Portugal has high fish and seafood consumption, and it is among the European countries with the highest intake of fishery and aquaculture products(1,2) . Fish/seafood is nutrient-dense foods, rich in high biological value proteins, n-3 long-chain PUFA (LCPUFA) and micronutrients such as iodine, Se and vitamins A and D, but are also a source of contaminants, such as methylmercury (MeHg). Thus, fish/seafood consumption is commonly associated with both benefits and risks concerning human health(Reference Hoekstra, Hart and Owen3–Reference Cardoso, Bandarra and Lourenço9).
There is convincing evidence for an effect of n-3 LCPUFA from fish/seafood on the reduction of CHD mortality(Reference Mozaffarian and Rimm4,5,10) and the neurodevelopment improvement in infants and young children derived from mother’s fish consumption during pregnancy(5,10) . Other benefits have also been suggested to be associated with fish consumption, namely, a probable effect on stroke incidence, a possible effect on depression and some, although insufficient, evidence concerning the incidence of some cancers(5).
On the contrary, exposure to MeHg during pregnancy is associated with adverse neurodevelopmental outcomes in infants and young children, since MeHg crosses the placental and blood-brain barriers, causing oxidative damage to the developing fetal central nervous system(Reference Clarkson and Magos11–13). Convincing evidence from epidemiological studies supports the deleterious effect of MeHg exposure during pregnancy on neurodevelopment and has been used to establish tolerable exposure levels(13). Thus, several European countries have advised pregnant women to balance their weekly fish intake and to avoid eating large predatory and older fishes, which typically have higher levels of MeHg occurrence(6).
Prior quantitative evidence suggests that the benefits of increasing fish consumption outweigh the risks(Reference Hoekstra, Hart and Owen3–5,10,Reference Thomsen, Pires and Devleesschauwer14–Reference Cohen, Bellinger and Connor18) ; however, those studies are usually performed in populations where fish consumption is low, contrasting with the Portuguese reality.
Considering the broad variety of contaminants’ levels within and between fish/seafood species and the consumption variability in different countries, European Food Safety Authority (EFSA) recommends that each country considers its pattern of fish/seafood consumption, especially the species consumed, and carefully assess the risk of exceeding the tolerable weekly intake (TWI) of methylmercury while obtaining the health benefits from consuming fish/seafood(6).
Thus, this study aims to quantify the health impact of different fish/seafood consumption scenarios on a high fish consumption population through a quantitative risk–benefit assessment (RBA) of several scenarios of fish consumption, combining the selected effects into a composite metric, the disability-adjusted life years (DALY). Furthermore, this study aims to evaluate and characterise the exposure to the hazardous MeHg and beneficial n-3 LCPUFA, namely EPA and DHA, in the Portuguese population using national representative consumption data from the Portuguese National Food and Physical Activity Survey (IAN-AF 2015–2016)(Reference Lopes, Torres and Oliveira19,Reference Lopes, Torres and Oliveira20) . Finally, the conclusions of this assessment will be considered to tailor Portuguese consumption advice for ffish/seafood consumption, as a major risk management instrument for fully achieving its beneficial effects whilst limiting the risks of mercury toxicity.
Methods
Study population
For this study, we used data from the IAN-AF 2015–2016 survey. Briefly, IAN-AF 2015–2016 is a national survey of the non-institutionalised Portuguese general population. It is composed of a sample of 5811 individuals from 3 months to 84 years of age that completed two dietary assessments. The sampling frame used to select the participants in this survey was the Portuguese National Health Registry, and the selection was performed by multistage sampling stratified by the seven Statistical Geographic Units of Portugal (NUTS II). Additionally, the sample was weighed according to sex and age group (< 1 year, 1–2 years, 3–9 years, 10–17 years, 18–34 years, 35–64 years, 65–74 years and 75–84 years) to be representative of the Portuguese population. Further details of the IAN-AF 2015–2016 methodology are described elsewhere(Reference Lopes, Torres and Oliveira19,Reference Lopes, Torres and Oliveira20) .
Data collection and dietary assessment
Two computer-assisted interviews were performed by trained dietitians using an electronic platform designed for the survey (‘You eAT&Move’), to collect socio-demographic, health-related, food intake and physical activity data. Data collection procedures followed the European guidelines from the EU-Menu project, to be harmonised with other countries surveys(21).
Dietary assessment of children, aged under 10 years, was accomplished by two non-consecutive, one-day food diaries that were filled in by the main caregiver. Following this, a face-to-face interview was conducted with the caregivers to collect additional details in food description and quantification. For the remaining age groups, dietary intake was obtained by two non-consecutive 24-h recalls, applied in a face-to-face interview separated by 8–15 d.
Detailed information and quantification of foods, recipes and supplements reported by the participants were collected using a validated electronic assessment tool, the eAT24 software(Reference Goios, Severo and Lloyd22). All foods reported by the participants were then categorised into food groups. Recipes were disaggregated into their components, and single food items were allocated to their respective food group.
Ethical standards
IAN-AF 2015–2016 was conducted according to the guidelines laid down in the Declaration of Helsinki and national legislation. All procedures involving human subjects were approved by the Portuguese National Commission for Data Protection and the Ethical Committee of the Institute of Public Health of the University of Porto. The participants were asked to provide their written informed consent and all documents with identification data were treated separately and stored in a different dataset.
Occurrence data of risk–benefit agents
National data on the occurrence of mercury (Hg) and MeHg in fish and seafood captured in Portuguese waters and marketed in Portugal (total n 1188 samples) were retrieved from the Portuguese National Sampling Plan(23) (n 693), carried out on an annual basis by the Portuguese Economic and Food Safety Authority (ASAE) and from databases of other Portuguese entities(Reference Afonso, Bernardo and Bandarra8) (n 495). To avoid underestimating MeHg exposure, we used a conservative approach by assuming that 100 % of Hg in fish/seafood is in the form of MeHg. Whenever data were left-censored, we used a middle-bound approach, assuming half of the value of the limit of detection or the limit of quantification.
Regarding EPA and DHA, national data (n 126 samples) were available only for a small share of the fish/seafood species consumed, thus, we retrieved information for raw food items from the FAO/INFOODS Global Food Composition Database for Fish and Shellfish Version 1·0(24) (n 134) and from the USDA National Nutrient Database for Standard Reference Legacy Release, April 2018 (n 3832)(Reference Haytowitz, Ahuja and Wu25).
Occurrence data of MeHg, EPA and DHA were available for more than 90 % of fish/seafood species consumed by the Portuguese population. All the food items included in the occurrence datasets were classified with the FoodEx2 classification system.
Scenarios’ definition
Six alternative scenarios of fish/seafood consumption were considered to compare the health risks and benefits with the current fish/seafood consumption, which is considered as the reference scenario. The characteristics of each scenario are described in detail in Table 1.
Table 1. Fish/seafood consumption scenarios characterisation
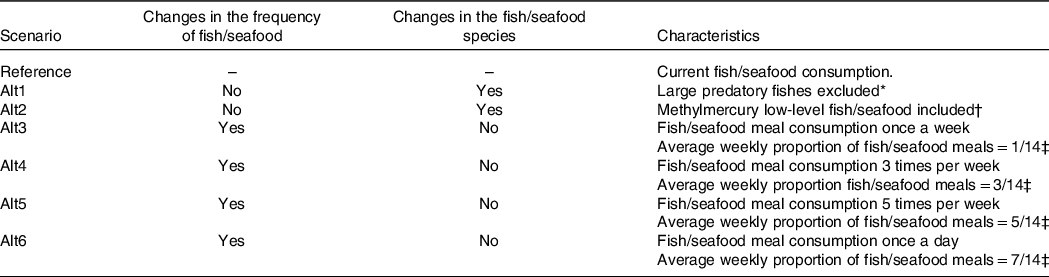
* Large predatory fishes considered: fresh tuna, rays, sharks, swordfish, scabbard fishes.
† Methylmercury low-level fishes: anchovies, Atlantic mackerel, cod, meagre, forkbeard, hake, horse-mackerel, monkfish, perch, pollock, pouting, rays, red mullet, salmon, sardines, Gilthead seabream, European seabass, sole, octopus, squid, mussels, clams, cockle, oyster, shrimp, lobster, crab, canned tuna, canned sardines, canned mackerel.
‡ Two daily meals considered, lunch and dinner, which results in fourteen meals per week.
First, we considered two alternative scenarios where the amount and frequency of fish/seafood were equal to the reference scenario, changing only the type of fish consumed. Thus, in the first alternative scenario (alt1), the consumption of large predatory fish species (see Table 1) was replaced by other fish species. A second, more conservative, alternative scenario (alt2) was defined replacing the consumption of fish/seafood with MeHg levels > 0·25 mg/kg by species with MeHg levels ≤ 0·25 mg/kg (Table 1). All the replacements were implemented according to the probability of consumption of the fish/seafood species within the Portuguese population, according to sex, age group and geographic region.
Another set of scenarios (alt3 to alt6) were created to represent different weekly frequencies of fish/seafood consumption: alt3 – once a week; alt4 – three times/week; alt5 – five times/week; and alt6 – seven times/week. We considered that the majority of fish/seafood consumption occurs mostly at lunch or dinner, and, in the reference scenario, we categorised the meal types at lunch and dinner in three possible categories. The categories considered were ‘Fish/Seafood’ (i.e. if any item consumed in the meal was from the fish/seafood food group), ‘Meat’ (i.e. if any item consumed in the meal was from the meat food group) or ‘Others’ (i.e. if no meat nor fish items were consumed in the meal, this category included egg meals and vegetarian meals). If both meat and fish or seafood items were part of the meal, the classification was based on the food category present in greater amount. Hence, for each alternative scenario, we replaced entire meals with other types to achieve the target weekly frequency of fish/seafood consumption (Table 1). The type of meal to be selected was modelled using a time-homogeneous Markov multistate model(Reference Jackson26), in which the ratio between ‘Meat’ meals and ‘Others’ meals was kept constant, regardless of the average weekly proportion of ‘Fish/seafood’ meals priorly defined for each scenario. Then, the content of each entire meal was imputed, at an individual and eating occasion level, based on the consumption of each meal type in the Portuguese population by sex, age group and geographic region.
All the statistical analyses described in this and throughout the following subsections were performed using R software version 3.4.1 for Windows(27). All results are representative of the Portuguese population and were estimated using the library ‘survey’(Reference Lumley28) from R software.
Exposure assessment to risk–benefit agents
To assess the exposure to MeHg, EPA and DHA, individual two-day food consumption data from the IAN-AF 2015–2016 was matched to the occurrence data using the FoodEx2 classification hierarchy system. Different values of MeHg, EPA and DHA within the occurrence datasets were randomly assigned each time a food item was reported in IAN-AF survey to deal with variability observed in the occurrence data. The attribution process was as follows. If more than one occurrence value matched a single consumption occasion or FoodEx2 code, one value was randomly selected. On the contrary, if there was not a direct match to one specific consumption occasion, an occurrence value from the closest item was selected, using the FoodEx2 hierarchy. Regarding EPA and DHA, besides fish/seafood consumption occasions, we applied the previously described methodology for the remaining food groups. All analyses were performed at the ingredient level, considering its raw weight. The exposure was then aggregated by day, and the two-day average individual exposure was estimated. The estimated population exposure was expressed as the mean daily intake for EPA and DHA and as the mean weekly exposure per kg of body weight (bw) for MeHg. Additionally, it was estimated the prevalence of the population at risk due to MeHg exposure, i.e. the percentage of the population that exceeded the TWI of 1·3 μg/kg bw(13).
This imputation process was repeated 10 times for each scenario and results were combined using Rubin’s rules(Reference Rubin29).
Health effects and disability-adjusted life year calculations
Identification and selection of health effects
To estimate the health impact of the scenarios, we first reviewed official assessments from the European Food Safety Authority (EFSA) and other institutions(5,10,13,30) to identify the most relevant effects associated with fish/seafood, its components, and meat, as the scenarios alt3-alt6 also reflect changes in meat consumption due to substitutions. Then, the health effects (HE) to be included in this RBA were selected based on the degree of evidence on the associations with the foods and components under study. The associations that were graded as convincing in the official reports were included. Finally, the measures of association to be used (Dose-Response/RR) were collected from the literature. Table 2 presents the selected HE, the population group in which the RBA was performed, and the dose-response approach applied.
Table 2. Health effects associated with the selected foods and components and data inputs for the risk–benefit assessment
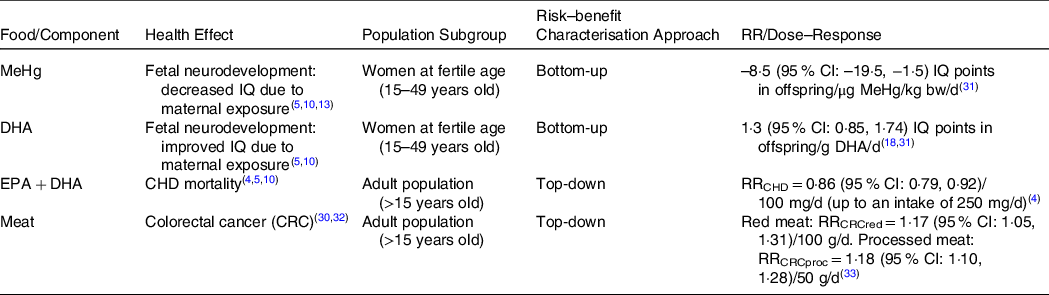
MeHg, methylmercury; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Quantification of scenarios health impact: DALY estimate
To quantify the health impact of the scenarios, we estimated the burden of disease using DALY for each HE, as expressed in the following equation:
YLD stands for years of life lived with disability, calculated as
where
![]() $I$
is the annual incidence of the HE in the population,
$I$
is the annual incidence of the HE in the population,
![]() ${\rm{DW}}$
is the disability weight for the HE and
${\rm{DW}}$
is the disability weight for the HE and
![]() $L$
is the average duration of the HE until remission or death, in years. A
$L$
is the average duration of the HE until remission or death, in years. A
![]() ${\rm{DW}}$
represents the magnitude of health loss associated with the outcome and in this paper
${\rm{DW}}$
represents the magnitude of health loss associated with the outcome and in this paper
![]() ${\rm{DWs}}$
were derived from the ones computed by the Global Burden of Disease 2017 study (GBD 2017)(34).
${\rm{DWs}}$
were derived from the ones computed by the Global Burden of Disease 2017 study (GBD 2017)(34).
YLL stands for years of life lost due to the HE under study and is calculated as
where
![]() $N$
is the annual number of deaths associated with the HE and
$N$
is the annual number of deaths associated with the HE and
![]() $RLE$
is the remaining life expectancy at the age of death, in years.
$RLE$
is the remaining life expectancy at the age of death, in years.
DALY for the reference scenario and their respective 95 % confidence interval (CI) were estimated considering the current values of incidence and mortality for the HE in the Portuguese population, assuming that it reflects the current intake of MeHg, EPA and DHA and red/processed meat. Depending on the available data, top-down and bottom-up approaches were applied to estimate the incidence and the mortality of the selected HEs considering the distributions of exposure to MeHg, EPA and DHA and red/processed meat in the different scenarios, as shown in Table 2. For the associations between the intake of EPA + DHA and Coronary Heart Disease (CHD) mortality, and the intake of red and processed meats and colorectal cancer incidence, top-down approaches were applied, since risk estimates (RR) from epidemiological studies were available. For the neurodevelopment outcome in offspring due to the maternal exposure to MeHg and DHA, where no risk estimates were available from the literature, a bottom-up approach was applied using dose-response models. The summary of the RR and dose-response inputs from the literature used is presented in Table 2, and the remaining data inputs used to calculate DALY for each health effect are given in the Appendix.
The difference in DALY between each alternative scenario and the reference scenario (ΔDALYalt), from all the HE, reflects the health impact of the change in the consumption of fish/seafood in each alternative scenario. If, a health loss is expected from the change in fish/seafood consumption. On the contrary, if , the change in fish/seafood consumption for the alternative scenario results in a populational health gain.
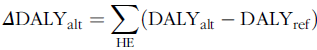 $$\it\Delta {\rm{DAL}}{{\rm{Y}}_{{\rm{alt}}}} = \mathop \sum \limits_{{\rm{HE}}} ({\rm{DAL}}{{\rm{Y}}_{{\rm{alt}}}} - {\rm{DAL}}{{\rm{Y}}_{{\rm{ref}}}})$$
$$\it\Delta {\rm{DAL}}{{\rm{Y}}_{{\rm{alt}}}} = \mathop \sum \limits_{{\rm{HE}}} ({\rm{DAL}}{{\rm{Y}}_{{\rm{alt}}}} - {\rm{DAL}}{{\rm{Y}}_{{\rm{ref}}}})$$
Bottom-up approach: methylmercury and DHA v. fetal neurodevelopment
To assess the effect of maternal exposure to MeHg and DHA on foetal neurodevelopment, we used cognitive impairment as the outcome, measured by the intelligence quotient (IQ). According to IQ definition, we assumed that, in the reference scenario, the Portuguese population IQ follows a normal distribution, with a mean of 100 and a standard deviation of 15, reflecting the current fish/seafood consumption. In the alternative scenarios, the respective changes in the fish/seafood consumption by the mothers will impact children’s IQ, according to the dose-response functions used(Reference Cohen, Bellinger and Connor18,Reference Zeilmaker, Hoekstra and van Eijkeren31) , causing a shift in the IQ distribution curve across the population of new-born children.
Different IQ values reflect different levels of disability according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR)(35) and the 10th revision of the International Classification of Diseases (ICD-10)(36). The five classes of cognitive impairment considered are borderline intellectual functioning (IQ: 70–84), mild intellectual disability (ID) (IQ: 50–69), moderate ID (IQ: 35–49), severe ID (IQ: 20–34) and profound ID (IQ: <20), with each class reflecting a specific DW according to GBD 2017(34) (see online supplementary material, Supplemental Table S5).
To estimate
![]() ${\rm{DAL}}{{\rm{Y}}_{{\rm{IQ}}}}\;$
we considered that no increased mortality is expected from this outcome, thus,
${\rm{DAL}}{{\rm{Y}}_{{\rm{IQ}}}}\;$
we considered that no increased mortality is expected from this outcome, thus,
![]() ${\rm{YL}}{{\rm{L}}_{{\rm{IQ}}}}\,{\rm{ = }}\,0$
and also no recovery is expected for a child with a low IQ level, thus the duration of the outcome (
${\rm{YL}}{{\rm{L}}_{{\rm{IQ}}}}\,{\rm{ = }}\,0$
and also no recovery is expected for a child with a low IQ level, thus the duration of the outcome (
![]() ${L_{IQ}}$
) would be equal to Portuguese life expectancy at birth (80·8 years)(37).
${L_{IQ}}$
) would be equal to Portuguese life expectancy at birth (80·8 years)(37).
![]() ${\rm{YL}}{{\rm{L}}_{{\rm{IQ}}}}$
, DWs for the classes and the duration of the effect are equal for all scenarios, thus, the difference in DALY between scenarios depends on the different incidences of each cognitive impairment class in the scenarios. To estimate the number of children within each class of impairment, we combined information of the fertility rates of the Portuguese women by age group(38) (see online supplementary material, Supplemental Table S1) and the number of women at fertile age at each age group(39) (see online supplementary material, Supplemental Table S1), with the probability of impairment, given by the IQ distributions from each scenario.
${\rm{YL}}{{\rm{L}}_{{\rm{IQ}}}}$
, DWs for the classes and the duration of the effect are equal for all scenarios, thus, the difference in DALY between scenarios depends on the different incidences of each cognitive impairment class in the scenarios. To estimate the number of children within each class of impairment, we combined information of the fertility rates of the Portuguese women by age group(38) (see online supplementary material, Supplemental Table S1) and the number of women at fertile age at each age group(39) (see online supplementary material, Supplemental Table S1), with the probability of impairment, given by the IQ distributions from each scenario.
Uncertainty in dose-response functions and DWs was described as PERT distributions and variability in the exposure to MeHg and DHA for each scenario as Gamma distributions. Second-order Monte Carlo simulation was used for DALY calculations with 1000 simulations for variability and 1000 iterations for uncertainty.
Top-down approach: EPA + DHA v. coronary heart disease mortality (CHD) and Red/Processed Meat v. colorectal cancer (CRC)
Regarding the top-down approaches, to account for EPA + DHA and red/processed meat intake variability, we divided the respective distributions into quartiles with each quartile representing an intake class (1–4). The intake of each class was set as the median value within each class. The RR for the effects (Table 2) was used to estimate a RR for each class, assuming a RR of 1 at zero consumption and a log-linear association between exposure and RR(Reference Berlin, Longnecker and Epidemiology40). Thus, the log-linear slope, β, and the RR for each class, j ∈ {1,2,3,4}, in each scenario, i ∈ {1,2,3,4,5}, were calculated according to the following equations:
To measure the fraction of DALY due to CHD and colorectal cancer (CRC) that could be altered by a given change in the intake of EPA + DHA and red and processed meat, respectively, the Potential Impact Fraction (PIF) was calculated. PIF was calculated for each alternative scenario by the RR shift methodology(Reference Barendregt and Veerman41) which assumes that the interventions are described by a change in the RR of the categories while keeping the proportion in each category constant:
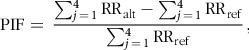 $${\rm{PIF}} = \;\displaystyle{{\sum\nolimits_{j \,= \,1}^4 {\rm{R}} {{\rm{R}}_{{\rm{alt}}}} - \sum\nolimits_{j\, = \,1}^4 {\rm{R}} {{\rm{R}}_{{\rm{ref}}}}} \over {\sum\nolimits_{j \,= \,1}^4 {\rm{R}} {{\rm{R}}_{{\rm{ref}}}}}},$$
$${\rm{PIF}} = \;\displaystyle{{\sum\nolimits_{j \,= \,1}^4 {\rm{R}} {{\rm{R}}_{{\rm{alt}}}} - \sum\nolimits_{j\, = \,1}^4 {\rm{R}} {{\rm{R}}_{{\rm{ref}}}}} \over {\sum\nolimits_{j \,= \,1}^4 {\rm{R}} {{\rm{R}}_{{\rm{ref}}}}}},$$
where RRref is the relative risk in the reference scenario and RRalt is the relative risk in each alternative scenario.
To estimate DALY due to CHD deaths in the reference scenario, it was assumed immediate death, thus,
![]() ${\rm{YL}}{{\rm{D}}_{{\rm{CHD}}}}\,{\rm{ = }}\,0$
. Regarding
${\rm{YL}}{{\rm{D}}_{{\rm{CHD}}}}\,{\rm{ = }}\,0$
. Regarding
![]() ${\rm{YL}}{{\rm{L}}_{{\rm{CHD}}}}$
estimate, we used CHD mortality rate in Portuguese population by age group(42) and the number of individuals in each age group(39) (see online supplementary material, Supplemental Table S2) to estimate the number of deaths, and the life-expectancy for the mean age in each age group to estimate
${\rm{YL}}{{\rm{L}}_{{\rm{CHD}}}}$
estimate, we used CHD mortality rate in Portuguese population by age group(42) and the number of individuals in each age group(39) (see online supplementary material, Supplemental Table S2) to estimate the number of deaths, and the life-expectancy for the mean age in each age group to estimate
![]() $RLE$
(37) (see online supplementary material, Supplemental Table S4).
$RLE$
(37) (see online supplementary material, Supplemental Table S4).
Concerning CRC, the DALY in the reference scenario were estimated using a three-stage model based on the methodological framework proposed by Soerjomataram et al.
(Reference Soerjomataram, Lortet-Tieulent and Ferlay43), illustrated in Fig. 1, and we assumed that all incident cases pass through a phase of diagnostic and treatment (p
1
). Incidence and mortality of CRC in the Portuguese population, for both sexes and several age groups, were retrieved from IARC(Reference Ferlay, Ervik and Lam44–Reference Ferlay, Colombet and Soerjomataram46) (see online supplementary material, Supplemental Table S3),
![]() ${\rm{DWs}}$
for the several stages of CRC were retrieved from GBD 2017 study(34) (see online supplementary material, Supplemental Table S5), the average duration of each stage was obtained in the cancer DALY framework study(Reference Soerjomataram, Lortet-Tieulent and Ferlay43) (see online supplementary material, Supplemental Table S6) and the remaining life expectancy in the case of death (
${\rm{DWs}}$
for the several stages of CRC were retrieved from GBD 2017 study(34) (see online supplementary material, Supplemental Table S5), the average duration of each stage was obtained in the cancer DALY framework study(Reference Soerjomataram, Lortet-Tieulent and Ferlay43) (see online supplementary material, Supplemental Table S6) and the remaining life expectancy in the case of death (
![]() $RLE$
) was estimated for the mean age at each age group considering the life expectancy for that age in the Portuguese population(37) (see online supplementary material, Supplemental Table S4). Regarding long-term sequelae, we considered that 13 % of CRC survivors will live until death with a stoma, according to what was described by Soerjomataram et al.
(Reference Soerjomataram, Lortet-Tieulent and Ferlay43).
$RLE$
) was estimated for the mean age at each age group considering the life expectancy for that age in the Portuguese population(37) (see online supplementary material, Supplemental Table S4). Regarding long-term sequelae, we considered that 13 % of CRC survivors will live until death with a stoma, according to what was described by Soerjomataram et al.
(Reference Soerjomataram, Lortet-Tieulent and Ferlay43).
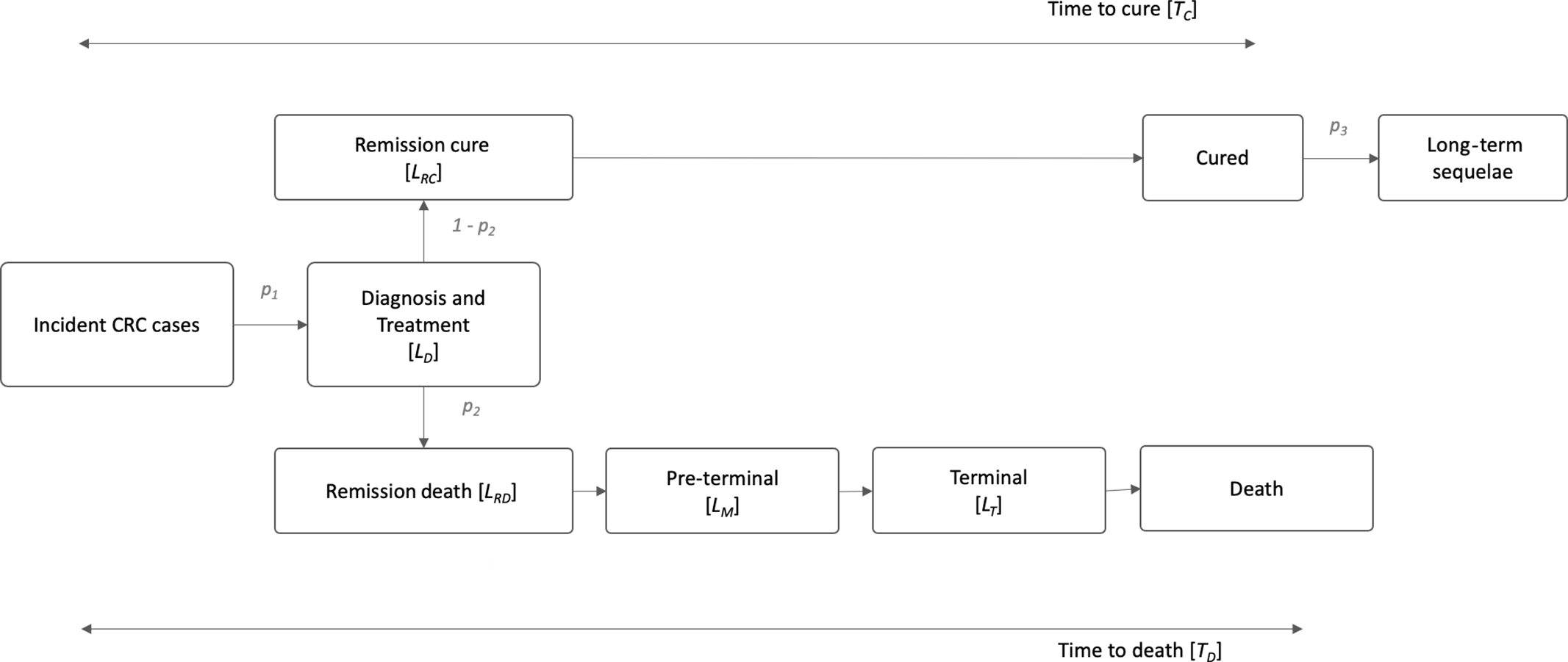
Fig. 1. Three-stage natural history for colorectal cancer (CRC), based on Soerjomataram et al. (2012). LD: duration of diagnosis and treatment; LR: duration of remission; LM: duration of preterminal/metastatic phase; LT: duration of terminal phase; p1: incidence of CRC; p2: case fatality of CRC; p3: probability of long-term sequelae; Tc=7 years and TD = 1·6 years.
We calculated the annual DALY change due to the differences in the intake of EPA + DHA and red and processed meat in the alternative scenarios by multiplying the estimated PIFs for each health effect in each alternative scenario by the DALY values previously calculated for the reference scenario. Uncertainty in RR values and DWs was described as PERT distributions, and Monte Carlo simulation was used for DALY calculations with 1000 iterations. The DALY estimation is represented by the median and the 95 % CI for uncertainty.
Results
Exposure to risk–benefit assessment agents
Methylmercury
The average MeHg concentration of fish and other seafood samples considered in this study was 0·25 mg/kg (range: 0·00–4·40 mg/kg; median: 0·06 mg/kg). The distribution of MeHg concentrations observed in the different species is presented in Supplemental Fig. S1 (Appendix).
Results on the weekly exposure to MeHg from fish/seafood consumption in the various scenarios are presented in Table 3. The current mean weekly exposure to MeHg is 0·65 µg/kg bw for the Portuguese general population, increasing significantly in children up to 5-years-old. These values are associated with a prevalence of exposure above the TWI of 13·7 % (95 % CI: 12·0, 15·4) for the general population (Table 4), being higher among young children from 2–5 years of age (36·6 %, 95 % CI: 26·9, 46·3). The exposure in the reference scenario represents an average frequency of consumption between 3–5 times/week.
Table 3. Mean exposure to methylmercury (MeHg) (µg/kg bw/week) and EPA + DHA (mg/d) in the Portuguese population for the fish/seafood consumption scenarios and respective 95 % confidence interval (95 % CI)
(Mean values and 95 % confidence intervals)
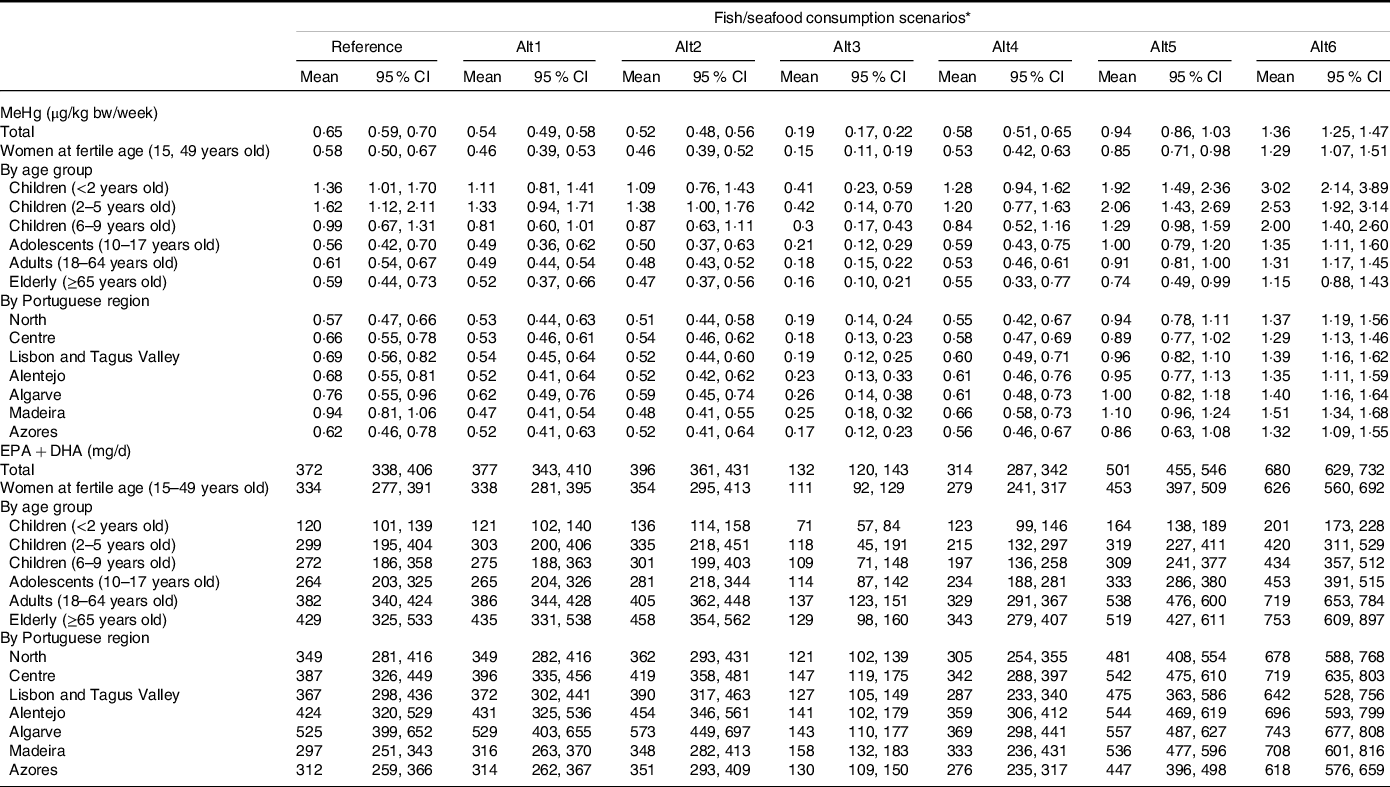
* Alt1: predatory fishes excluded; Alt2: MeHg low-level fishes included; Alt3: fish/seafood meal consumption once a week; Alt4: fish or seafood meal consumption 3×/week; Alt5: fish or seafood meal consumption 5×/week; Alt6: Fish or Seafood meal consumption 7×/week.
Table 4. Prevalence of methylmercury (MeHg) exposure above the tolerable weekly intake (TWI) in the Portuguese population for the fish/seafood consumption scenarios and respective 95 % confidence interval (95 % CI)
(Mean values and 95 % confidence intervals)
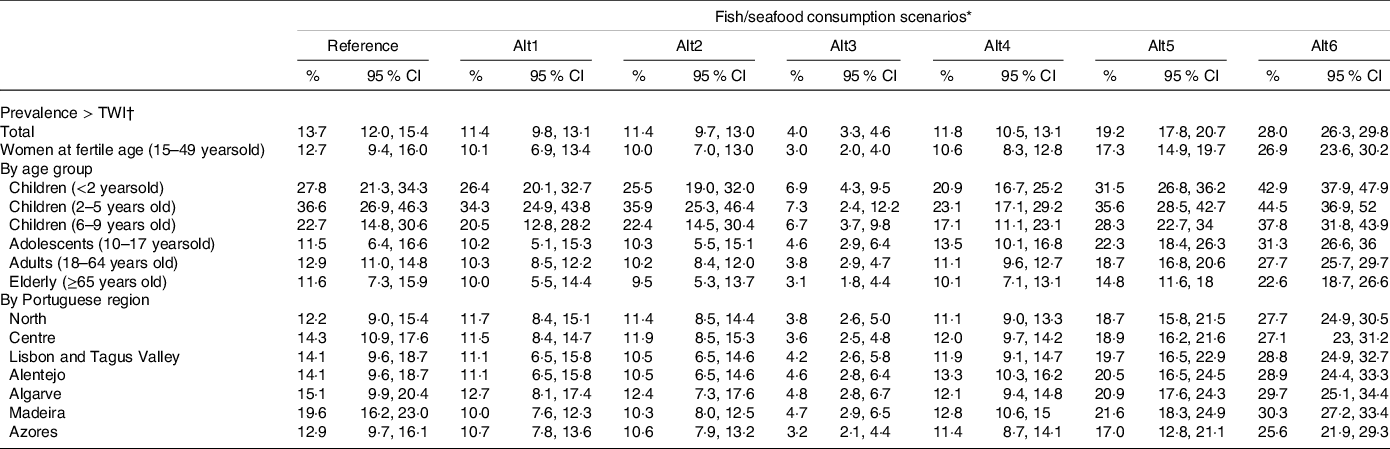
* Alt1: predatory fishes excluded; Alt2: MeHg low-level fishes included; Alt3: fish/seafood meal consumption once a week; Alt4: fish or seafood meal consumption 3×/week; Alt5: fish or seafood meal consumption 5×/week; Alt6: fish or seafood meal consumption 7×/week.
† TWI = 1·3 µg MeHg/kg bw/week.
Replacing certain fish species by species with lower MeHg levels (scenarios alt1 and alt2) does not considerably lower the prevalence of exposure higher than the TWI, considering the general population or the different age groups. However, at the regional level, the Madeira region would benefit from the fish species replacement, decreasing the prevalence of exposure above the TWI from 19·6 % (95 % CI: 16·2, 23·0) in the reference scenario to 10·0 % in alt1 (95 % CI: 7·6, 12·3) or 10·3 % (95 % CI: 8·0, 12·5) in alt2 (Table 4). As expected, by reducing the number of fish/seafood consumption occasions to once a week (alt3 scenario), the prevalence of exposure above the TWI decreases to 4·0 % (95 % CI: 3·3, 4·6) in the general population. On the other hand, in the alt5 and alt6 scenarios, the prevalence of population with exposure levels above the TWI increases.
EPA + DHA
The average concentration of EPA + DHA in the fish and other seafood samples used in this study was 0·70 g/100 g (range: 0·00–7·87 g/100 g; median: 0·33 g/100 g). The distribution of EPA + DHA concentrations observed in the different species is presented in Supplemental Fig. S1 (Appendix). Regarding the other food groups, the average concentration of these fatty acids observed was close to 0 g/100 g.
The current mean daily intake of EPA + DHA is 372 mg (95 % CI: 338, 406) for the general Portuguese population (Table 3), which is higher than the value of Adequate Intake (AI), defined for these nutrients (250 mg/d). Replacing fish/seafood consumed with lower MeHg contaminated species (alt2 scenario) slightly increases the mean daily intake of n-3 LCPUFA.
Considering the change in the frequency of fish/seafood consumption (scenarios alt3-alt6), consuming it only once a week would significantly decrease mean EPA + DHA intake to an average level lower than the AI. This level would increase when consuming fish/seafood 5 or 7 times a week (Table 3).
Health effects and DALY calculations
Table 5 presents the results of ΔDALY estimates for the alternative scenarios by HE. The scenario that represented a higher change in the burden of disease is the one that represents an average frequency of fish/seafood intake of seven times per week, with an estimated average of 11 445 healthy years saved in one year within the Portuguese population. Additionally, increasing fish consumption to a weekly average of 5 times would also result in an estimated health gain of 5361 healthy years saved per year. The greatest health gain is expected due to the intake of EPA + DHA and decreased risk of CHD mortality. On the contrary, decreasing fish consumption (alt3 and alt4) resulted in an increase in the burden of disease with an annual estimate of 14 106 and 3124 lost healthy years, respectively, in the Portuguese population.
Table 5. Total and outcome specific disability adjusted life years difference (ΔDALY) in one year, in the Portuguese population, for each alternative scenario compared with the reference scenario
(Mean values and 95 % confidence intervals)
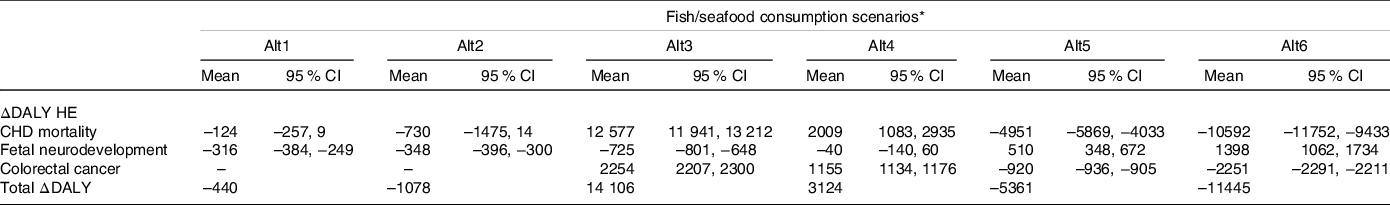
DALY, disability adjusted life years; HE, health effects.
* Alt1: large predatory fishes excluded; Alt2: MeHg low-level fishes included; Alt3: fish/seafood meal consumption once a week; Alt4: fish/seafood meal consumption 3×/week; Alt5: fish/seafood meal consumption 5×/week; Alt6: fish/seafood meal consumption 7×/week.
Changing fish type consumed, as described for the alternative scenarios alt1 and alt2, resulted in a slight decrease in DALY compared with the reference scenario. Specifically, regarding the HE foetal neurodevelopment, it was found a small but significant decrease in the burden of disease by decreasing consumption of highly MeHg contaminated species. For this HE, the highest health gain was found in alt3.
Discussion
In this study, we estimated the Portuguese exposure to MeHg and EPA + DHA using a national representative sample from IAN-AF 2015–2016. We estimated that about 14 % of the Portuguese population has a MeHg exposure above the established TWI.
A slightly lower prevalence of 11 % in the Portuguese population was reported in a previous study(Reference Jacobs, Sioen and Jacxsens47), which may be explained due to methodological differences between the two studies. First, Jacobs et al., considered only the adult population (18–75 years old), not considering children, which we found to be the population group at a higher risk. In our study, adults and the elderly had a risk prevalence of 12·9 and 11·6 %, respectively, values closer to the results from Jacobs et al. Furthermore, in our study, food consumption was assessed with two 24 h dietary recalls, where participants reported the type of fish, and the specific amount consumed, using food pictures for quantification. Differently from the Jacobs et al. study, where a food-frequency questionnaire was applied considering only 32 fish species and an average portion of fish/seafood was used for all intake occasions, in the IAN-AF 2015–2016, average standard portions were used only when no other information was available. Finally, the differences in the occurrence data used may have also contributed to this difference. Regarding MeHg occurrence, we used data from a large number of fish/seafood samples that were available in the Portuguese market, which is a strength of this study. For EPA and DHA the nationally available data was scarcer, thus there was a need to search for data from other sources to increase the accuracy of the assessment. However, a limitation can arise from this, since the fatty acid composition of fish/seafood may vary with the fishing ground and feeding practices of aquaculture products(Reference Rittenschober, Stadlmayr and Nowak48,Reference Khalili Tilami and Sampels49) . Nevertheless, there was national analytical data available for the most consumed fatty fish species, which we assume to be enough to overcome this limitation. Another important strength of our methodology regarding exposure assessment is the probabilistic approach used to input concentration values to individual eating occasions, rather than a deterministic approach using a point estimate for all individuals. Applying a probabilistic approach acknowledges the variability in the occurrence of food components and the food consumption between and within individuals.
Moreover, in this study, we applied an RBA to estimate the health impact of several hypothetical scenarios of fish consumption in the Portuguese population, considered a population with high fish consumption(1,2) . Our results show that the scenario with higher fish/seafood consumption frequency (seven times per week) was the one that represented the highest health gains, and that decreasing fish consumption frequency (once to 3 times per week) would represent a health loss in the Portuguese population. The HE that most contributed to the change in DALY was CHD mortality, which may happen due to its high incidence, as CHD is the second main cause of death in Portugal(37). Nonetheless, this scenario presented a deleterious impact considering foetal neurodevelopment.
The scenarios reflecting changes in fish/seafood type to low-contaminated species had a lower impact in decreasing the health burden and the change in DALY was significant only for the ‘foetal neurodevelopment’ effect. This finding may be explained because the majority of fish consumed by the Portuguese population are species typically less contaminated with MeHg, such as cod, hake or salmon. In some regions like Madeira, however, it is expected that these scenarios have a greater impact. In line with findings from a study performed on pregnant women from Madeira(Reference Caetano, Branco and Cavaco50), our results show that this is the region with the highest prevalence of exposure to MeHg in the reference scenario. We hypothesize that the specific reduction in the risk prevalence estimated in the alternative scenarios alt1 and alt2 observed in Madeira is due to the typical higher consumption of specific predatory fish species (particularly black-scabbardfish and fresh tuna) in that region, also shown in other study(Reference Caetano, Branco and Cavaco50). Thus, the change for these scenarios would especially benefit this region.
Our results suggest that official guidelines of fish consumption may recommend daily fish consumption for the general population. However, some population groups, as pregnant women and small children should be a target of special considerations. According to our results, children younger than 5 years old are susceptible to a high prevalence of MeHg exposure above the TWI, particularly in the alternative scenarios with an increased average frequency of fish consumption. Furthermore, there is an increase in the health burden considering the HE ‘foetal neurodevelopment’ by increasing average fish consumption frequency, suggesting a negative effect on the IQ of children due to maternal fish consumption during pregnancy. This is in line with the findings of the RBA studies from Cohen et al and Zeilmaker et al. (Reference Cohen, Bellinger and Connor18,Reference Zeilmaker, Hoekstra and van Eijkeren31) , from where we derived the dose-response models for MeHg and DHA effects. On the contrary, another quantitative RBA study considering fish substitutions in Denmark(Reference Thomsen, Pires and Devleesschauwer14), using a different approach, found opposite results concerning neurodevelopment, by applying a dose-response to fish intake as a whole(5,Reference Hibbeln, Davis and Steer51) , instead of only to DHA intake. In fact, according to EFSA, the benefits of fish consumption in neurodevelopment during pregnancy cannot be exclusively attributed to DHA, but also other nutrients such as iodine, thus, we cannot rule out the possibility of underestimation of the neurodevelopment benefits of fish in our study. Additionally, fish/seafood are a source of highly bioavailable selenium (SE)(Reference Cardoso, Bernardo and Bandarra7–Reference Cardoso, Bandarra and Lourenço9,Reference Fox, Van den Heuvel and Atherton52) , which may contribute to a beneficial net-effect of fish on neurodevelopment, since previous evidence from animal studies have shown a countereffect of dietary SE in MeHg toxicity(Reference Watanabe53–Reference Bjørklund, Aaseth and Ajsuvakova57). Evidence on this protective concurring effect of SE regarding MeHg from epidemiologic studies in humans is, however, conflicting(Reference Kosta, Byrne and Zelenko58–Reference Lemire, Fillion and Frenette63). Thus, for this study, we decided to apply a more conservative approach considering only DHA dose-response from randomized clinical trials to isolate its effect, but this may be a limitation since it may produce an underestimation of the benefits of fish/seafood consumption.
An important remark must be done concerning the methodological approach of using foods’ raw weights to estimate the exposure to the RBA agents. For MeHg, this is not an issue as there is little impact on the content of mercury in foods after cooking or processing, according to EFSA’s Scientific Opinion(13). On the contrary, regarding EPA and DHA, by considering it in raw food items only, we are overlooking the potential losses (e.g., due to oxidation) caused by heat. Several authors have studied the effect of cooking on n-3 fatty acid profile of different fish species, and while some found a decrease in these fatty acids(Reference Türkkan, Cakli and Kilinc64–Reference Weber, Bochi and Ribeiro66), many others described a not very wide variation in fish’s fatty acid composition and that n-3 fatty acids were well preserved(Reference Gladyshev, Sushchik and Gubanenko67–Reference de Castro, Pinheiro Sant’Ana and Campos72). Thus, we consider this limitation most likely has little impact on our results.
Further limitations of this assessment should be addressed. First, we recognise that not all HE and fish/seafood components were considered for this RBA. Other contaminants such as dioxin and dioxin-like polychlorinated biphenyls may be present in fish/seafood and may pose risks to humans. As already discussed, some nutrients such as iodine, Se and iron, which may have important benefits, were not accounted for in our health impact assessment. Moreover, to quantify the health impact in DALY we rely on available data from different sources and different years. We used the most recently available national data on the incidence and mortality of the HE, fertility rate and life expectancy, which were not all from the same period but were apart only 1–2 years, a timeframe that can be considered short enough to exclude significant changes. Furthermore, there are many sources of uncertainty, namely on dose-response, disability-weights, incidence and mortality rates, and other data, that we tried to account for whenever possible, however, we cannot rule out the possibility of some unquantified uncertainty to impact our results, despite that in general, we used a conservative approach, overestimating the risks. Finally, we used the distribution based on the 2-day average intake to compute the prevalence of inadequate exposure to MeHg, as data shown to be unsuitable to estimate the usual intake. Thus, the obtained prevalence may be slightly overestimated due to a heavy-tailed distribution of the 2-days assessment.
Despite the limitations, our findings, showing greater benefit in the scenarios with average higher fish/seafood consumption frequency, are in line with previous quantitative RBA studies on fish consumption that also showed an overall health benefit of increasing fish consumption(Reference Hoekstra, Hart and Owen3,5,Reference Thomsen, Pires and Devleesschauwer14,Reference Cohen, Bellinger and Connor18,Reference Thomsen, de Boer and Pires73) . However, a quantitative comparison with these studies is not possible due to differences in the alternative scenarios considered and other methodological aspects, namely the components and HE selected for the assessment as well as the model for food substitutions in the alternative scenarios. A relevant strength of our study is the probabilistic approach used to perform the substitutions in the alternative scenarios that allowed to account for variability in food substitution behaviour. It is not expected that all individuals make substitutions in the same way, thus the models for the substitutions to achieve the average weekly fish/seafood frequencies in the several alternative scenarios took into account variables such as sex, age group and geographic region. In the scenarios’ development, to consider food type and portion sizes for the substitutions, we imputed meals classified as ‘Meat’, ‘Fish/seafood’ and ‘Others’ as they were reported in the survey, by sex, age group and region. This imputation process in the alternative scenarios was performed in a way to vary average ‘Fish/seafood’ weekly frequency, keeping the ratio between ‘Meat’ v. ‘Others’ meals the same as the reference scenario, according to sex, age group and region using multistate models. By applying this approach, the replacements were not at random, and we assume a more realistic substitution to build the alternative scenarios rather than a deterministic one, where all individuals replace food in the same manner.
Reflecting on our results and previous evidence from regulatory bodies, as EFSA(10), we consider that for greatly vulnerable population groups (young children and pregnant women), about 3 to 4 weekly meals of fish should be recommended by the Portuguese national guidelines, which are in line with the current national average of consumption. Along with the frequency recommendation of fish/seafood consumption, the choice of smaller non-predatory fish species should be promoted. We found a small but significant decrease in the health burden in the alternative scenarios where selected predatory fish species were excluded, thus we acknowledge that the type of fish has an impact on the health burden and risk prevalence, as shown in previous studies and guidelines(Reference Thomsen, Pires and Devleesschauwer14,Reference Jacobs, Sioen and Jacxsens47,Reference Caetano, Branco and Cavaco50,Reference Anual, Maher and Krikowa74–77) .
Besides the HE considered in this RBA, increasing fish/seafood consumption may also have environmental benefits. Scenarios with higher average fish/seafood frequency have lower levels of meat consumption that has typically higher environmental footprints(Reference Willett, Rockström and Loken78–Reference Scarborough, Appleby and Mizdrak81). Thus, considering the relevance of sustainability in our current food systems and the impact of climate change on human health, we acknowledge that further research should focus on quantifying the scenarios’ environmental footprint and integrating it in the RBA.
Conclusions
Our findings support a recommendation for the general population to increase fish/seafood consumption up to seven times a week, as it allows to save more than 10k healthy years in the Portuguese population per year. For pregnant women and children, however, the recommendation should not exceed the 3–4 times per week, which is the current frequency of fish/seafood consumption, to avoid potential risks on foetal neurodevelopment due to MeHg exposure. The Portuguese national recommendations should also promote the choice of fish species with lower MeHg levels (as small pelagic fish, i.e., sardine, atlantic horse mackerel, mackerel) to minimise the MeHg exposure, especially in vulnerable populations and regions.
Acknowledgements
The researchers acknowledge all the institutions and people involved in all phases of the IAN-AF 2015–2016 Survey, as well as to participants. The survey was conducted by a Consortium: Faculty of Medicine, University of Porto, Portugal; EPIUnit, Institute of Public Health, University of Porto, Portugal; Faculty of Nutrition and Food Sciences, University of Porto, Portugal; National Health Institute Doutor Ricardo Jorge, Portugal; Institute of Preventive Medicine and Public Health, Faculty of Medicine, University of Lisbon, Portugal; CIAFEL, Faculty of Sports, University of Porto, Portugal; Faculty of Human Kinetics, CIPER, University of Lisbon, Portugal; SilicoLife, Lda, Portugal; Faculty of Medicine University of Oslo, Norway. The Survey had institutional support from the General Directorate of Health (DGS), the Regional Health Administration Departments, the Central Administration of the Health System (ACSS) and from the European Food Safety Authority (CFT/EFSA/DCM/2012/01-C03).
The authors also acknowledge all the institutions that contributed with national methylmercury and n-3 fatty acids occurrence data, namely: the General Directorate of Food and Veterinary (DGAV); the Portuguese Institute for Sea and Atmosphere, IP (IPMA, IP); the National Institute of Health Doutor Ricardo Jorge, IP (INSA, IP) and the Portuguese Economic and Food Safety Authority (ASAE).
This work was supported by the EEA Grants Program, Public Health Initiatives (PT06 – 000088SI3); the Operational Programme Factors of Competitiveness – COMPETE from FEDER and national funding from the Foundation for Science and Technology – FCT (Portuguese Ministry of Education and Science) under the project ‘FOCAcCIa’ (POCI-01–0145-FEDER-031949); the Epidemiology Research Unit (UIDB/04750/2020) (POCI-01–0145-FEDER-006862) and the FCT doctoral grant (SFRH/BD/146078/2019) (CC).
C. C.: investigation, methodology, writing – original draft; D. C.: investigation, methodology, Formal analysis, writing – review & editing; M. S.: methodology; C. A.: data curation, writing – review & editing; N. B.: data curation, writing – review & editing; S. G.: data curation; H. L.: data curation; M. G. D.: data curation, writing – review & editing; L. M. O.: data curation, writing – review & editing; P. N.: data curation, writing – review & editing; P. C.: data curation, writing – review & editing; S. M.: data curation, writing – review & editing; M. B.: data curation, writing – review & editing; C. L.: conceptualisation, investigation, supervision, writing – review & editing, project administration; D. T.: conceptualisation, investigation, methodology, supervision, writing – review & editing, project administration.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114521004773









