecia (see later) and the remaining specimen, described here, to Leptoecia sp.
Leptoecia vivipara has previously only been reported from Antarctica, and the records reported by Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) would be the first for the Falkland Islands and the most northerly records to date. However, the characters present in the specimen described, although aligning it with Leptoecia, do not conform to any of the currently described species or their known variations. It differs from L. vivipara in the shape of the prostomium (pointed not rounded), having pseudocompound falcigers on two anterior chaetigers (not one) and dorsal cirri reduced to a nodule by chaetiger 9 (not 20).
The pseudocompound falcigers on both chaetigers 1 and 2 set the specimen apart from all the other described Antarctic species except for a single, incomplete specimen of ‘Leptoecia sp.’ from the Wilkes abyssal plain mentioned by Orensanz (Reference Orensanz1990). However, on that specimen, all falcigers were bidentate whereas those on chaetiger 1 are unidentate in the present specimen.
The pointed (‘helmet’-shaped) prostomium is as described and illustrated for Leptoecia oxyrincha (Kucheruk, Reference Kucheruk1978), although there is no dorsal tubercle (as described for the species in Orensanz, Reference Orensanz1990). It also differs from that species in several characters including the start of the subacicular hooks (chaetiger 4 not 15–17), presence of dorsal cirri (reduced but continuing to posterior in Leptoecia sp., absent from posterior in L. oxyrincha) and the anterior falcigers (parapodia of chaetigers 1–2 in Leptoecia sp. as opposed to just parapodia of chaetiger 1 in L. oxyrincha). Leptoecia benthaliana (McIntosh, Reference McIntosh1885) is described as having a variable prostomial shape with some specimens having a blunter, bilobed form and others a more pointed one. The ‘pointed’ form is not as smoothly-shaped as in Leptoecia sp. and anterior falcigers are present on first pair of parapodia only and are bidentate (unidentate on chaetiger 1 in Leptoecia sp. as well as having bidentate falcigers on parapodia of chaetiger 2).
The very small size of the specimen of Leptoecia sp. (less than 7 mm long) makes it unclear as to whether it is a juvenile or not and thus how characters such as the start of the subacicular hooks, and the presence of branchiae and dorsal cirri may develop. The prostomial shape and presence of pseudocompound falcigers on two anterior chaetigers clearly set it apart from the other known species of Leptoecia, however without further specimens, Leptoecia sp. is considered the best identification possible at this time.
Distribution
East Falklands Basin in 1842 m (Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) (Figure 1).
Leptoecia cf. benthaliana (McIntosh, Reference McIntosh1885)
Figure 1, 9A–G; Table 1; S1
Hyalinoecia benthaliana McIntosh, Reference McIntosh1885: 339, pl.21a, figs 15,16
Hyalinoecia tubicola. — Hartman, Reference Hartman1967: 89 (in part: stn 377)
Leptoecia cf. benthaliana. — Orensanz, Reference Orensanz1990: 55–58, fig. 16, pl.13a–n
Type Locality (Leptoecia benthaliana)
Southeast Indian Ocean; −50.016667, 123.066667; 3240 m
Diagnosis
Prostomium pointed or bilobed; peristomial cirri absent. Eyes absent. Dorsal cirri present throughout. Ventral cirri on chaetigers 1–2. Branchiae absent. Bidentate simple falcigers present on chaetiger 1 only. Bidentate subacicular hooks from chaetiger 48. Pectinate chaetae flat, transverse, 8–10 denticles, up to 10 per parapodium.
Material Examined
USNM 58018 (2).
Description
Two complete specimens (Figure 9A, B), 80 and 95 chaetigers, L10 = 4.1–4.9 mm, W10 = 0.8–1.1 mm, TL = 23.1–30.4 mm. Prostomium pointed on one specimen with frontal lips absent, slightly bilobed on the other with reduced frontal lips (Figure 9C, D). Antennae with short antennophores with two rings and long, slender styles (Figure 9C). Palps short, to chaetiger 1 only, median antenna long, reaching to chaetiger 11 or 14, lateral antennae to chaetiger 7 or 9 (Figure 9B, C). Eyes absent. Peristomium half as long as first chaetiger; peristomial cirri absent.

Figure 9. Leptoecia cf. benthaliana: USNM 58018 (A) whole specimen in tube; (B) whole specimen; (C) dorsal view; (D) ventral view; (E) simple, bidentate falciger, chaetiger 1; (F) pectinate chaeta, chaetiger 74; (G) subacicular hook, chaetiger 73. Scale bars: A, 10 mm; B–D, 1 mm; E–G, 20 μm.
First pair of parapodia modified, enlarged, directed anteriorly, elongated with auricular prechaetal lobes (Figure 9C, D); postchaetal lobes digitiform. Second pair of parapodia modified, slightly enlarged (Figure 9C), not directed anteriorly. First chaetiger twice length of second (Figure 9C). Dorsal cirri reduced from chaetiger 18 onwards but present to end of body. Ventral cirri subulate, chaetigers 1–2 (Figure 9D). Branchiae absent.
First pair of parapodia with three simple bidentate falcigers with short, blunt hoods (Figure 9E). Second pair of parapodia with up to four limbate chaetae only. Third pair of parapodia with limbate chaetae and up to 10 flat, transverse pectinate chaetae with up to 8–10 denticles (Figure 9F). Subacicular hooks (Figure 9G) from chaetiger 48: one on chaetigers 48–49 or 50, two from chaetiger 50 or 51. Tube quill-like (Figure 9A).
Remarks
Hartman (Reference Hartman1967) identified specimens from three stations around the Falkland Islands as Hyalinoecia tubicola (Müller, Reference Müller1776), including three from a sample taken in a deep trench north of Burdwood Bank in the southern part of the Falkland Islands zone. Orensanz (Reference Orensanz1990) examined and re-identified the specimens from that station (377), stating that they ‘may belong to’ Leptoecia cf. benthaliana (the remaining specimens from the other samples were attributed to Hyalinoecia artifex Verrill, Reference Verrill1881 as discussed later). Re-examination of the specimens does not find any reason to suspect Orensanz' original placement of the specimens and it is thought that his tentative placement was more due to their locality, L. benthaliana being originally described from south of the Great Australian Bight. The different prostomial shapes exhibited in the specimens are as described by Orensanz for the other specimens he discussed, and all other characteristics fall within the ranges described. Most records of the species are from the Antarctic, particularly the deep southeast Pacific Basin (Orensanz, Reference Orensanz1990), but it is also reported from the Scotia Sea, south of the Falkland Islands region. The record is also at the shallow end of the reported depth range and so a more tentative assignation is considered appropriate. This is the first and, so far only, report of the species from the Falkland Islands region and differs from the only other specimen here attributed to the genus (Leptoecia sp., see earlier) in having simple, unidentate falcigers on chaetiger 1 only (Leptoecia sp. has pseudocompound falcigers on first two pairs of parapodia with those on chaetiger 1 being unidentate and those on chaetiger 2 bidentate) and a pointed, ‘helmet-shaped’ prostomium (as opposed to slightly bilobed or pointed).
Distribution
Falkland Islands (Figure 1): Northeast of Burdwood Bank (south of the Islands) in 1879–1886 m (Hartman, Reference Hartman1967). Wider distribution: circum-Antarctic in deep water (1879–4946 m; Orensanz, Reference Orensanz1990).
Genus Nothria Malmgren, Reference Malmgren1867
Type species Onuphis conchylega Sars, Reference Sars1835
Diagnosis (from Budaeva, Reference Budaeva, Purschke, Westheide and Böggemann2021)
Body short, up to 100 segments. Antennae with antennophores consisting of 2–5 rings and short to moderately long styles. Median antenna longer than lateral antennae. Nuchal organs straight with narrow middorsal separation. Anterior 2–3 pairs of parapodia enlarged, directed anteroventrally with large auricular prechaetal lobes. Ventral cirri subulate on first 2–3 chaetigers. Branchiae present or absent, single to up to five filaments. Uni-, bi- or tridentate simple or pseudocompound falcigers on first 2–3 pairs of anterior parapodia. Pectinate chaetae wide with rolled margins, so-called ‘scoop-shaped’, from chaetigers 2–3, in one species from chaetiger 9; rarely pectinate chaetae flat. Paired hooded subacicular hooks from chaetigers 7–15. Maxillae V present; Mx VI absent. Tubes dorsoventrally flattened with thin inner parchment-like layer covered with large shell fragments, small stones, and shells foraminiferans.
Nothria anoculata Orensanz (Reference Orensanz1974a)
Figure 1, 10A–H; Table 1; S1
Nothria conchylega anoculata Orensanz, Reference Orensanz1974a: 99, pl. 8.
Nothria nr conchylega. — Hartman, Reference Hartman1967: 90 (in part: stns 350, 369).
?Paronuphis antarctica. — Hartman, Reference Hartman1967: 96–97 (in part).
Nothria anoculata. — Orensanz, Reference Orensanz1990: 44–48, pl. 9a–m, fig. 14. — Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020: 66.
Type Locality
Western Atlantic: off Argentina, Buenos Aires Province; 700–900 m.
Diagnosis
Eyes absent. Ventral cirri on chaetigers 1–2. Branchiae present from chaetiger 10–14, single filament. Simple and pseudocompound falcigers present: unidentate, simple on chaetiger 1; unidentate simple and unidentate and bidentate pseudocompound on chaetiger 2; bidentate pseudocompound on chaetiger 3. Bidentate subacicular hooks from chaetigers 12 or 13. Pectinate chaetae scoop-shaped, flat, up to 10 denticles, up to 19 per parapodium, number reducing posteriorly.
Material Examined
as ?Paronuphis antarctica: USNM 58439 (1); as Nothria anoculata: USNM 58193 (32); USNM 98048 (1); NHMUK 2018.23592 (1); NHMUK 2018.23473 (1); NHMUK 2018.23538 (1).
Description
Three specimens were available to examine from Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020), two originally identified as Nothria anoculata and one that had been re-identified from Anchinothria cf. pycnobranchiata. In addition, 34 specimens from Hartman (Reference Hartman1967) were examined – one juvenile re-identified from ?Paronuphis antarctica (see earlier), one from east of the Islands and 32 from stn 350, south of Burdwood Bank just outside of the region. The two specimens from Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020), correctly identified as Nothria anoculata, were complete, one with eggs. The remaining specimens, that had previously been misidentified, were posteriorly incomplete. The following description encompasses all specimens excluding those from stn 350 outside of the region although those are discussed in the Remarks.
Two complete specimens (Figure 10A) with 30 and 36 chaetigers, L10 = 2.5–3.6 mm, W10 = 1.1–1.5 mm, TL = 11.6–14.7 mm; three posteriorly incomplete specimens with 15–21 chaetigers, L10 = 2.4–4.6 mm, W10 = 1.1–1.3 mm, TL = 3.8–5.75 mm. Prostomium with rounded anterior margin, very weakly incised; frontal and upper lips oval. Palpo- and antennophores with two short and one long ring with moderately long styles. Palps reaching chaetiger 1, lateral antennae reaching chaetiger 7–9 (4 in juveniles), median antenna reaching chaetiger 12–13 (6 in juveniles) (Figure 10B, C). Eyes absent. Peristomium half as long as first chaetiger. Peristomial cirri slender, slightly longer than peristomium, inserted distally on peristomium in line with lateral antennae.

Figure 10. Nothria anoculata: NHMUK 2018.23473 (A) whole specimen in tube; (B) dorsal view; (C) lateral view; (D) simple, unidentate falciger, chaetiger 1; (E) simple, unidentate falciger, chaetiger 2; (F) bidentate pseudocompound falciger, chaetiger 3; (G) pectinate chaeta, chaetiger 5; (H) subacicular hook, chaetiger 11. Scale bars: A–C, 1 mm; D–F, H, 50 μm; G, 50 μm.
First two pairs of parapodia modified, enlarged, directed anteroventrally (Figure 10B), first pair more than second, with large auricular prechaetal lobes. Ventral cirri subulate on chaetigers 1–2 (Figure 10B). Branchiae present, from chaetiger 10–14, single strap-like filament only.
First pair of parapodia with 3–5 simple, unidentate falcigers (Figure 10D). Second pair of parapodia with 1–2 simple, unidentate falcigers (Figure 10E) and 2–3 pseudocompound, uni- or bidentate falcigers, 1–2 limbate chaetae and 10–17 flat, scoop-shaped pectinate chaetae with up to 10 denticles. Third pair of parapodia with 3 bidentate, pseudocompound falcigers (Figure 10F), 2 limbate chaetae, and up to 19 scoop-shaped pectinate chaetae (Figure 10G). From chaetiger 4 with up to 4 limbate and 3–8 pectinate chaetae; bidentate subacicular hooks (Figure 10H) from chaetiger 12 or 13. Tubes with thin, inner parchment-like layer with large irregular pebbles attached (Figure 10A).
Remarks
Nothria anoculata was not described when Hartman identified the specimens for her 1967 publication and she clearly recognized that they did not fit any description available at the time, placing those from stn 350, just outside the Falkland Islands region, as Nothria nr conchylega but not reporting those from station 558. Orensanz (Reference Orensanz1990) noted that several specimens from Hartman's stn 350 showed some variation and could represent a different species, however, of those specimens examined, all fit within the description for Nothria anoculata and were consistent with the specimens examined from the other stations. The Falkland Islands specimens (Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020), from up to 1837 m depth just within Orensanz' considered range, also conform to the original description. Some falcigers in chaetiger 1 of the smallest specimens, did demonstrate a ‘semi-pseudocompound’ appearance as opposed to having simple hooks only in chaetiger 1. Orensanz (Reference Orensanz1990), however, illustrated three stages of development in the species, the second of which could demonstrate both simple and pseudocompound hooks in chaetigers 1 and 2. He also stated that the pseudocompound falcigers of chaetiger 2 were replaced by simple ones later in development, as also documented in more detail by Budaeva and Paxton (Reference Budaeva and Paxton2013). Due to the small size of the Falkland Islands specimens, it is likely that the variation noted in chaetiger 1 is due to the incomplete development of the specimen and, in the mature specimen (indicated by the presence of eggs in the tube), simple falcigers are evident in chaetiger 2 as well as pseudocompound ones. These variations in the falcigers are therefore considered to be due to the incomplete development of the specimens and are not considered significant. All specimens fit the description for the species in all other respects.
Distribution
Falkland Islands (Figure 1): East of the Falkland Islands and East Falklands Basin, 646–1837 m (Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) and just outside the region to the south of Burdwood Bank in 2452 m. Wider distribution: subantarctic areas around Tierra del Fuego, off Argentina, north of Drake Passage, South Georgia shelf, Pacific-Antarctic, and Macquarie ridges, Antipodes-Bounty and Prince Edward-Marion shelfs (75–1887 m) (Orensanz, Reference Orensanz1990).
Genus Hyalinoecia Malmgren, Reference Malmgren1867
Type species Nereis tubicola Müller, Reference Müller1776
Diagnosis (from Budaeva, Reference Budaeva, Purschke, Westheide and Böggemann2021)
Small to large-sized worms up to 20 cm long with up to 200 chaetigers. Prostomium anteriorly rounded with oval or ovoid frontal lips. Antennae with antennophores with 2–5 rings and long styles reaching chaetiger 8–20. Median antennae longer and thicker than lateral antennae. Nuchal organs are straight with small to moderately large middorsal separation. Peristomial cirri absent. Anterior 2–3 pairs of parapodia modified, moderately prolonged, with large auricular prechaetal and subulate postchaetal lobes. Ventral cirri are subulate on anterior 3–4 chaetigers. Branchiae from chaetiger 18–33, single, strap-like filaments, or absent. Pseudocompound falcigers on modified parapodia uni- to bidentate, simple to pseudocompound, with or without hoods. Flat pectinate chaetae from chaetiger 2, with up to 20 denticles. Paired bidentate hooded subacicular hooks from chaetiger 15–30. Maxillae V present; MxVI absent. Tubes round in transverse section, translucent quill-like, completely secreted by worm and lacking external covering of foreign particles. Anterior and posterior ends of the tube with 2–4 internal valves.
Hyalinoecia falklandica sp. nov.
Figure 1, 11A–E, 12A–I; Table 1, 3; S1
Hyalinoecia tubicola. – Hartman, Reference Hartman1967: 89 (in part: stns 557, 558).
Hyalinoecia artifex. — Orensanz, Reference Orensanz1990: 52–54, pl.12a–l, fig. 15.
Leptoecia vivipara. — Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020: 66 (in part).
Type Locality
East Falkland Islands; −51.942, −56.642; 855–866 m.
Diagnosis
Peristomial cirri absent. Eyes absent. Ventral cirri on chaetigers 1–3 or 4. Branchiae present from chaetiger 26–27, single filament. Simple, unidentate falcigers on chaetigers 1–2. Two bidentate subacicular hooks from chaetigers 23–37. Pectinate chaetae flat, transverse, up to 12 denticles, up to 5 per parapodium.
Type Material
USNM 1682921 (holotype); USNM 1682922 (2 paratypes).
Additional Material Examined
as Hyalinoecia stricta: USNM 058016 (9); as Hyalinoecia artifex: USNM 058019 (6); as Leptoecia vivipara: NHMUK 2018.23562 (1).
Description
Description based on holotype unless otherwise stated. Holotype complete (Figure 11A) with 153 chaetigers, L10 = 15 mm, W10 = 4 mm, TL = 97 mm, tube = 175 mm; two paratypes complete with 105 chaetigers, L10 = 14–15 mm, W10 = 4 mm, TL = 80–85 mm.

Figure 11. Hyalinoecia falklandica sp. nov.: Holotype USNM 1682921 (A) whole specimen, dorsal view, with tube; (B) dorsal view; (C) ventral view; Paratype USNM 1682921 (D) close-up view of chaetiger 1, illustrating auricular prechaetal lobe and simple falcigers; Holotype USNM 1682921 (E) subacicular hooks, emergent aciculae and limbate chaetae of chaetigers 88–89. Scale bars: A, 10 mm; B–E, 1 mm.
Dark brown pigmentation is present (holotype only) on anterodorsal and anteroventral region of prostomium (Figure 11B, C), dorsal region of upper lips around the posterior edge of ventral lips, around ventral and lateral border of palpophores and on anterior surface of parapodia from chaetiger 1; cuticle iridescent. Prostomium with rounded anterior margin; frontal lips and upper lips globose. Palpo- and antennophores short (Figure 11B, C) with two basal rings and one slightly longer ring, styles long; palps reaching to chaetiger 1, lateral antennae to chaetiger 9, median antenna to chaetiger 12 (Figure 11B, C). Eyes absent. Peristomium less than half as long as first chaetiger, with middorsal anterior fold. Peristomial cirri absent.
First four pairs of parapodia are modified, enlarged, with auricular prechaetal and subulate postchaetal lobes, directed anteroventrally (Figure 11B–D). Chaetiger 1 ~⅓ longer than chaetiger 2. Postchaetal lobes long, reducing in size from about chaetiger 13, shorter than the prechaetal and acicular lobes from around chaetiger 30–35, absent by chaetiger 50. Ventral cirri subulate on chaetiger 1–3 or 4 (Figure 11C, 12A–B). Branchiae from chaetiger 27 to end of body, single strap-like filament (Figure 12C), reaching to or just past mid-line of body.

Figure 12. Hyalinoecia falklandica sp. nov.: Paratype USNM 1682921 (A) chaetiger 1, posterior view; (B) chaetiger 2, posterior view; (C) chaetiger 37, anterior view; USNM 58019 (D) simple, unidentate falciger, chaetiger 1; (E) pectinate chaeta, chaetiger 105; (F) subacicular hook, chaetiger 105; USNM 58016 (G) simple, bidentate falciger, chaetiger 2; (H) pectinate chaeta, chaetiger 79; (I) subacicular hook, chaetiger 80. Scale bars: A–C 1 mm; D, 100 μm; E–F, 10 μm; G, 20 μm; H–I, 20 μm.
First two pairs of parapodia with simple, unidentate falcigers (Figures 11D, 12A–B, 12D), 4 or 5 in each parapodium; limbate and pectinate chaetae present from chaetiger 2. Limbate chaetae (Figure 11E) present throughout body, except chaetiger 1, up to 14 per parapodium. Pectinate chaetae flat, transverse (Figure 12E, H), up to 5 per parapodium in anterior chaetigers with up to 12 denticles. Three blunt aciculae emerging from chaetiger 7, to end of body (Figure 11E); bidentate subacicular hooks (Figures 11E, 12F, 12I) from chaetiger 37, two per parapodium, teeth angled approximately 45° to main axis.
Pygidium with two long, thin anal cirri, ventrally inserted. Tube tough, translucent, cylindrical, smooth, slightly curved.
Variation
USNM 58016 consists of nine specimens in tubes, all of similar size, most in poor condition due to inadequate in-tube preservation; USNM 58019 consists of six specimens, none with tubes. In addition, a single juvenile specimen from Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020), previously mis-identified as Leptoecia vivipara (see earlier) is here re-assigned to Hyalinoecia falklandica sp. nov.
Twelve incomplete specimens with 64–112 chaetigers, L10 = 6.7–18 mm, W10 = 1.6–5 mm, TL = 22–82 mm; four complete specimens with 66–174 chaetigers, L10 = 4.7–16 mm, W10 = 0.8–4 mm, TL = 22.5–135 mm; tubes 48–76 mm long. Antennophores with 1–2 basal rings, lateral antennae reaching to chaetiger 5–14, median antenna to chaetiger 9–14. Branchiae present from chaetiger 26 or 27, consistent across all sizes examined. Smaller specimens (USNM 58016: TL 23–34 mm) with up to four simple, bidentate falcigers (Figure 12G) with short, pointed hoods on chaetiger 1, chaetiger 2 with up to three simple, bidentate falcigers on each side; smallest specimen (NHMUK 2018.23562) with both pseudocompound and simple bidentate falcigers in chaetigers 1 and 2. Bidentate, subacicular hooks present from chaetiger 23–37, from chaetiger 12 in the smallest specimen; up to four in posterior chaetigers of some larger specimens. Small specimens (USNM 58016) with teeth of subacicular hooks angled at 30° to main axis (Figure 12I).
Remarks
Specimens identified by Hartman (Reference Hartman1967) as Hyalinoecia tubicola, from the station 558, were re-assigned to H. artifex by Orensanz (Reference Orensanz1990) after his examination, along with others from station 557 that he did not examine. Specimens of H. tubicola from the station 377, he re-assigned to Leptoecia cf. benthaliana (see earlier). A search of the collection catalogue at the Smithsonian National Museum of Natural History also brought to light additional specimens that had been identified as Hyalinoecia stricta by Hartman, also from stations 557 and 558, although never published as such nor mentioned by Orensanz in his later works. Orensanz (Reference Orensanz1990) recorded in his publication that he did not examine the Hyalinoecia specimens from station 557, however, he re-assigned them to H. artifex along with those from station 558. No other specimens of Hyalinoecia from stations 557 or 558 were found when searching the catalogue, suggesting that those examined here are the same as those recorded by both Hartman and Orensanz.
Morphological characters were consistent between specimens from station 557 and those from station 558 that Orensanz (Reference Orensanz1990) had re-identified as H. artifex. Eyes are absent from all specimens, branchiae are consistent in their start on either chaetiger 26 or 27 and the large falcigers of chaetiger 1 are all simple and unidentate. Small specimens, previously identified as H. stricta from stn 558, differ slightly from the larger ‘H. stricta’ and ‘H. artifex’ (also from station 558), in the presence of simple bidentate falcigers on chaetiger 2 and subacicular hooks with teeth at a more acute angle (30° vs 45°) to the main axis; all other characters however fell within the same boundaries.
The smallest specimen, a specimen previously misidentified as L. vivipara (NHMUK 2018.23562), bears some resemblance to Orensanz’ (Reference Orensanz1974a, Reference Orensanz1990) southwest Atlantic variety of H. tubicola, with more rounded frontal lips and subacicular hooks starting from chaetiger 12, well before the earliest start of chaetiger 23 on the smallest of the USNM specimens. However, on the aforementioned NHMUK specimen, branchiae start from chaetiger 26 as with the other specimens documented (as opposed to chaetigers 23–24 in Orensanz' southwest Atlantic H. tubicola) and the apical tooth of the bidentate anterior falcigers is larger and more rounded than that figured by Orensanz (Reference Orensanz1974a) for his southwest Atlantic variety. The specimen is therefore included within the new species as it is considered that these characters are most likely a result of the very small size and juvenile status of the specimen, although the potential of there being more than one taxon represented is noted.
Despite Orensanz' comparison to H. artifex, several differences are apparent between that description and the current specimens. In Table 3 selected characters are compared for H. falklandica sp. nov. H. artifex, H. stricta and H. tubicola . From the table, it can be seen that H. falklandica differs from H. artifex in the reduction of the postchaetal lobes, number of anterior chaetigers with ventral cirri and the shape of the subacicular hooks, from H. stricta in the shape of the falcigers of chaetigers 1 and 2 and the subacicular hooks and from H. tubicola in the presence of eyes, the shape of the chaetiger 1 and 2 falcigers and the shape of the subacicular hooks. Orensanz (Reference Orensanz1974a) considered characters such as the start of the branchiae (within limits) and relative sizes of cirri and antennae to be unimportant due to individual variation, however, when such characters show a consistent and reliable difference, particularly for animals of an equivalent size, they are here considered of high taxonomic value. Ontogenetic variation in falcigers is noted by Orensanz (Reference Orensanz1990), and further detailed by Budaeva and Paxton (Reference Budaeva and Paxton2013), for Nothria anoculata, with falcigers developing from pseudocompound to simple and from bidentate to unidentate. Although not specifically detailed for Hyalinoecia, this genus may have a similar chaeta progression pattern in ontogeny explaining the discrepancy in the falcigers between the small and the large animals that otherwise share other characters. The start of the subacicular hooks was also noted as having significant variation by Orensanz (Reference Orensanz1990), and by Mangum and Rhodes (Reference Mangum and Rhodes1970) for H. artifex although not for H. tubicola, however, the shape and presence of a notch proximal to the teeth in H. tubicola provide further distinction between the species.
Table 3. Comparison of morphological characters for H. falklandica sp. nov. with H. artifex (as defined by Mangum and Rhodes (Reference Mangum and Rhodes1970)), H. stricta (as defined by Moore (Reference Moore1911) with additions from Fauchald (Reference Fauchald1968*)) and H. tubicola (as defined by Mangum and Rhodes (Reference Mangum and Rhodes1970)). Range of some characters provided in brackets where known.

Range of some characters provided in brackets where known.
Amongst the currently described species of Hyalinoecia, the combination of simple unidentate falcigers, ventral cirri present to chaetigers 3 or 4, branchiae present from chaetigers 26 or 27 to the end of the body (a consistent character across the size range investigated) and subacicular hooks with teeth positioned at an acute angle to the main axis is unique. The presence of some pseudocompound falcigers in the smallest specimen is considered a juvenile character, especially as some simple falcigers are also in place. The presence of bidentate falcigers in the USNM 58016 lot is more problematic and it is not clear if this indicates the presence of a different species in the examined material. The specimens are, however, from the same station as USNM 58019, in which the much larger specimens align with the type specimens here described and so are believed to be part of the same population, just separated due to their size.
None of the currently described species of Hyalinoecia were originally described from the southwest Atlantic: 10 of the 19 species were described from the Pacific, two from Australia and New Zealand, six from the north Atlantic, and one from South Africa. Recent investigation of the reproductive traits of Hyalinoecia robusta (Arias and Paxton, Reference Arias and Paxton2022) found them unlikely to support widespread dispersal of the species with the conclusion that reports of that species from outside of its native northeast Atlantic range are unlikely to be correct. Although H. artifex has been recorded from Patagonia, Strait of Magellan, and the Argentinean slope (Orensanz, Reference Orensanz1990) and H. tubicola reported as present further north from San Sebastian Island to Uruguay (Orensanz, Reference Orensanz1974a), the type locality of H. tubicola is off Norway in the northeast Atlantic and the type locality of H. artifex is off New England in the northwest Atlantic. The research by Arias and Paxton (Reference Arias and Paxton2022) adds to the doubt that either taxa might be likely to occur in the southwest Atlantic or any of the other currently described taxa. Despite the lack of molecular data, the morphological data is deemed strong enough to warrant the description of H. falklandica as a new species and promote a start toward a more accurate knowledge of Hyalinoecia in the southwest Atlantic and reduce the perpetuation of inaccurate species records. All other southwest Atlantic records of Hyalinoecia, including those recorded by Orensanz (Reference Orensanz1974a, Reference Orensanz1990) and Hartmann-Schröder (Reference Hartmann-Schröder1983), should now be re-evaluated.
Etymology
Hyalinoecia falklandica is named for the Falkland Islands region from where the specimens were collected.
Habitat
Habitat type unknown; slope depths in 571–866 m.
Distribution
Falkland Islands (Figure 1): eastern slope in 646–866 m (Hartman, Reference Hartman1967) and southeast sector of the region in 571 m (Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020).
Family Eunicidae Berthold, Reference Berthold1827
Genus Leodice Lamarck, Reference Lamarck1818
Type species Leodice antennata Lamarck, Reference Lamarck1818
Diagnosis (from Zanol and Budaeva, Reference Zanol, Budaeva, Purschke, Westheide and Böggemann2021)
Median, lateral antennae and palps present with regular or irregular articulations. Prostomium steep truncate or round. Peristomial cirri present. Maxillae with four or five paired plates and one unpaired plate. Mandibles flat. Limbate chaetae, thin pectinate chaetae, compound bidentate or tridentate falcigers, aciculae, and subacicular hooks present. Aciculae light or dark. Dark aciculae vary in colour along body; anteriormost always lightest but maintain same colour shade. Subacicular hooks light or dark, bidentate, or tridentate. Lateral black dots between parapodia present or absent.
Leodice sp.
Figure 1, 13A–K; Table 1; S1
Eunice pennata: Monro (Reference Monro1930): 118–120, fig. 42. – Orensanz (Reference Orensanz1990): pl. 17a–f, fig. 18.
Eunice cf. pennata. – Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020): 60.
Diagnosis
Ventral cirri present. Branchiae pectinate, up to five filaments, from chaetiger 3. Bidentate, compound, hooded falcigers present from chaetiger 1. Aciculae yellow with bent tip. Single subacicular hook, yellow, from chaetigers 15 to 37, start size dependent. Pectinate chaetae present from chaetiger 2, up to 3 per parapodium.
Material Examined
as Eunice pennata: NHMUK 1930.10.8.1434 (1); as Eunice cf. pennata: NHMUK 2018.23524 (1); NHMUK 2018.23525 (1); NHMUK 2018.23547 (2).
Description
Description based on Monro's (Reference Monro1930) specimen. Additional notes in Variation section detail Neal et al.'s (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) specimens.
Single specimen posteriorly incomplete with 48 chaetigers, L10 = 9.5 mm, W10 = 2.35 mm, TL = 36 mm. Colour cream in alcohol, no pigmentation present. Prostomium bilobed, lobes frontally rounded (Figure 13A, B). Prostomial appendages in semicircle. Palps, lateral, and median antennae long with irregular, long articulations, with ring-shaped bases. Median antenna reaching chaetiger 4, lateral antennae reaching chaetiger 3, and palps reaching to first peristomial ring (Figure 13A, B).

Figure 13. Leodice sp.: NHMUK 1930.10.8.1434 (A) dorsal view; (B) ventral view; (C) mid-body region showing pectinate branchiae, chaetigers 26–29; (D) post-branchial end of specimen, chaetigers 46–48; (E) pseudocompound falciger, chaetiger 3; (F) pseudocompound falciger, chaetiger 21; (G) pseudocompound falciger, chaetiger 47; (H) pectinate chaeta, chaetiger 3; (I) aciculum, chaetiger 21; (J) aciculum, chaetiger 44; (K) subacicular hook, chaetiger 47. Scale bars: A–B, 5 mm; C–D, 1 mm; E–H 20 μm; I–K, 50 μm.
Second peristomial ring approximately half length of first, shorter than following chaetigers; rings clearly separated all round. Peristomial cirri long, articulated, inserted anteromedially on segment, reaching middle of chaetiger 2 (Figure 13A).
Branchiae pectinate with up to five filaments (Figure 13C), from chaetiger 3 to 40; filaments shorter than notopodial cirri to chaetiger 7, thereafter equal to or longer than notopodial cirri. Notopodial cirri long, articulated, increasing in length from chaetigers 1 to 3 (Figure 13A) afterwards stable to end of fragment; articulation clear in the pre-branchial region, becoming fainter to almost indistinguishable in the remaining fragment. Ventral cirri present, digitate on chaetigers 1–4 (Figure 13B), developing defined conical tips over chaetigers 5–8, with oblong inflated bases, and conical tips from chaetiger 9. In postbranchial chaetigers, ventral cirri reverting back to digitate over several chaetigers and reducing in size. Prechaetal lobes short, postchaetal lobes as long as or very slightly shorter than chaetal lobes along length of fragment, chaetal lobes rounded.
First pair of parapodia with compound hooded, bidentate falcigers (Figure 13E, F, G) with short blade, ventral to aciculae. Limbate chaetae supra-acicular only, from chaetiger 1. Pectinate chaetae (Figure 13H) from chaetiger 2, up to 3 per parapodium. Aciculae yellow, darkening slightly in post-branchial region; stout, single on first chaetiger, two thereafter, developing pronounced bent tips (Figure 13I–J). Subacicular hooks (Figure 13K) from chaetiger 31, yellow (of similar hue to aciculae), one per parapodium.
Variation
The specimens from Loligo station 1MFA are very small, one whole specimen of 38 chaetigers and one anterior fragment of 11 chaetigers. Both have branchiae from chaetiger 3. In the complete specimen, branchiae end on chaetiger 16 with maximally two filaments. Antennae and palps are articulated. Aciculae are yellow, blunt with bent tips. Subacicular hooks are bidentate, starting from chaetiger 15.
Remarks
Only three specimens represent this species from the Falkland Islands region, one of those is posteriorly incomplete, two more are juvenile. Two additional specimens, published as Eunice cf. pennata by Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) from the Inflexible exploration area, were re-assigned to Onuphidae indet. and are not discussed here.
Fauchald (Reference Fauchald1974) noted a wide variability in reports of Leodice pennata and suggested that they indicated that more than one species may be involved. Later (1992), Fauchald discussed the wide-ranging bi-polar distribution, stating that reports of the species from the southern hemisphere (Hartman, Reference Hartman1964, Reference Hartman1967) required confirmation. Zanol and Budaeva (Reference Zanol, Budaeva, Purschke, Westheide and Böggemann2021) state that although worldwide distribution has been reported for some species, more modern investigative work is now finding that such taxa are, generally, complexes of cryptic species with more narrow distributions. Orensanz (Reference Orensanz1990) also referred to Fauchald's comments regarding the potential for additional species but could not find any definitive differences in those specimens he determined as L. pennata to identify them as a different species. In a review of Eunice species (1992), Fauchald stated the identifying character for L. pennata as the presence of ring-shaped bases in posterior notopodia (a character only shared by one other species). Orensanz (Reference Orensanz1990) did provide a figure of a posterior parapodium (the 60th) as part of the description of his L. pennata specimens, however, a ring-shaped base was not apparent nor mentioned. Examination of Monro's (Reference Monro1930) and Neal et al.'s (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) specimens found no evidence of ring-shaped bases in posterior notopodia (Figure 13D) either. Fauchald's description of ring-shaped bases was only that they were ‘post-branchial’, and, except for the short anterior fragment, specimens still had a significant portion of post-branchial chaetigers so it is therefore assumed that this feature should have been present for them to represent L. pennata sensu stricto.
Leodice antarctica (Baird, Reference Baird1869), described from Antarctic Seas (actual type locality unclear), also has branchiae starting on chaetiger 3, yellow aciculae and subacicular hooks, and cylindrical articulations on the antennae. Historically, L. antarctica has been synonymized with L. pennata (Monro, Reference Monro1936; Hartman, Reference Hartman1964) and, after examining the type of material, Orensanz (Reference Orensanz1990) also considered it so. Fauchald (Reference Fauchald1992), however, also found the species to be very similar to L. pennata but determined there to be verifiable differences in the branchiae (up to five filaments that are shorter than notopodial cirri in L. antarctica but up to 12 filaments that are longer than notopodial cirri in L. pennata) and the articulations of the notopodial cirri (present throughout the body in L. antarctica but absent from the branchial region on L. pennata). Arguably, the specimens here are closer to L. antarctica than to L. pennata, with branchiae with five filaments and the absence of the post-branchial cirrophores. However, there are still discrepancies with L. antarctica: the branchial filaments are only shorter than the notopodial cirri in the pre-branchial region and articulation of notopodial cirri becomes unclear through the branchial region with most of the post-branchial region absent. This, combined with the limited distribution capacity of most Eunice species (Zanol and Budaeva, Reference Zanol, Budaeva, Purschke, Westheide and Böggemann2021) making the presence of L. antarctica around the Falkland Islands doubtful, means that identification was deemed better left at genus level than to assign a poorly-matched species name and potentially introduce another doubtful record to the area.
In the wider Magellan region, Eunice magellanica McIntosh, Reference McIntosh1885, is recorded from southwest Chile to Argentina (Rozbaczylo, Reference Rozbaczylo1985; Orensanz, Reference Orensanz1974b, Reference Orensanz1990) but has branchiae from chaetiger 7 and black aciculae. Eunice frauenfeldi Grube, Reference Grube1866, also recorded from the region, has branchiae from chaetiger 6 but was originally described from St Paul's Island in the Indian Ocean, with records probably incorrectly attributed to the name due to the previous synonymy of E. magellanica.
Distribution
Falkland Islands (Figure 1): east (115 m; Monro, Reference Monro1930) and northeast (1321 m; Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) of the Islands.
Genus Marphysa de Quatrefages, 1866 (Reference de Quatrefages1865)
Type species Nereis sanguinea Montagu, Reference Montagu1813
Diagnosis (from Zanol and Budaeva, Reference Zanol, Budaeva, Purschke, Westheide and Böggemann2021)
Median antenna, lateral antennae, and palps present. Peristomial cirri absent. Maxillae with four paired plates and one unpaired plate. MxI falcal arch extended rectangular; basal inner edge lacking a curvature. Mandibles flat. Branchiae distributed along most of the body. Neuropodial postchaetal lobes longer than chaetal lobes at least in anteriormost parapodia. Limbate chaetae, pectinate chaetae, aciculae, and subacicular hooks present; bidentate falcigers and spinigers present or absent. Thin pectinate chaetae with both outer teeth longer than inner teeth; inner teeth of equal length. Thick pectinate chaetae present. Subacicular hooks light or dark, falcate, or bidentate.
Marphysa sp.
Figure 1, 14A–J; Table 1; S1
Marphysa corallina. – Fauvel, Reference Fauvel1916: 432–3, Pl.XI fig. 50–52
Marphysa aenea. – Orensanz, Reference Orensanz1990: 70, Pl.18, figs a–f. – Darbyshire, Reference Darbyshire2018: 31, 37.
Diagnosis
Ventral cirri on chaetigers 1–2. Branchiae present from chaetiger 10 to 14, single filament. Supra-acicular limbate chaetae and bidentate compound falcigers with short blade on all parapodia. Anterior chaetigers with isodont pectinate chaetae with thin shafts, narrow blade, and short teeth; median chaetigers with isodont pectinate chaetae with thin shafts and narrow and wide blades. Posterior chaetigers with isodont pectinate chaetae with thin shafts and wide blade and up to three asymmetrical anodont chaetae, with thick, short shaft and wide blade and up to 6 long, thick teeth. Subacicular hooks single, unidentate from chaetiger 16–34.
Material Examined
East Falkland: NMW.Z.2011.039.0233 (1); NMW.Z.2011.039.0234–235 (2); NMW.Z. 2011.039.0236 (1); NMW.Z.2012.082.0232–235 (8); NMW.Z.2012.082.0236–237 (4); NMW.Z.2012.082.0219, 0220, 0223 (24); NMW.Z.2012.082.0224 (1); NMW.Z.2012.082.0225–227 (3); NMW.Z.2012.082.0221 (1); NMW.Z.2012.082.0228 (2); NMW.Z.2015.002.0009 (1); West Falkland: NMW.Z.2012.082.0222, 0238–240 (6); NMW.Z.2012.082.0229 (1); NMW.Z.2012.082.0230–231 (4).
Comparative Material Examined
Marphysa corallina: NHMUK 1928.4.26.181 (1); Marphysa aenea: NHMUK 1963.3.1 (1).
Description
Complete specimens with 58–255 chaetigers, L10 = 2.15–16.25 mm, W10 = 0.5–6.25 mm, TL = 10–169 mm. Description based on best specimen (complete, best representing all characters: NMW.Z.2012.082.0232) with variations shown detailed in following section.
Prostomium bilobed, 1.5 mm long, 3.5 mm wide, lobes anteriorly rounded (Figure 14A, B); median sulcus shallow, reaching ⅓ length of prostomium, and ventral sulcus deep (Figure 14A, B). Palps, lateral and median antennae short, blunt (but see Variation below), reaching second peristomial ring where complete (Figure 14A). Palpo- and antennophores ring-shaped, short, thick; styles tapering, without articulation. Eyes present, situated between palps and lateral antennae.

Figure 14. Marphysa sp.: NMW.Z.2012.082.0225. (A) dorsal view; (B) ventral view; (C) lateral view; NMW.Z.2012.082.0226 (D) Maxillae; (E) lateral view of left maxillae II–IV; (F) mandibles; NMW.Z.2011.039.0234. (G) aciculae, chaetiger 71; (H) subacicular hook, chaetiger 71; (I) anodont pectinate chaeta, chaetiger 212; NMW.Z.2012.082.0231. (J) pygidium. Scale bars: A–C, 5 mm; D–F, J, 1 mm; G, 20 μm; H–I, 50 μm.
Peristomium slightly longer than prostomium, first ring nearly twice as long as second, separation between rings distinct on all sides (Figure 14A–C). Second peristomial ring slightly shorter than first chaetiger. Peristomial ventrolateral lips present as elevated surfaces (Figure 14B).
Maxillary apparatus (Figure 14D, E) with MF = 1 + 1, 6 + 5, 7 + 0, 4 + 6, 1 + 1. Maxillary carriers half the length of MI with pair of oval wings situated at lateral margins. MI forceps-like, maxilla with falcal arch extended, well developed; MII with distal teeth largest; MIII short, curved, with blunt teeth. Left MIV longer than wide, teeth blunt; right MIV with second and third teeth larger. MV rectangular, longer than wide (Figure 14E). Mandibles dark, rectangular with whitish cutting plates (Figure 14F).
Branchiae pectinate, up to 4 filaments, from chaetiger 14, absent from posterior fifth of body. Branchial filaments longer than notopodial cirri except in posteriormost parapodia.
Notopodial cirri without articulation; longer than ventral cirri, digitiform, decreasing in size after branchiae start. Prechaetal lobes as transverse folds in all chaetigers. Chaetal lobes rounded, with aciculae emerging in midline, longer than other lobes. Postchaetal lobes best developed in pre-branchial chaetigers then reduced in size, becoming shorter than acicular lobe by chaetiger 40. Ventral cirri elliptical in chaetigers 1–2, becoming globular with obtuse tip from chaetiger 4 onwards then more pad-like with small tip from start of branchiae; digitiform in posteriormost chaetigers. Aciculae blunt, straight, black (Figure 14G), up to 5 per parapodium, highest number of aciculae in pre-branchial chaetigers.
Limbate chaetae supra-acicular only. Pectinate chaetae present: isodont chaetae with thin shafts, narrow blade, and short teeth in both anterior and median chaetigers; wide blade isodont chaetae with thin shafts and short teeth in both median chaetigers and posterior chaetigers. Up to three asymmetrical anodont chaetae, with thick, short shaft and wide blade and up to 6 long, thick teeth (Figure 14H) present in posterior chaetigers also. Compound falcigers bidentate with short blade, both teeth blunt, distal marginally smaller, no significant variation along body. Subacicular hooks unidentate (Figure 14I), paler than aciculae, slightly curved distally, from chaetiger 33 (L), 31 (R), one per parapodium.
Pygidium with anus terminal. Two pairs of ventral cirri, dorsal pair longer than ventral pair (Figure 14J). Ventral pair missing in majority of specimens.
Variation
Several morphological features of Marphysa have previously been shown to vary relative to body size (Molina-Acevedo and Carrera-Parra, Reference Molina-Acevedo and Carrera-Parra2015, Reference Molina-Acevedo and Carrera-Parra2017) including first branchial chaetiger (12–24), number of branchial filaments (1–4), number of aciculae (1–6), and start of subacicular hooks (chaetigers 16–34) which frequently appear on different segments of an animal by 1–2 chaetigers.
Juveniles with less than around 60 chaetigers possessed a second pair of minute eyespots at the anterior of the prostomium (larger animals with single pair only as in main description) as well as occasionally lacking or having poorly developed lateral antennae. Palps and antennae on larger animals frequently appeared blunted giving a generally digitiform appearance, however the tips of the styles are easily broken.
Subacicular hooks were bidentate in smaller specimens of less than around 100 chaetigers, becoming unidentate thereafter, generally 1 per parapodium, occasionally 2. Aciculae were always unidentate, even in juveniles.
Remarks
Fauvel (Reference Fauvel1916) described specimens of Marphysa from a single intertidal location in the Falkland Islands, calling the species Marphysa corallina, a species originally described from Hawaii (Kinberg, Reference Kinberg1865). Orensanz (Reference Orensanz1990) later re-assigned Fauvel's specimens to Marphysa aenea, a species described from the Pacific coast of Chile, although he did not re-examine any of the specimens. One specimen from Fauvel's original description was available from Natural History Museum, London and was examined and confirmed as the same as those described here.
The specimens from the Falkland Islands are part of the Marphysa-aenea group (sensu Glasby and Hutchings, Reference Glasby and Hutchings2010) with composite falcigers only in the sub-acicular region and branchiae present into the posterior of the body (Group C2 of Fauchald, Reference Fauchald1970). Within that group, the deeply divided prostomium, dark (black) unidentate aciculae and pale subacicular hooks that are slightly curved distally, distinguish it from most other species in the group and place it as most similar to Marphysa aenea, Marphysa capensis (Schmarda, Reference Schmarda1861), Marphysa gayi Quatrefages, 1866 and Marphysa peruviana Quatrefages, 1866. Marphysa quadrioculata (Grube, Reference Grube1856) would also fall into this category however it was designated as indeterminable by Fauchald (Reference Fauchald1970) as well as being described as lacking branchiae.
In agreement with Orensanz (Reference Orensanz1990), M. corallina is discounted for the identification due to having bidentate aciculae (unidentate in Marphysa sp.) but M. aenea, described with bidentate subacicular hooks (unidentate in Marphysa sp. for equivalent sized specimens), can also be discounted. Unfortunately, the other comparable species (M. gayi, M. peruviana, M. capensis) are represented by very old descriptions lacking details and are in need of modern re-description. The type specimens of M. gayi and M. peruviana, housed in Paris, were unavailable for loan and no type of material of M. capensis could be located. Most, if not all, currently accepted diagnostic characters are missing from the descriptions and no genetic work has been carried out making a definitive identification impossible at the current time. Collaboration is now planned with other researchers looking into these species to provide a more definitive identification in the future.
Distribution
Around the Falkland Islands in intertidal and shallow water rocky habitats (0–18 m) (Figure 1).
Discussion
The majority of publications that have reported taxa of Eunicoidea from the Falkland Islands region date back to the 1960s and earlier. Since that time, taxonomy and knowledge of the two families have changed substantially, many new taxa have been described and others have been synonymized or revised and, consequently, much of the information in those early publications is out of date. The ten taxa reviewed here were represented by another 17 taxon names in the past that were either incorrectly used for them or changed since the original publications, resulting in a challenge for anyone attempting to determine the current situation.
The genus Kinbergonuphis currently includes around 40 species and is found worldwide, with most species reported from the western Atlantic Ocean in shallow and intertidal waters (Fauchald, Reference Fauchald1982a). Six species are recorded from the southwest Atlantic in particular, although only two of these were recorded specifically from the Falkland Islands region, Kinbergonuphis dorsalis in shallow slope depths (around 100 m, Monro, Reference Monro1930) and Kinbergonuphis oligobranchiata in deep water (1000 m+, Neal et al., Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020). The other four species, Kinbergonuphis fragilis (Kinberg, Reference Kinberg1865), Kinbergonuphis difficilis (Fauchald, Reference Fauchald1982b), Kinbergonuphis orensanzi (Fauchald, Reference Fauchald1982b), and Kinbergonuphis tenuis (Hansen, Reference Hansen1882) occur off northern Argentina and Uruguay. No Onuphidae have previously been recorded from shallow (50 m or less) or intertidal habitats around the Islands, although K. dorsalis has been recorded intertidally from Punta Arenas (Magellan Strait) and the Argentinean coast (Ehlers, Reference Ehlers1897; Hartmann-Schröder, Reference Hartmann-Schröder1962). Previous studies have shown a high similarity between Falkland Islands fauna and other areas of the Magellan biogeographic region (Knox and Lowry, Reference Knox, Lowry and Dunbar1977; Montiel et al., Reference Montiel, Gerdes, Arntz, Arntz, Lovrich and Thatje2005a, Reference Montiel, Gerdes, Hilbig and Arntz2005b; Darbyshire, Reference Darbyshire2018) as well as a high affinity between Falkland Islands and South Georgia polychaetes (Knox and Lowry, Reference Knox, Lowry and Dunbar1977). It might therefore be expected that, similar to K. dorsalis, other onuphid taxa that occur along the Atlantic coasts of Chile and southern Argentina also occur in the Falkland Islands. It is less likely to find the species reported from north of the Magellan region boundary. The majority of Kinbergonuphis species are either known only from their original type locality or local region and so K. dorsalis is unusual in its wide distribution, being recorded from the Pacific coastline of Chile (Wesenberg-Lund, Reference Wesenberg-Lund1962) as well as the Atlantic coasts of Chile and Argentina (Ehlers, Reference Ehlers1897; Monro, Reference Monro1936; Hartman-Schröder, Reference Hartmann-Schröder1962; Orensanz, Reference Orensanz1974a, Reference Orensanz1974b, Reference Orensanz1990) and the Falkland Islands. No other Kinbergonuphis taxa occur along the Magellan Atlantic coast, although others, as previously mentioned, are recorded north of the area as well as from the Pacific coast of Chile. The wide geographic distribution and variability of several characters, demonstrated by K. dorsalis, could indicate a species complex.
A recent study by Hektoen et al. (Reference Hektoen, Willassen and Budaeva2022) on Diopatra in the East Atlantic, found that despite the genus being well-studied globally, morphological similarities and high intraspecific variation of characters have led to diversity in the genus potentially being significantly underestimated. Using molecular techniques, the study recovered an additional 17 undescribed species, many of which were found to have restricted distributions. The findings are no longer unusual in the field of polychaete research with many species previously thought to be widely distributed, being instead found to represent multiple species with more narrow distributions, sometimes affecting species knowledge over a wide geographic area (e.g. Bleidorn et al., Reference Bleidorn, Kruse, Albrecht and Bartolomaeus2006; Barroso et al., Reference Barroso, Klautau, Solé-Cava and Paiva2010; Simon et al., Reference Simon, van Niekerk, Burghardt, ten Hove and Kupriyanova2019) but sometimes in more localized areas too (e.g. Nygren et al., Reference Nygren, Parapar, Pons, Meißner, Bakken, Kongsrud, Oug, Gaeva, Sikorski, Johansen, Hutchings, Lavesque and Capa2018; Grosse et al., Reference Grosse, Bakken, Nygren, Kongsrud and Capa2020). Although attempts to sequence COI in Kinbergonuphis sp. were unsuccessful, despite attempts with more than one set of primers, sequences of 16S were obtained. Unfortunately, in the case of Kinbergonuphis, genetic information is only available for one other species, K. pulchra (Budaeva et al., Reference Budaeva, Schepetov, Zanol, Neretina and Willassen2016), and so cannot help resolve the question of whether multiple species exist under the K. dorsalis species name. In the future though, perhaps those sequences obtained will be able to offer some help to other studies.
Except for O. pseudoiridescens and K. dorsalis, the other onuphid species reported by Orensanz (Reference Orensanz1974b), for the Atlantic portion of the Magellan region, were originally described from Pacific or northern hemisphere localities. All have since been re-assigned (Orensanz, Reference Orensanz1990) to other southern hemisphere species (Table 1) whose distribution in the region is more generally accepted or, in the case of H. artifex, re-described as a new species.
Other species that occur within close-by parts of the Antarctic and subantarctic that could be considered as having potential to occur within the Falkland Islands zone include one other species of Kinbergonuphis, Kinbergonuphis notialis (Monro, Reference Monro1930), found in Antarctic waters and the Scotia Sea, including South Georgia, but it is not known outside of the Antarctic convergence (Orensanz, Reference Orensanz1990). Other subantarctic taxa reported from the Scotia Sea, South Georgia, or other nearby subantarctic islands are, in fact, few. The removal of Hartman's 1967 record from the Falkland Islands now restricts the distribution of Paradiopatra antarctica (Monro, Reference Monro1930) to Antarctic and sub-Antarctic waters (Budaeva and Fauchald, Reference Budaeva and Fauchald2011). Paradiopatra ehlersi (McIntosh, Reference McIntosh1885), a widely distributed taxon around deep areas of the Southern Ocean and elsewhere, is not currently known from the South Atlantic above the subantarctic zone (Budaeva and Fauchald, Reference Budaeva and Fauchald2011). Additionally, a single unidentified species of Diopatra (for the most part, a warmer water genus: Paxton et al., Reference Paxton, Fadlaoui and Lechapt1995) was recorded from South Georgia (Orensanz, Reference Orensanz1990), Rhamphobrachium ehlersi Monro, Reference Monro1930 is also known from South Georgia and other subantarctic islands (Paxton, Reference Paxton1986b; Orensanz, Reference Orensanz1990) and Nothria abyssia Kucheruk, Reference Kucheruk1978 is recorded widely including from the Scotia Sea (Orensanz, Reference Orensanz1990) and the Atlantic sector of Antarctica (Budaeva and Paxton, Reference Budaeva and Paxton2013).
The situation in the Eunicidae is much the same as for Onuphidae, with very few additional species reported for the Magellan region, although more records are known for northern Argentina and the Pacific coast of Chile. Eunice magellanica was originally described from muddy sediment in the Magellan Strait (McIntosh, Reference McIntosh1885) and is also noted as having an association with Macrocystis holdfasts (Orensanz, Reference Orensanz1990), neither of which habitat was sampled around the Falkland Islands. Mud is uncommon but Macrocystis holdfasts offer a wealth of potential habitat that has not yet been investigated so it is feasible that the species could be present in the shallow waters of the Islands but so far unsampled. Hartman (Reference Hartman1964) synonymized E. magellanica with Eunice frauenfeldi (Hartman, Reference Hartman1964) until it was re-established by Fauchald (Reference Fauchald1992). Eunice frauenfeldi, though, was described from the Indian Ocean and is not considered a valid species for the region here, records more likely to have been incorrectly attributed due to the synonymy of E. magellanica. Further south, Leodice antarctica, as discussed earlier, is also considered unlikely to occur outside of the region due to the limited distribution capacity of most Eunice species (Zanol and Budaeva, Reference Zanol, Budaeva, Purschke, Westheide and Böggemann2021). No other Eunice or Leodice taxa are currently reported for the Magellan region, or any other Eunicidae genera except for Marphysa.
The identification of the Marphysa specimens collected from the Falkland Islands remains in question. Originally identified as M. corallina by Fauvel (Reference Fauvel1916) and then reassigned to M. aenea by Orensanz (Reference Orensanz1990), the species is shown here to clearly not be the latter either, although M. aenea is the only Marphysa species currently reported for the Magellan Atlantic coasts. Species of Marphysa have come under scrutiny in recent years following the re-description (Hutchings and Karageorgopoulos, Reference Hutchings and Karageorgopoulos2003) of the widely recorded and considered-to-be cosmopolitan species Marphysa sanguinea (Montagu, Reference Montagu1813), with that species now considered far more restricted in its distribution. In South Africa too, another place where M. sanguinea had previously been recorded but is now known to be a different species (Lewis and Karageorgopoulos, Reference Lewis and Karageorgopoulos2008), research is showing that other Marphysa species recorded there have been historically mis-identified and have more restricted distributions than previously believed (Kara et al., Reference Kara, Molina-Acevedo, Zanol, Simon and Idris2020). One of those species, M. capensis, is part of the poorly described group that is most similar to the Falkland Islands specimens. It is hoped that collaboration with researchers in South Africa, as well as South America where the other closely related but poorly defined species M. gayi and M. peruviana originate, albeit from the Pacific, may resolve taxonomic issues for all the species. It seems apparent that Marphysa species generally, are not found over wide geographic areas, but those from South Africa and South America must still be investigated appropriately and so it is essential to get new morphological and molecular data to resolve the situation.
The lack of clarity in some of the species included in this review is a reflection of the problems of working with damaged or juvenile specimens where that is the only material available. Many of the Neal et al. (Reference Neal, Paterson, Blockley, Scott, Sherlock, Huque and Glover2020) specimens are from deep water sites where retrieval of good quality specimens is more challenging, some taxa were small-bodied or juvenile and generally few specimens were available to enable a comprehensive comparison of characters. Polychaete diversity in the deep sea is known to be highly underestimated and deep sea investigations often report large numbers of new species (e.g. Fiege et al., Reference Fiege, Ramey and Ebbe2010; Brasier et al., Reference Brasier, Wiklund, Neal, Jeffreys, Linse, Ruhl and Glover2016) with mis-identifications common due to physically damaged specimens and the pressures involved in identifying large numbers of specimens in a restricted timeframe (Brasier et al., Reference Brasier, Wiklund, Neal, Jeffreys, Linse, Ruhl and Glover2016). The vast majority of specimens collected are also fixed or preserved with formaldehyde or other denaturing solutions, as was the case here for all the comparative material borrowed from other institutions, rendering them unusable for genetic studies that might have mitigated the problem of damaged or juvenile specimens. The correction of several mis-identified specimens, the description of a new species of Hyalinoecia, and a thorough review of the current names of confirmed taxa will help to improve current and future knowledge of the Eunicoidea in the Falkland Islands and facilitate more accurate identification. It is to be hoped that the information provided here may also prompt further research or provide a stepping stone for further descriptions if new specimens become available. The fauna of the Falkland Islands region is shown to be more diverse than previously recognized and potentially harbours undescribed species.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315423000966.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or its supplementary materials].
Acknowledgements
The work in the Falkland Islands was funded by both the Shackleton Scholarship Fund (2011, 2013; Falkland Islands) and Amgueddfa Cymru–Museum Wales (2011, 2013, 2015) with additional logistical support provided by the Falkland Islands Government Fisheries Department, the Shallow Marine Surveys Group (SMSG) and the South Atlantic Environmental Research Institute (SAERI). Thanks are also due to Emma Sherlock (Natural History Museum, London), Jenna Moore (Zoological Museum, Hamburg) and Karen Osborn and Bill Moser (Smithsonian Institution) for facilitating access to and loan of specimens; to Joana Zanol for advice and assistance with sequencing of Kinbergonuphis and Isabel Molina-Acevedo and Jyothi Kara for advice on Marphysa morphology and identification. Also to the anonymous referees for their extensive comments and advice that improved the quality of this manuscript.
Author contributions
Teresa Darbyshire: collection and preliminary identification of specimens, project concept, DNA sequencing and analysis, review of Kinbergonuphis specimens and description of all non-Kinbergonuphis specimens; manuscript preparation. Jacob Cameron: analysis, imaging and illustration of Kinbergonuphis specimens; DNA sequencing and analysis; manuscript preparation
Financial support
This work was supported by two Shackleton Scholarship Fund grants (2011 and 2013, grant numbers not assigned).
Conflict of interest
The authors declare none.
Ethical standards
No vertebrates or regulated invertebrates were involved in this study.



















