Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterised by sustained hyperglycaemia. Hyperglycaemia in T2DM is due to insulin resistance, resulting from impaired sensitivity of tissues for insulin, and relative insulin deficiency. The prevalence worldwide of T2DM will increase from its present level of 225 million to as many as 552 million people by 2030( Reference Guariguata, Whiting and Weil 1 ). Diabetes accelerates the development of other chronic diseases such as CVD, cognitive problems and decreased quality of life. Therefore, it is crucial to prevent diabetes and improve health status by identifying modifiable lifestyle factors including diet( Reference Zimmet 2 ).
Previous studies have demonstrated positive associations of circulating total free fatty acids with pancreatic β-cell dysfunction( Reference Carpentier, Mittelman and Bergman 3 ) and insulin resistance( Reference Kashyap, Belfort and Berria 4 ). Other studies have suggested a negative association between dietary dairy-derived fatty acids and diabetes( Reference Mozaffarian, de Oliveira Otto and Lemaitre 5 – Reference den Besten, Bleeker and Gerding 7 ). Up to now, five meta-analyses( Reference Elwood, Givens and Beswick 8 – Reference Tong, Dong and Wu 12 ) have summarised the results of prospective studies( Reference Tong, Dong and Wu 12 – Reference Mozaffarian, Cao and King 15 ) and randomised controlled trials( Reference Nikooyeh, Neyestani and Farvid 16 , Reference Stancliffe, Thorpe and Zemel 17 ) on dairy product consumption and diabetes risk. Generally, these studies revealed an inverse relationship between consumption of total dairy products and T2DM.
Despite the presence of several meta-analyses on dairy consumption and T2DM, there is still a striking lack of knowledge regarding the role of specific dairy foods in the aetiology of T2DM, and studies have revealed mixed results on the relationship between specific dairy products and diabetes. For example, most studies have observed inverse associations between yogurt consumption and T2DM risk( Reference Aune, Norat and Romundstad 10 – Reference Tong, Dong and Wu 12 , Reference Diaz-Lopez, Bullo and Martinez-Gonzalez 18 ). In addition, cheese consumption was not associated with the incidence of the metabolic syndrome and/or T2DM( Reference Fumeron, Lamri and Abi Khalil 13 ). However, cheese intake showed a, although not statistically significant, inverse association with diabetes incidence in the European Prospective Investigation into Cancer and Nutrition (EPIC) – InterAct Study( Reference Sluijs, Forouhi and Beulens 19 ) and the Malmö Diet and Cancer cohort( Reference Ericson, Hellstrand and Brunkwall 20 ). Furthermore, within Europe, substantial differences across countries exist with regard to consumption amounts of both total and specific dairy products( Reference Hjartaker, Lagiou and Slimani 21 ). For instance, total dairy product intake is relatively high in Spain and the Netherlands, whereas yogurt consumption is relatively high in Sweden and the Netherlands compared with other European countries( Reference Hjartaker, Lagiou and Slimani 21 ). Moreover, none of the publications so far have included impaired glucose metabolism (IGM), which is associated with an increased T2DM risk as an outcome( Reference Tabak, Herder and Rathmann 22 ). In line with the expected rise in incidence of T2DM( Reference Wild, Roglic and Green 23 ), data from the 1999–2010 National Health and Nutrition Examination Survey revealed a 21 % increase in IGM prevalence from 1999 through 2010( Reference Bullard, Saydah and Imperatore 24 ), whereas IGM prevalence will rise further from 344 million in 2010 to an estimated 472 million in 2030( Reference Hu 25 ). The aetiological hypothesis that dairy product intake is inversely associated with diabetes risk would be strengthened if associations between dairy product intake and IGM, a potential pre-stage of diabetes, have similar direction and magnitude compared with associations between dairy product intake and diabetes. The Maastricht Study is an extensive phenotyping study that focuses on the aetiology of T2DM, its classic complications and its emerging co-morbidities( Reference Schram, Sep and van der Kallen 26 ). Within the framework of this cohort, we applied an extensive FFQ capturing fifty specific dairy products. The presence of data from an oral glucose tolerance test (OGTT), performed in all participants, also including individuals with normal glucose metabolism (NGM), allowed us to investigate associations of the consumption of specific dairy products with both IGM and newly diagnosed (ND) T2DM. In the present study, we included only individuals with IGM and ND T2DM because all individuals with pre-existing T2DM would have received advice on how to change their lifestyle, including their diet( 27 ).
Methods
Study design, study area and population
In this study, we used data from the Maastricht Study, an observational prospective population-based cohort study. The rationale and methodology have been described previously( Reference Schram, Sep and van der Kallen 26 ). In brief, the study focuses on the aetiology, pathophysiology, complications and co-morbidities of T2DM and is characterised by an extensive phenotyping approach. Eligible for participation were all individuals aged between 40 and 75 years and living in the southern part of the Netherlands. Participants were recruited through mass media campaigns and from the municipal registries and the regional Diabetes Patient Registry via mailings. Recruitment was stratified according to known T2DM status for reasons of efficiency. The present report includes cross-sectional data from the first 3451 participants, who completed the baseline survey between November 2010 and September 2013. The examinations of each participant were performed within a time window of 3 months. All participants filled out a FFQ of the Maastricht Study after their first visit to the study centre and returned their completed FFQ by the third visit. Upon return of the FFQ, participants were informed about their glucose metabolisms status (e.g. normal glucose tolerance, IGM or diabetes). Participants who had previously been diagnosed with T2DM (n 883) were excluded from the present analyses as they already would have received dietary advice( 27 ), which likely would have changed their dietary habits. From the remaining 2568 participants, 117 individuals had not returned their FFQ, and sixty individuals had implausible energy intakes (<3347 or >17 573 kJ/d (<800 or >4200 kcal/d) for men and <2092 or >14 644 kJ/d (<500 or >3500 kcal/d) for women)( Reference Willett 28 ), and were therefore excluded as well. Finally, a total of 2391 participants (1796 with normal glucose tolerance, 470 with IGM and 125 with ND T2DM) were included in the present analyses.
The present study was approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of the Netherlands, on the basis of the Health Council’s opinion (Permit 131088-105234-PG). All the participants gave their written informed consent.
Data collection
Oral glucose tolerance test
To determine glucose metabolism status, all the participants (except those who use insulin) underwent a standardised seven-point OGTT after an overnight fast. Blood samples collected at baseline and 120 min after ingestion of a 75-g glucose drink were used to define diabetes status according to the World Health Organization 2006 criteria( 29 ) into NGM, impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and T2DM. In the present study, individuals with IFG or IGT were combined into one category – that is, IGM.
Dietary intake
Dietary intake was assessed by a tailor-made FFQ developed using the Dutch national FFQ tool( Reference Molag, de Vries and Duif 30 ). The FFQ used in the Maastricht Study is one of the most extensive FFQ used so far in any study. It contains 101 questions on consumption over the last year, comprising twenty-three product groups and 253 items. The FFQ collected information on the intake of major food groups such as vegetables, fruits, meat, fish, dairy products and alcohol. Dairy product intake was appraised by fifty items covering milk (four items), chocolate milk (one item), coffee creamer (four items), ready-to-eat porridge (one item), cheese (nine items), yogurt (seven items), drink yogurt (three items), curd cheese (seven items), custard (six items), butter (two items), cream (four items) and ice cream (two items). Moreover, the FFQ captured differences between full cream, semi-skimmed and skimmed products, as well as fermented and non-fermented products (Table 1). Intake of energy and specific nutrients (SFA, Ca and lactose) was calculated using the Dutch NEVO food composition table, version 2011( 31 ).
Table 1 Categorisation of fifty dairy food items into specific categories

Other relevant health parameters
All measurements were performed by trained research assistants during visits to the Maastricht Study research centre using standardised protocols. During study visits, office blood pressure (mmHg) was measured, and weight and height were measured to the nearest 0·1 cm and 0·5 kg( Reference Schram, Sep and van der Kallen 26 ), respectively, to calculate BMI (kg/m2). Information on age and sex was extracted from study files, and information on education level (low, middle, high), physical activity, smoking habits (never, former, current) and self-reported medical history of CVD (yes, no) was derived from self-reported health questionnaires. Laboratory assessments included total cholesterol, LDL-cholesterol and HDL-cholesterol and TAG. Details about these measurements have been presented elsewhere( Reference Schram, Sep and van der Kallen 26 ).
Statistical analysis
Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc.). Logistic regression models were fitted to estimate OR and their 95 % CI for the association between dairy product consumption and presence of IGM and ND T2DM. Crude models (model 1) were adjusted for age and sex. Fully adjusted multivariable models (model 2) were adjusted for sex, age, education (low, middle, high), BMI (continuous), physical activity (h/week), smoking (never, former, current) and intakes of energy, alcohol, vegetables, fruits, meat and fish. Associations were analysed with consumption of dairy product products expressed both as continuous measures (per serving for specific dairy products, and per 100 g increment for combined dairy categories) and as categorical variables, with tertile cut-off points based on intake of the NGM participants in our study population. All tests were two-tailed, and P values<0·05 were considered statistically significant.
Results
Characteristics of study population
Of the 2391 participants included in this study, 1796 individuals had NGM, 470 had IGM and 125 were ND with T2DM. The mean age was 59·9 (sd 8·2) years and 50·9 % were men. Altogether, 12·6 % of the participants were current smokers and 17·0 % reported a medical history of CVD. Compared with individuals with NGM, individuals with IGM or ND T2DM were more frequently male, were older and had higher BMI, lower physical activity, higher blood pressure and a higher prevalence of CVD. Moreover, individuals with IGM or ND T2DM tended to have lower intakes of fruits and vegetables and higher intakes of meat, whereas fish consumption was similar across subgroups of glucose metabolism status (Table 2).
Table 2 Population characteristics (Mean values and standard deviations)

NGM, normal glucose metabolism; IGM, impaired glucose metabolism; ND T2DM, newly diagnosed type 2 diabetes mellitus.
Intake (g/d) of dairy products
The mean intake of total dairy products was 159 (sd 137) g/d in individuals with normal glucose tolerance compared with an average intake of 119 (sd 93) g/d in individuals with ND T2DM (P for difference<0·01). Cheese was consumed by all participants and yogurt by approximately 80 %. Just over 50 % of the total study population consumed milk and approximately 40 % consumed butter. Butter milk, drink yogurt and curd cheese were consumed by a smaller proportion of the participants (<35 %). The consumption of milk was essentially the same for NGM, IGM and ND T2DM. The most pronounced differences across groups of glucose metabolism status were observed for the amount of consumption of yogurt, fermented, skimmed and semi-skimmed products and for total dairy product intake, with the highest intakes in individuals with NGM and the lowest intakes in individuals with ND T2DM (data not shown, online Supplementary Table S1).
Individuals in the highest tertile of yogurt intake (≥63 g/d) had higher physical activity levels, were less often current smokers and consumed more fruits and vegetables and less meat compared with individuals in the lowest tertile of yogurt intake (≤10·5 g/d). Similar patterns were observed for individuals in the highest intake tertile of fermented, skimmed and semi-skimmed products and total dairy products (data not shown).
Associations between consumption of dairy products with the presence of impaired glucose metabolism or newly diagnosed type 2 diabetes mellitus
Age- and sex-adjusted analyses revealed significant inverse associations of total dairy, skimmed and fermented dairy products, as well as of cheese (both for total cheese and Dutch cheese) and yogurt, with IGM. After further adjustment for BMI, physical activity, smoking status, education and intakes of energy, vegetables, fruits, meat and fish, the associations of total dairy product intake and cheese with IGM were no longer statistically significant. The other associations remained significant, with OR, comparing the third with the first tertile, of 0·73 (95 % CI 0·55, 0·96) for skimmed products, 0·74 (95 % CI 0·54, 0·99) for fermented products and 0·67 (95 % CI 0·50, 0·90) for yogurt. In continuous analyses, the OR per 100 g increment in fermented products and per serving of yogurt were only statistically significant in the age- and sex-adjusted models, whereas fully adjusted OR was 0·88 (95 % CI 0·80, 0·97) per serving (20 g increment) of Dutch cheese consumption (Table 4). Semi-skimmed, full-fat and non-fermented products, as well as milk and curd cheese, were not associated with IGM in any of the models (Table 3).
Table 3 Cross-sectional associations between intake of dairy products and impaired glucose metabolism (Odds ratios and 95 % confidence intervals)Footnote *
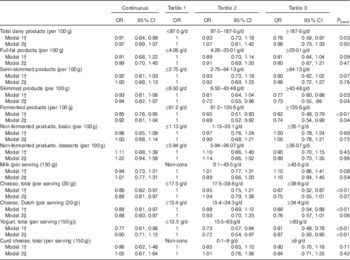
Non-cons, non-consumers.
* Continuous analyses: milk, cheese, yogurt and curd cheese per serving increment, all combined dairy categories per 100 g increment.
† Model 1 adjusted for age and sex.
‡ Model 2 additionally adjusted for BMI, physical activity, smoking status, education and intakes of energy, vegetables, fruits, meat and fish.
Age- and sex-adjusted analyses of the association between dairy products and ND T2DM revealed significant inverse associations for total dairy products, semi-skimmed products, fermented products and yogurt, whereas full-fat products were positively associated with ND T2DM. Fully adjusted models comparing the third with the first tertile revealed OR of 0·50 (95 % CI 0·26, 0·93) for total dairy product intake, but an increased OR of 2·01 (95 % CI 1·16, 3·47) for full-fat dairy products. Even though fully adjusted OR comparing the third v. the first tertile were not significant for total dairy product intake, fermented products and yogurt, the fully adjusted continuous models showed OR of 0·76 (95 % CI 0·61, 0·95) per 100 g increment in total dairy product intake, 0·69 (95 % CI 0·50, 0·94) per 100 g increment in fermented products and 0·47 (95 % CI 0·24, 0·89) per serving (150 ml) for yogurt (Table 4), respectively. Non-fermented products, cheese and curd cheese were not associated with ND T2DM (Table 4).
Table 4 Cross-sectional associations between intake of dairy products and newly diagnosed type 2 diabetes mellitus (Odds ratios and 95 % confidence intervals)Footnote *
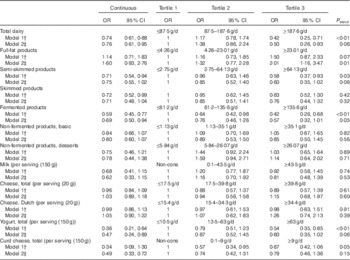
Non-cons, non-consumers.
* Continuous analyses: milk, cheese, yogurt and curd cheese per serving increment, all combined dairy categories per 100 g increment.
† Model 1 adjusted for age and sex.
‡ Model 2 additionally adjusted for BMI, physical activity, smoking status, education and intakes of energy, vegetables, fruits, meat and fish.
Mutual adjustment for the associations of full-fat products with skimmed products and vice versa and of fermented products with non-fermented products did not alter the observed associations with IGM and ND T2DM.
Discussion
This is the first epidemiological study on the association of specific dairy products with both ND T2DM and IGM as outcomes. Detailed information on dairy product intake was collected using an extensive, 253-item, tailor-made FFQ that included fifty dairy items, and associations were extensively adjusted for potential confounders. To prevent reporting bias, participants filled out the FFQ before results of the OGTT became available, and only individuals with ND T2DM (screen-diagnosed diabetes) were included. Results showed a strong inverse association between total dairy product intake and ND T2DM, with an OR of 0·50 (95 % CI 0·26, 0·93) for the highest v. lowest tertile of intake, but a marked positive association between full-fat dairy products and T2DM (OR 2·01; 95 % CI 1·16, 3·47). Remarkably, these associations were completely absent for IGM (OR 0·98 for the highest tertile v. lowest of total dairy and 0·90 for full-fat products). Fermented products and yogurt were inversely associated with T2DM (OR<0·60) and the same was observed for IGM (OR<0·75). An inverse association with IGM was found for skimmed dairy products (OR for the top v. bottom tertile of intake: 0·73 (95 % CI 0·55, 0·96)), which was non-significant for ND T2DM despite a similar OR (0·76 (95 % CI 0·44, 1·32)). Finally, the consumption of cheese showed a significant negative association with IGM (OR per serving (20 g): 0·88 (95 % CI 0·80, 0·97)) but not with T2DM (OR per serving: 1·05 (95 % CI 0·90, 1·22)).
Four meta-analyses( Reference Elwood, Givens and Beswick 8 , Reference Aune, Norat and Romundstad 10 – Reference Tong, Dong and Wu 12 ) revealed combined hazard ratio of 0·85( Reference Elwood, Givens and Beswick 8 ) and 0·86( Reference Tong, Dong and Wu 12 ) for highest v. lowest categories of total dairy consumption – 0·80( Reference Gao, Ning and Wang 11 ) to 0·93( Reference Aune, Norat and Romundstad 10 ) for 200 and 400 g increment of total dairy product intake( Reference Gao, Ning and Wang 11 ), respectively. Our results confirmed this inverse association, but associations in our study were more pronounced with an estimated 50 % lower odds of T2DM in individuals in the third tertile (total dairy product intake >188 g/d) as compared with individuals in the first tertile (total dairy product intake <87 g/d). Published studies also showed inverse associations of both skimmed and full-fat dairy products( Reference Malik, Sun and van Dam 14 ) with T2DM. The present study showed a positive association of full-fat products with ND T2DM, but not with IGM. In addition, skimmed products were significantly inversely associated with IGM, but not with ND T2DM. This is in contrast with a recent prospective study showing an inverse association of full-fat dairy products with T2DM risk and no association between skimmed products and T2DM risk( Reference Ericson, Hellstrand and Brunkwall 20 ). Several other studies showed a negative association of skimmed products with T2DM risk but no association of full-fat dairy products with T2DM risk( Reference Choi, Willett and Stampfer 32 , Reference Liu, Choi and Ford 33 ). Although it is unclear why full-fat products were positively associated with T2DM in our study, but not with IGM, one possible reason may have been the relatively low number of individuals with ND T2DM (n 125), which may have led to a false-positive finding. In addition, the heterogeneity of the IGM population may have attenuated the association, which may explain this difference. Future studies need to further investigate associations of skimmed and full-fat dairy products with IGM and T2DM risk.
There is very less information on the association between single dairy products and T2DM risk. Meta-analyses and a recent study have shown that milk consumption was inversely( Reference Elwood, Givens and Beswick 8 ) or not associated with the risk of T2DM in fully adjusted models( Reference Aune, Norat and Romundstad 10 – Reference Tong, Dong and Wu 12 , Reference Ericson, Hellstrand and Brunkwall 20 ). In our study, the null finding for milk consumption was confirmed for both T2DM and IGM. Although some studies did not reveal an association of cheese consumption with the incidence of the metabolic syndrome and/or T2DM( Reference Fumeron, Lamri and Abi Khalil 13 , Reference Ericson, Hellstrand and Brunkwall 20 ), a meta-analysis showed an 8( Reference Aune, Norat and Romundstad 10 ) to 20 %( Reference Gao, Ning and Wang 11 ) reduction in relative risk per 50( Reference Aune, Norat and Romundstad 10 ) or 30 g( Reference Gao, Ning and Wang 11 ) increment in cheese consumption. In our population, cheese consumption was not associated with ND T2DM, but a significantly lower odds of IGM of 11 % was observed per serving (20 g) of cheese, which corresponded with a lower odds of IGM of 26 and 16 % per 50 and 30 g increment (data not shown), respectively. In line with a potential inverse association of cheese consumption on diabetes risk, a recent randomised controlled trial showed significant decreases in total cholesterol and waist circumference after increased cheese consumption for a period of 8 weeks in individuals with the metabolic syndrome( Reference Nilsen, Hostmark and Haug 34 ). Our finding that consumption of yogurt and fermented products was inversely associated with both T2DM and IGM is also consistent with previous studies( Reference Aune, Norat and Romundstad 10 – Reference Tong, Dong and Wu 12 , Reference Diaz-Lopez, Bullo and Martinez-Gonzalez 18 ). In line with this, a cross-sectional study performed within the Framingham Heart Study Offspring and Third Generation cohorts revealed that individuals who consumed yogurt had a better diet quality as compared with non-consumers. In addition, yogurt consumption was associated with lower circulating levels of glucose and TAG, as well as with lower systolic blood pressure and insulin resistance( Reference Wang, Livingston and Fox 35 ). Furthermore, fermentation may enhance the nutritional value of yogurt and provide it with unique properties that enhance the bioavailability of some nutrients( Reference Astrup 36 ) and reduce blood pressure and LDL-cholesterol( Reference Agerholm-Larsen, Raben and Haulrik 37 ). In contrast, the recently published Seguimiento Universidad de Navarra (SUN) study revealed no associations between yogurt consumption and the incidence of the metabolic syndrome during a 6-year follow-up period( Reference Sayon-Orea, Bes-Rastrollo and Marti 38 ).
The mechanisms underlying alleged protective effects of total dairy products or fermented products on T2DM are not well understood. It has been hypothesised that dairy product intake may be inversely associated with T2DM through a protective role of vitamin D, Ca, Mg and whey proteins present in dairy products( Reference Weaver 39 ). As dairy products in the Netherlands are not fortified with vitamin D, this vitamin is unlikely to play a role in the present study results. Ca increases insulin secretion and is essential for insulin-responsive tissues such as skeletal muscle and adipose tissue and may reduce insulin resistance( Reference Pittas, Lau and Hu 40 ). Furthermore, Mg intake has been associated with reduced diabetes risk in epidemiological studies( Reference Dong, Xun and He 41 ) and with improved insulin sensitivity in some experimental studies, although data are limited( Reference Volpe 42 ). Dairy products also contain whey proteins, which in animal models have been shown to reduce body weight gain and to increase insulin sensitivity( Reference Belobrajdic, McIntosh and Owens 43 ). In contrast with these potentially protective nutrients, dairy products are also rich sources of SFA, which were associated with insulin resistance in some but not all controlled trials and prospective studies( Reference Micha and Mozaffarian 44 ). In the recent EPIC-InterAct study, plasma levels of even-chain SFA were positively associated with incident T2DM, whereas odd-chain SFA were inversely associated with incident T2DM( Reference Forouhi, Koulman and Sharp 45 ). As SFA can be synthesised in the human body, the physiological implication of this finding remains to be elucidated. Two studies revealed that higher plasma levels of trans-palmitoleic acid, which is indicative of dairy fat consumption, were associated with a reduced diabetes risk( Reference Mozaffarian, de Oliveira Otto and Lemaitre 5 , Reference Mozaffarian, Cao and King 15 ). In addition, animal studies showed that butyrate consumption, one of the main fatty acids in dairy products, could protect from obesity and increase insulin sensitivity( Reference den Besten, Bleeker and Gerding 7 ).
It is possible that the observed inverse association of Dutch cheese, yogurt, skimmed and fermented products with IGM and of total dairy product intake and high-fat dairy product intake with ND T2DM reflects a general difference in lifestyle habits. For instance, in our study population, higher intake of total dairy products was associated with healthier lifestyle reflected by higher levels of physical activity, lower prevalence of smoking and overweight/obesity, lower intakes of meat and higher intakes of fruits and vegetables. Even though associations between specific dairy products remained significant after adjustment for these variables, the possibility of residual confounding cannot be fully disregarded.
Our study has several strengths and limitations. A major limitation is the cross-sectional design of our study, which formally rules out causal inference. Nevertheless, we believe that our analyses do add to the existing evidence on the relationship between dairy product intake and T2DM, as all participants completed the FFQ before being informed about their glucose metabolism status, which minimised the risk of reporting bias. Furthermore, subjects with known pre-existing T2DM (n 883) were excluded from the present analyses, which makes it quite unlikely that subjects with ND T2DM could have adapted their diet to their disease status.
It may be noted that our results are generally in line with associations observed in previous prospective studies, which corroborates the integrity of our findings. A strength of our study is the fact that we have reported unique data on IGM, which have not been published before. Nevertheless, of note, IGM is a mixed population defined as the presence of either an elevated fasting glucose level, an IGT test or the combination of both. Furthermore, extensive information on lifestyle factors was collected for each cohort member, allowing the adjustment for potential confounders. Another important strength of this study is the extensive collection of dairy product intake data. We believe that the FFQ used in the Maastricht Study optimally estimated dairy product intake as our extensive FFQ captured fifty dairy food items.
In conclusion, this is the first study on the associations of dairy products with both ND T2DM and IGM. Intakes in the top tertiles of yogurt and fermented products were cross-sectionally associated with lower odds of presenting ND T2DM and IGM by 25–40 % relatively to intakes in the bottom tertiles. This observed association is consistent with the hypothesis that yogurt and fermented products may play a role in the aetiology of diabetes. However, associations of total dairy product intake, full-fat products, skimmed products and Dutch cheese did not reveal similar direction and magnitude for ND T2DM and IGM. Therefore, our results warrant further assessment of the relationship between single dairy products and the incidence of IGM and T2DM in a prospective design to elucidate their role in T2DM development.
Acknowledgements
The authors thank all the voluntary participants from the Maastricht Study as well as the funding bodies.
The Maastricht Study is supported by the European Regional Development Fund as part of OP-ZUID, the province of Limburg, the Department of Economic Affairs of the Netherlands (grant no. 31O.041), Stichting the Weijerhorst, the Pearl String Initiative Diabetes, the Cardiovascular Center Maastricht, Cardiovascular Research Institute Maastricht (CARIM), School for Nutrition, Toxicology and Metabolism (NUTRIM), Stichting Annadal, Health Foundation Limburg and by unrestricted grants from Janssen, Novo Nordisk and Sanofi. Furthermore, the authors thank FrieslandCampina for their financial support of the current study.
The contributions of the authors are as follows: M. T. S., S. J. S. S., C. J. v. d. K., A. K., N. S., R. M. A. H., C. D. A. S. and P. C. D. participated in the design and implementation of the Maastricht Study. M. C. J. M. v. D., N. W., L. d. B., S. J. P. M. E. and P. C. D. collected and cleaned the dietary intake data. S. J. P. M. E. and P. C. D. performed data analyses and wrote the initial draft of the manuscript. S. J. P. M. E., M. C. J. M. v. D., N. W., L. d. B., S. J. W. H. O. E., C. M. S.-P., M. T. S., S. J. S. S., C. J. v. d. K., A. K., N. S., R. M. A. H., C. D. A. S. and P. C. D. critically read the manuscript and approved the final version.
S. J. W. H. O. E. and C. M. S.-P. are employees at FrieslandCampina. All the other authors have no potential conflicts of interest to declare.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516000313







