Atherosclerosis is the main pathophysiological mechanism of CVD, the most common cause of morbidity and mortality worldwide. Platelet-activating factor (PAF) is a potent lipid mediator of inflammation and platelet aggregation that participates in the initiation and prolongation of atherosclerosis( Reference Demopoulos, Karantonis and Antonopoulou 1 ). PAF levels are balanced via its enzymatic biosynthesis by the remodelling and the de novo pathways and its catabolism (Fig. 1)( Reference Snyder 2 ). Briefly, initiation of the remodelling pathway leads phospholipase A2 to generate lyso-PAF which is then acetylated by acetyl-CoA:lyso–platelet-activating factor acetyltransferases (Lyso-PAF AT) to form PAF. Τwo isoforms of Lyso-PAF AT are known, one of them is activated under inflammatory conditions, while the other one is Ca independent and does not participate in inflammatory processes( Reference Snyder 2 , Reference Harayama, Shindou and Ogasawara 3 ). The main enzyme of the de novo pathway is cytidine 5'-diphospho-choline:1-alkyl-2-acetyl-sn-glycerol cholinephosphotransferase (platelet-activating factor–cholinephosphotransferase (PAF-CPT)) which catalyses the synthesis of PAF from 1-O-alkyl-2-acetyl-glycerol( Reference Snyder 4 ). Concerning PAF catabolism, an intracellular platelet-activating factor-specific acetylhydrolase (PAF-AH) and its plasma isoform lipoprotein-associated phospholipase A2 (LpPLA2) are the key enzymes for the cleavage of the acetyl chain at the sn-2 position forming lyso-PAF( Reference Stafforini 5 ). PAF is also produced non-enzymatically along with oxidised phospholipids (oxPL) during LDL oxidation( Reference Liapikos, Antonopoulou and Karabina 6 ). The concomitant LpPLA2 inactivation on oxidised LDL particles leads to augmentation of PAF and oxPL levels( Reference Liapikos, Antonopoulou and Karabina 6 ).
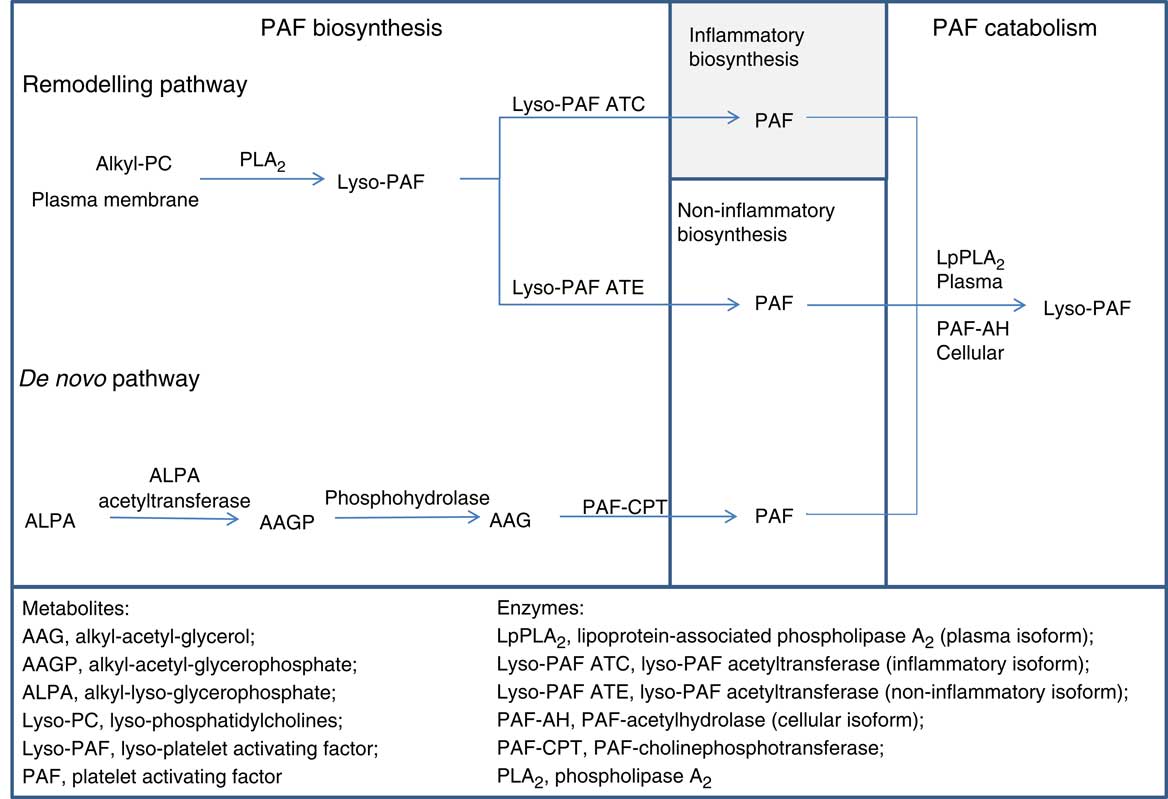
Fig. 1 Main enzymatic pathways of platelet-activating factor (PAF) metabolism. Metabolites: AAG, alkyl-acetyl-glycerol; AAGP, alkyl-acetyl-glycerophosphate; ALPA, alkyl-lyso-glycerophosphate; Lyso-PC, lyso-phosphatidylcholines; Lyso-PAF, lyso-platelet-activating factor; PAF. Enzymes: LpPLA2, lipoprotein-associated phospholipase A2 (plasma isoform); Lyso-PAF ATC, lyso-platelet-activating factor acetyltransferase in the presence of Ca2+ (inflammatory isoform); Lyso-PAF ATE, lyso-platelet-activating factor acetyltransferase in the presence of EDTA (non-inflammatory isoform); PAF-AH, platelet-activating factor–acetylhydrolase (cellular isoform); PAF-CPT, platelet-activating factor–cholinephosphotransferase; PLA2, phospholipase A2.
PAF is a crucial component of inflammatory cell signalling and its actions are mediated through a specific G protein-linked receptor( Reference Zimmerman, McIntyre and Prescott 7 ). Overexpression of adhesion molecules along with PAF is required for recruitment and tight adhesion of platelets and leucocytes on endothelium. PAF regulates P-selectin translocation via both autocrine and intracrine signalling( Reference Rollin, Lemieux and Maliba 8 ) and mediates soluble vascular cell adhesion molecule 1 (sVCAM)-induced secretion of leukotriene C4 in eosinophils( Reference Tsuruta, Cobb and Mastrangelo 9 ). Furthermore, PAF in coordination with other agonists like ADP and thrombin through their receptors promote signal transduction pathways, which raise intracellular concentration of Ca2+, alter platelets shape and lead to platelets aggregation( Reference Savage, Cattaneo and Ruggeri 10 ).
Several studies demonstrate the existence of food bioactive components that could interfere with endothelial function and platelet aggregation( Reference Vilahur and Badimon 11 ). Foods, such as tomatoes, vegetables, garlic, onions, olive oil and fish, have been shown in vitro to inhibit platelet aggregation via different mechanisms( Reference Vilahur and Badimon 11 ). From this point of view, we previously identified compounds that inhibit PAF-induced platelet aggregation in vitro, namely, PAF inhibitors, in several foods, including virgin olive oil, fish and red/white wines( Reference Nomikos, Fragopoulou and Antonopoulou 12 ). We have also reported that wine extracts and phenolic compounds inhibit Lyso-PAF AT and PAF-CPT activities in U937 monocytes under basal and inflammatory conditions( Reference Xanthopoulou, Asimakopoulos and Antonopoulou 13 , Reference Vlachogianni, Fragopoulou and Stamatakis 14 ). In human studies, consumption of traditional Greek Mediterranean meals containing PAF inhibitors reduced platelet sensitivity against PAF in patients with type 2 diabetes mellitus and in healthy subjects( Reference Antonopoulou, Fragopoulou and Karantonis 15 ). Furthermore, the consumption of wines, which contained PAF inhibitors, along with a meal, ameliorates postprandial platelet sensitivity against PAF( Reference Xanthopoulou, Kalathara and Melachroinou 16 ) as well as the activities of Lyso-PAF AT and PAF-CPT in leucocytes of healthy men( Reference Argyrou, Vlachogianni and Stamatakis 17 ). All the above support the idea that the consumption of Mediterranean foods that contain PAF inhibitors could have a protective effect on inflammation and thrombosis( Reference Nomikos, Fragopoulou and Antonopoulou 18 ). However, in our days the intense pace of life disrupts the pattern of balanced diet, and nutritional plant supplements are more often used, without always clinical evidences. Especially, as far as PAF actions and metabolism are concerned, limited data exist( Reference Cha, Kim and Kim 19 ).
The aim of the present study is to examine whether a combination of phytochemicals and vitamins with established anti-oxidant effect( Reference Fragopoulou, Gavriil and Argyrou 20 ) may have an impact on PAF actions and metabolism. For this purpose, a double-blind, randomised and placebo-controlled clinical trial was undertaken in apparently healthy adults.
Methods
Supplement
The supplement Mind Master and a look-alike placebo were custom prepared and donated by LR Healthy and Beauty Systems Ltd. The supplement contained per dose of 80 ml, Aloe barbadensis miller gel (USA/Mexico 36 %), grape juice (32·5 %), Polygonum cuspidatum extract (which contains 10 % resveratrol), green tea extract, 1·1 mg vitamin B1, 2·5 µg vitamin B12, 12 mg vitamin E (α-tocopherol equivalents), coenzyme Q10, 200 µg folic acid, ascorbic acid, 27·5 µg Se and 4·2 mg Fe. The placebo comprised low concentration of A. barbadensis Miller gel (USA/Mexico 3·6 %), ascorbic acid and some excipients.
Study protocol
This involves a double-blind, block randomised, parallel-arm, placebo-controlled study, and its duration was 8 weeks. A detailed description of the clinical study has already been reported( Reference Fragopoulou, Gavriil and Argyrou 20 ) and a brief description is presented in the online Supplementary material. A total of fifty-eight healthy volunteers completed the study. The study was conducted at the Metabolic Unit of the Department of Nutrition and Dietetics, Harokopio University, according to the guidelines laid down in the Declaration of Helsinki. Volunteers gave informed consent and the University Ethics Committee approved the experimental protocol. ClinicalTrials.gov Identifier for this study is NCT02837107.
Blood sample collection
Venous blood was collected after a 12 h fasting at the beginning of the study (0 weeks), at 4 weeks and at the end of the intervention (8 weeks) from the brachial vein of the volunteers. For the isolation of serum, venous blood samples were drawn into evacuated glass tubes and serum was collected after 45 min incubation at room temperature by centrifugation at 1500 g for 10 min. For plasma isolation, EDTA vacutainers were used and blood was centrifuged immediately at 1500 g for 10 min. For the isolation of leucocytes, 5 ml of heparinised blood were obtained from each volunteer and leucocytes were isolated as previously described( Reference Detopoulou, Nomikos and Fragopoulou 21 ). Protein concentrations of all preparations were determined according to the Bradford method( Reference Bradford 22 ), with the use of bovine serum albumin as protein standard. All biological samples were immediately aliquoted and stored at approximately –80°C.
Haematological parameters and classic biochemical measurements
Haematological parameters such as total leucocyte count, erythrocyte count, Hb level, haematocrit, erythrocyte indices such as mean corpuscular volume, mean corpuscular Hb, mean corpuscular Hb concentration, erythrocyte distribution width, platelet count and mean platelet volume were determined by a Mindray Hematology Analyzer (BC 3000 plus). Enzymatic methods were used to define glucose, TAG, uric acid, total cholesterol, LDL-cholesterol and HDL-cholesterol, as previously described( Reference Fragopoulou, Gavriil and Argyrou 20 ).
Measurement of sP-selectin, soluble vascular cell adhesion molecule-1 and IL-6
Sandwich ELISA kit were used for measurement of sP-selectin, sVCAM-1 (Duoset ELISA; R&D Systems) and IL-6 (Quantikine HS ELISA; R&D Systems). The intra-assay CV was <10 % for sVCAM/sP-selectin and <7 % for IL-6. The inter-assay CV was <12 % for sVCAM/sP-selectin and <9·8 % for IL-6.
Ex vivo human platelet-rich plasma aggregation
Blood was centrifuged at 170 g for 15 min to collect the supernatant platelet-rich plasma (PRP). Platelet-poor plasma (PPP) was obtained by recentrifugation of the pellet at a higher force. Platelet count of PRP was adjusted to 300 000/ml with PPP. Samples were incubated at 37°C, with a stirring rate of 1000 rpm. Chronolog Aggregometer (Model 440VS) was used for determining aggregation responses based on light transmittance method against various concentrations of PAF and ADP as well as against 2 µm of thrombin receptor activating peptide (TRAP). For PAF and ADP, the maximum reversible or the least not reversible aggregation was estimated to evaluate the 100 % aggregation. The plot of percentage aggregation (ranging from 20 to 80 %) v. different concentrations of agonist is linear. Based on this curve, EC50 value meaning concentration of PAF or ADP that induces 50 % of maximum aggregation was calculated. In the case of TRAP, the percentage change in platelet aggregation (against 2 µm of TRAP) v. baseline was calculated.
Assay of lyso-platelet-activating factor acetyltransferase activity in leucocyte homogenate
Two isoforms of Lyso-PAF AT were measured, the one is inducible and activated by inflammatory stimulation and the other is constitutively expressed. Isolated leucocyte homogenates, 15 µg of total protein, were incubated for 10 min at 37°C with 4 nmol of lyso-PAF and 40 nmol of acetyl-CoA. Final reaction volume of 200 µl was obtained by adding 50 mm Tris–HCl buffer (pH 7·4) containing 0·25 mg/ml of bovine serum albumin( Reference Fragopoulou, Iatrou and Demopoulos 23 ). In the case of inducible Lyso-PAF AT, the assay was performed in the presence of CaCl2 2·8 mm (Lyso-PAF ATC); and in the case of the constitutively Lyso-PAF AT, the assay was performed in the presence of EDTA 1·4 mm (Lyso-PAF ATE). Cold chloroform:methanol (2 % acetic acid) was added for stopping the reaction. All assays were performed in duplicate.
Assay of platelet-activating factor-cholinephosphotransferase activity in leucocyte homogenate
In all, 15 µg of isolated leucocyte homogenate protein was incubated at 37°C for 5 min with 100 mm Tris–HCl (pH 8·0), 15 mm dithiothreitol, 0·5 mm EDTA, 20 mm MgCl2, 1 mg/ml of bovine serum albumin, 100 µm of cytidine 5'-diphospho-choline and 100 µm of 1-O-hexadecyl-2-acetyl-sn-glycerol in total reaction volume of 200 µl. Cold chloroform:methanol (2 % acetic acid) was added for stopping the reaction. All assays were performed in duplicate.
Extraction and quantification of platelet-activating factor
The levels of assay produced PAF were extracted by the acid Bligh–Dyer method( Reference Bligh and Dyer 24 ) and determined with liquid chromatography (LC)-MS. The enzymatic activity was expressed as specific activity pmol PAF/mg per min.
Extracted samples were inserted on Hypersil GOLD™ (5 µm, 150×4·6 mm; Thermo Scientific) for the analysis of assay product (PAF). The mobile phase contained 98 % methanol LC-MS, 2 % 1 mm aqueous ammonium acetate and the flow rate was 0·1 ml/min. MS analysis was performed using an Exactive™ Plus Orbitrap Mass Spectrometer (Thermo Scientific) with an electrospray ionisation source. Quantification was performed using the transition m/z 524·37 at retention peak time of 10 min. A calibration curve, with range 1·6–24 pmol PAF, was routinely performed every two batches and a control PAF sample was included in each batch. Inter-day and intra-day precision was 15·9 and 11·8 %. Mass spectra were processed using the Xcalibur 4.0 (Thermo Scientific) software.
Measurement of platelet-activating factor–acetylhydrolase activity in leucocyte homogenate and lipoprotein-associated phospholipase A2 activity in serum
Determination of PAF-AH activity was done in 30 µg leucocyte homogenate protein based on the trichloroacetic acid precipitation method using [3H] PAF as a substrate( Reference Detopoulou, Nomikos and Fragopoulou 21 ). Leucocyte homogenate was incubated with 4 nmol of [3H] PAF (20 Bq/nmol), 100 mm Tris–HCl buffer (pH 7·2) plus 1 mm ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) for 15 min at 37°C. Final reaction volume was 200 µl and the reaction was stopped by adding cold trichloroacetic acid (10 % final concentration). Afterwards, samples were incubated in ice bath for 30 min and then were centrifuged at 15 000 g for 2 min. Liquid scintillation counter was used for the measurement of [3H]-acetate which was released into the aqueous phase. All assays were performed in duplicate. The enzyme activity was expressed as specific activity pmol/mg per min.
Serum LpPLA2 activity was measured by a commercial kit using 2-thio PAF as a substrate (Cayman Chemical). The intra-assay CV was <4 % and the inter-assay CV was <10 %. All assays were performed in duplicate. The enzyme activity was expressed as specific activity nmol/min per ml.
In vitro platelet aggregation in plasma rich in platelets
The in vitro effect of supplement and its plant extracts (Aloe, grape skin, grape seed, extract that contain resveratrol) on platelet aggregation against PAF, ADP and TRAP was determined on PRP from healthy volunteers; 0 % inhibition was considered as platelet aggregation without the addition of the examined sample. Plot of percentage inhibition (ranging from 20 to 80 %) v. different concentrations of the examined sample is linear, and it was used to calculate the concentration of the sample that induced 50 % inhibition against each agonist. This value was defined as the IC50, namely, inhibitory concentration producing 50 % inhibition.
Statistical analysis
Statistical analysis was done using SPSS 18 (SPSS Inc.) software. The primary outcome of the present study was a significant reduction in platelet aggregation, while the secondary outcome was a significant alteration of PAF metabolic enzyme activity. Considering the primary outcome, statistical power analysis revealed that nineteen participants in each arm was adequate to achieve statistical power equal to 95 % at 5 % significance level of two-sided hypotheses that evaluated 1 sd differences based on EC50 values of platelet aggregation. The Kolmogorov–Smirnov criterion was used for testing normality and mean values and standard deviations are used for the presentation of normally distributed continuous variables, and medians and quartiles (25th–75th percentiles) for skewed variables. Comparisons of the baseline characteristics of our population are based on the independent-samples t test for normally distributed variables, and the Mann–Whitney test for skewed variables. Furthermore, in skewed variables, the supplement and placebo groups at 4 and 8 weeks were compared by the Mann–Whitney test to illuminate a potential trial effect, while Friedman’s two-way ANOVA by ranks was performed for testing the time effect within each trial. The Wilcoxon test for paired samples was also performed for testing the time effect within each trial. Repeated-measures ANOVA was applied for normally distributed variables for examining the trial effect, time effect and time×trial effect. Comparison between different biomarkers is based on Pearson. The significance level for P values is 5 %.
Results
Biochemical markers, lifestyle characteristics and haematological parameters
Biochemical markers and lifestyle characteristics of volunteers have been previously reported( Reference Fragopoulou, Gavriil and Argyrou 20 ). Briefly, baseline anthropometric and biochemical markers of the study population did not differ between the two groups. No difference was observed at the levels of glucose, total cholesterol, HDL-cholesterol, LDL-cholesterol, TAG and uric acid between groups at 4 weeks or at 8 weeks of the intervention (online Supplementary Table S1)( Reference Fragopoulou, Gavriil and Argyrou 20 ). Haematological parameters of volunteers at baseline and during the intervention are presented in Table 1. No significant changes were detected between the two groups.
Table 1 Levels of basic haematological markers before the intervention and during the interventionFootnote * (Mean values and standard deviations)
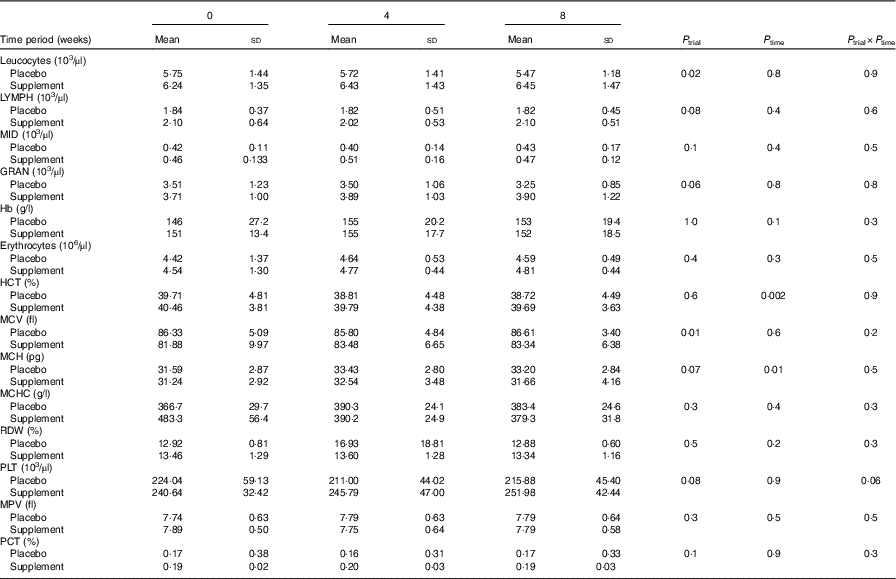
LYMPH, lymphocytes; MID, monocytes, eosinophils, basophils, blasts and other precursor white cells; GRAN, granulocytes; HCT, haematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular Hb; MCHC, mean corpuscular Hb concentration; RDW, erythrocyte distribution width; PLT, platelets; MPV, mean platelet volume; PCT, plateletcrit.
* Data are presented as means and standard deviations for normally distributed variables. Repeated-measures ANOVA was used for comparisons.
Platelet-activating factor enzymes activities, adhesion molecules and IL-6 at baseline
To evaluate PAF metabolism, the activities of the biosynthetic enzymes, namely, PAF-CPT and the two isoforms of Lyso-PAF AT, namely, Lyso-PAF ATE (in the presence of EDTA) and Lyso-PAF ATC (in the presence of Ca2+) as well as of the catabolic ones, namely, LpPLA2 and PAF-AH, were measured. PAF metabolic enzyme activities did not differ between the two examined groups at baseline (Table 2). The activities of both isoforms of Lyso-PAF AT, Lyso-PAF ATE and Lyso-PAF ATC, were positively correlated with PAF-CPT (r 0·608, P<0·001, r 0·346, P=0·01, respectively) and with each other (r 0·625, P<0·001). The activity of LpPLA2 was negatively correlated with the activity of Lyso-PAF ATC (r –0·404, P=0·003), while a trend of correlation was observed with the activity of Lyso-PAF ATE (r –0·237, P=0·09). When the above correlations were adjusted for age, BMI, LDL (in the case of LpPLA2) and sex, the correlations remained significant and also the one between LpPLA2 and Lyso-PAF ATE (r –0·309, P=0·03) became significant and a trend of positive correlation was observed between LpPLA2 and PAF-AH (r 0·283, P=0·06).
Table 2 Levels of adhesion molecules, IL-6 and platelet-activating factor (PAF) enzyme activity before the interventionFootnote * (Mean values and standard deviations; medians and 25th–75th percentiles)
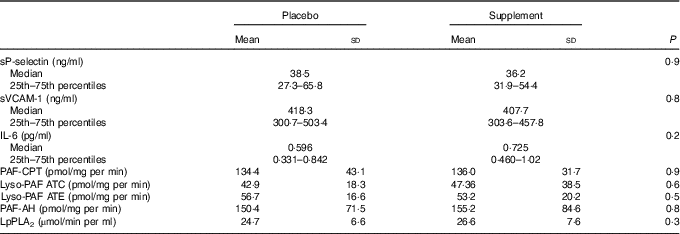
sVCAM, soluble vascular cell adhesion molecule; PAF-CPT, platelet-activating factor-cholinephosphotransferase; Lyso-PAF ATC; lyso-platelet-activating factor acetyltransferase in the presence of Ca2+; Lyso-PAF ATE; lyso-platelet-activating factor acetyltransferase in the presence of EDTA; PAF-AH, platelet-activating factor-acetylhydrolase; LpPLA2, lipoprotein-associated phospholipase A2.
* Data are presented as means and standard deviations for normally distributed variables and as medians (25th–75th percentiles) for skewed variables. ANOVA or the Mann–Whitney test was used for comparisons, respectively.
Νo differences were observed in adhesion molecules sP-selectin and sVCAM-1 levels or in IL-6 levels at the initiation of the study between the placebo and supplement groups (Table 2). Baseline levels of sP-selectin were positively correlated with the activities of LpPLA2 (r 0·337, P=0·01), PAF-CPT (r 0·342, P=0·01) and Lyso-PAF ATE (r 0·246, P=0·07). Baseline levels of sVCAM-1 were negatively correlated with the activity of LpPLA2 (r –0·266, P=0·05) and the levels of IL-6 were negatively correlated with Lyso-PAF ATC (r –0·293, P=0·03). After adjustment for age, sex and LDL-cholesterol (in the case of LpPLA2), the above correlations were not retained with the exception of the one between sP-selectin and PAF-CPT.
Effect of intervention on endothelial cell adhesion molecules and IL-6
No significant time or trial effect was observed concerning the levels of sVCAM-1, sP-selectin and IL-6 at 4 and 8 weeks of supplementation (Table 3).
Table 3 Effect of intervention on the levels of adhesion molecules and IL-6 (Medians and lower–upper quartiles (25th–75th percentiles) for skewed variables)
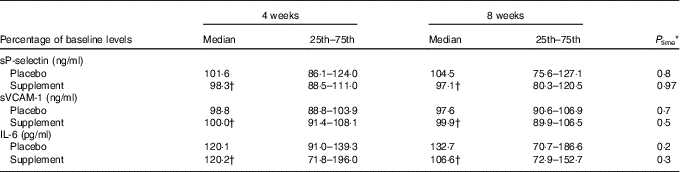
sVCAM, soluble vascular cell adhesion molecule.
* P time: Friedman’s two-way ANOVA by ranks was used for the estimation of the time effect within the placebo or supplement group.
† P trial: the Mann–Whitney U independent test was used for comparison of the supplement v. placebo group at 4 or 8 weeks.
Effect of intervention on the activity of platelet-activating factor metabolic enzymes
A significant time effect was observed on PAF-AH activity and a trend on Lyso-PAF ATC activity (Table 4). Specifically, PAF-AH activity was increased at 4 and 8 weeks in the supplement group compared with baseline (P 0–4=0·008, P 0–8=0·01) and a trend for increase was also detected in Lyso-PAF ATC activity in the placebo group compared with baseline (P 0–4=0·07).
Table 4 Effect of intervention on platelet-activating factor (PAF) metabolism enzyme activityFootnote * (Mean values and standard deviations for normally distributed variables)
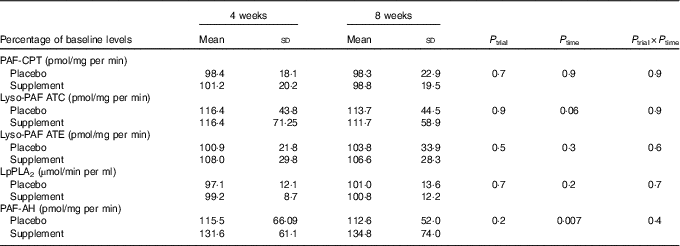
PAF-CPT, platelet-activating factor–cholinephosphotransferase; Lyso-PAF ATC, lyso-platelet-activating factor acetyltransferase in presence of Ca2+; Lyso-PAF ATE; lyso-platelet-activating factor acetyltransferase in the presence of EDTA; LpPLA2, lipoprotein-associated phospholipase A2; PAF-AH, platelet-activating factor–acetylhydrolase.
* A repeated-measures ANOVA was used for the comparisons.
Effect of intervention on ex vivo platelet aggregation against platelet-activating factor, ADP and thrombin receptor activating peptide
A significant time effect was revealed only in the supplement group, which was agonist dependent. In specific, a significant time effect was observed on platelet aggregation against PAF (P=0·001), a trend for time effect on platelet aggregation against ADP (P=0·06), while no time effect was observed on platelet aggregation against TRAP (Table 5). Compared with baseline, the EC50 values against PAF in the supplement group were significantly increased at 4 (P<0·001) and 8 weeks (P=0·01), indicating lower platelet sensitivity. Compared with baseline levels, the EC50 values against ADP in the supplement group were also increased at 4 (P=0·03) and 8 weeks (P=0·003, respectively).
Table 5 Effect of intervention on platelet aggregation against platelet-activating factor (PAF), ADP and thrombin receptor activating peptide (TRAP) (Medians and lower–upper quartiles (25th–75th percentiles) for skewed variables)
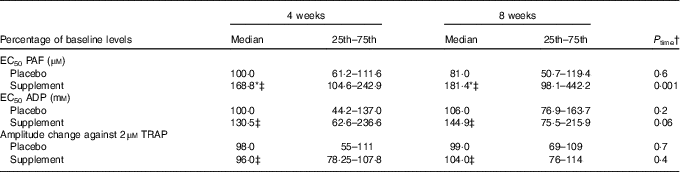
EC50, concentration that induces 50 % of maximum aggregation.
* P<0·05.
† P time: Friedman’s two-way ANOVA by ranks was used for the estimation of the time effect in the placebo or supplement trial.
‡ P trial: the Mann–Whitney U independent test was used for comparison of supplement v. placebo at 4 or 8 weeks.
A significant trial effect at 4 and 8 weeks of supplementation was only observed against PAF-induced aggregation. In specific, EC50 values against PAF were higher at both 4 (P=0·02) and 8 weeks (P=0·02) v. placebo group (Table 5).
Effect of total supplement and its main individual components on in vitro platelet aggregation
The in vitro anti-thrombotic ability of the supplement’s extract components against platelet aggregation induced by PAF, ADP and TRAP in healthy volunteers’ PRP was also performed (Table 6). The in vitro results revealed that the supplement inhibited platelet aggregation against all agonists. The examination of each extract separately showed that grape juice (seed and skin extract) and especially grape skin exerted the most potent anti-platelet activity against PAF. In contrast, Aloe vera gel exhibited very slight anti-platelet activity that never reached 50 % inhibition regardless of the agonist.
Table 6 Effect of total supplement and its individual extract components on in vitro platelet aggregationFootnote *

IC50, inhibitory concentration producing 50 % inhibition; PAF, platelet-activating factor; TRAP, thrombin receptor activating peptide; ND, not detected.
* Data are expressed as IC50 values (µl of supplement or mg of extract) and is the average of two independent experiments.
Discussion
Atherosclerosis is a multifactorial pathological situation which includes prolonged inflammation, endothelial dysfunction and platelet aggregation. PAF is a potent inflammatory mediator that is implicated in platelet aggregation and degranulation, leucocyte–platelet interactions, endothelium permeability and subsequently in the infiltration of leucocytes into the sub-endothelial matrix( Reference Demopoulos, Karantonis and Antonopoulou 1 ). The objective of this study was to evaluate the effect of 8-week supplementation mainly with plant extracts along with vitamins on PAF actions and metabolism, inflammatory and endothelial function biomarkers.
Previous data support the idea that PAF production is implicated in the adhesion of leucocytes to endothelium( Reference Zimmerman, McIntyre and Mehra 25 ) and probably PAF acts complementary to P-selectin to activate inside-out signalling of b2 integrins on neurophiles( Reference Zimmerman, McIntyre and Prescott 26 ). As was described, the volunteers had a medium adherence to the Mediterranean diet and were overweight( Reference Fragopoulou, Gavriil and Argyrou 20 ). At baseline, the levels of the soluble adhesion molecules (sVACM, sP-selectin) and IL-6 were in the physiological range for healthy subjects( Reference Thompson, Hosking and Pederick 27 , Reference Lee, Min and Chun 28 ). As far as PAF metabolism is concerned, the activities of PAF-CPT and LpPLA2 were similar with the previous ones reported for the Greek population( Reference Detopoulou, Nomikos and Fragopoulou 29 ), while no data exist for the activities of Lyso-PAF ATC and Lyso-PAF ATE that were strongly and positively correlated with the activity of PAF-CPT of the de novo pathway, indicating a parallel regulation of the two pathways. This result is in accordance with a previous publication in the general Greek population where total acetyltransferase activity (Lyso-PAF AT) was correlated with PAF-CPT( Reference Detopoulou, Nomikos and Fragopoulou 29 ). The activity of the catabolic enzyme LpPLA2 was also negatively correlated with the activity of Lyso-PAF ATC and Lyso-PAF ATE. In the present study, biosynthetic enzyme PAF-CPT was positively correlated with sP-selectin independently of age, BMI and sex, supporting the idea that PAF acts complementary to P-selectin.
The platelet aggregation assay, which is the most common bioassay evaluating PAF activity( Reference Bossant, Ninio and Delautier 30 ), showed that the EC50 values against PAF-induced platelet aggregation at baseline levels were positively correlated to the corresponding ones of ADP, supporting a parallel sensitivity of platelets against these two agonists. The EC50 values against ADP-induced platelet aggregation (higher EC50 value means lower sensitivity) were negatively correlated with sVCAM levels, indicating a parallel activation of platelets and endothelial cells.
The present study used a supplement that contains natural plant extracts mainly A. vera gel (36 %) and grape juice (32·5 %) but also P. cuspidatum that contains 10 % resveratrol along with vitamins. The results revealed that 4- and 8-week supplementation did not affect the levels of sP-selectin, sVCAM-1 and IL-6 in healthy subjects. Concerning A. vera, no previous reports exist comparing the above results. In vitro data reported that grape seed proanthocyanidin extract inhibits VCAM-1 expression in human endothelial cells stimulated by TNF-α ( Reference Sen and Bagchi 31 ) and reduces the expression of P-selectin and VCAM-1 in rats( Reference Zhang, Shi and Wang 32 ). However, in vivo reports concerning the effect of natural products consumption on VCAM levels are in agreement with the results of the present study since 8 weeks’ daily consumption of resveratrol (250 mg)( Reference Gliemann, Schmidt and Olesen 33 ) and 4 weeks’ consumption of green tea( Reference Lee, Min and Chun 28 ) did not alter sVCAM-1 levels in healthy volunteers. Concerning sP-selectin, in contrast with the present study, it was reported that consumption of anthocyanin capsules reduced P-selectin expression measured by flow cytometer in a sedentary population( Reference Thompson, Hosking and Pederick 27 ) and 4 weeks consumption of green tea decreased sP-selectin levels in male smokers( Reference Lee, Min and Chun 28 ). It should be mentioned that in the first study different methodology was used( Reference Thompson, Hosking and Pederick 27 ), and in the second one( Reference Lee, Min and Chun 28 ) baseline values of P-selectin were double compared with the values of the present study. Focusing in clinical trials with vitamin supplementation, much higher dosages than the ones presented in this study were used, without always have a beneficial effect on the levels of the adhesion molecules in healthy populations( Reference van Dijk, Enneman and Swart 34 – Reference Koh, Blum and Hathaway 36 ).
As far as PAF metabolic enzymes are concerned, the supplementation did not alter the activity of its biosynthetic enzymes in the leucocyte homogenate of volunteers’ activity of plasma LpPLA2. However, the activity of intracellular PAF-AH in volunteers’ leucocytes homogenate was increased in the supplement group at 4 and 8 weeks compared with the baseline levels, indicating a reduction in PAF levels. Unfortunately, comparisons with other reports are difficult, since most studies are restricted to cell cultures. In vitro data support that resveratrol and wine extracts inhibit Lyso-PAF AT and PAF-CPT activities in U-937 monocytes under basal and inflammatory conditions( Reference Vlachogianni, Fragopoulou and Stamatakis 14 ) and flavonoids inhibit Lyso-PAF AT activity in endothelial cells( Reference Yanoshita, Chang and Son 37 , Reference Balestrieri, Castaldo and Balestrieri 38 ). Also, wine consumption parallel with a meal has been reported to reduce PAF biosynthetic enzymes in postprandial state( Reference Argyrou, Vlachogianni and Stamatakis 17 ). Concerning PAF catabolic enzymes, tea extract is reported to inhibit in vitro LpPLA2 ( Reference Zeng, Yan and Luo 39 ) and in vivo in the metabolic syndrome subjects after supplementation with red yeast rice olive( Reference Hermans, Van der Auwera and Breynaert 40 ). No data exist concerning vitamins in vivo effects on PAF metabolism.
Activated platelets are an important contributor of atherothrombosis development. Anti-platelet drugs have been reported to have side effects and increase resistant in target populations; therefore, the use of natural extracts is a potential alternative( Reference Vilahur and Badimon 11 ). The supplementation in the present study resulted in reduction of platelet sensitivity against PAF (higher EC50 values) at 4 and 8 weeks, compared with the placebo group and reduction of platelet sensitivity against both PAF and ADP at 4 and 8 weeks compared with the baseline values. In contrast, no results were observed with regard to the sensitivity against TRAP that activates platelets thought thrombin receptors; however, due to the limited blood volume collected, no EC50 values were calculated in the case of TRAP. In vitro data support that grape juice extract, green tea extract and resveratrol reduce platelet aggregation( Reference Borriello, Cucciolla and Della Ragione 41 , Reference Vitseva, Varghese and Chakrabarti 42 ), while no data exist concerning the effect of A. vera on platelet aggregation. In this context, it has been shown that resveratrol is capable of reducing platelet aggregation against PAF( Reference Fragopoulou, Nomikos and Antonopoulou 43 ). Also, 4 weeks’ consumption of grape juice with resveratrol inhibited platelet aggregation against thrombin in healthy subjects( Reference Pace-Asciak, Rounova and Hahn 44 ) and anthocyanin capsules supplementation reduced ADP-induced whole blood platelet aggregation in a sedentary population( Reference Thompson, Hosking and Pederick 27 ), although the consumption of a grape seed extract rich in low-molecular-weight polyphenolic compounds did not alter platelet aggregation in subjects with hypertension( Reference Ras, Zock and Zebregs 45 ). Furthermore, the consumption of wines, which contain PAF inhibitors, along with a meal ameliorates postprandial platelet sensitivity against PAF in healthy men( Reference Xanthopoulou, Kalathara and Melachroinou 16 ). The observed effect on platelet aggregation could be attributed to the synergistic effect of all extracts along with vitamins with some extracts playing more crucial role since our in vitro experiments revealed that grape skin and seed extracts along with resveratrol but not Aloe gel inhibit platelet aggregation against PAF, ADP and TRAP in a dose-dependent manner. However, we should consider the fact that extracts’ micro-constituents could undergo modifications through their absorption and metabolism and the observed effect may be attributed to the initial compounds or to their metabolites( Reference Velderrain-Rodríguez, Palafox-Carlos and Wall-Medrano 46 ). Concerning the effect of vitamins, it is reported that vitamin E in dosages of 200–600 mg/d has no significant effect on platelet aggregation in vivo, although in vitro results supported this idea( Reference Steiner 47 ). In contrast, 5 months’ consumption of supplement that combined 600 mg ascorbic acid, 300 mg α-tocopherol, 27 mg carotene and 75 mg Se reduced aggregation of platelets against ADP in men( Reference Salonen, Salonen and Seppanen 48 ). It should be mentioned that the dosages in the above studies were much higher, so comparison with the present study is difficult.
The limitations of the present study are that our population is healthy with medium adherence to Mediterranean diet; thus, our results are difficult to generalise in patients or in healthy subjects with balanced diet. Compliance of participants’ to the study protocol was estimated by the assessment of the supplement’s volume that has not been consumed and not by methods that measured vitamins or other nutrients. The study design does not allow conclusions regarding the effect of each component of the supplement on the observed results.
In conclusion, the consumption of a supplement that contains mainly plant extracts along with vitamins reduces platelet sensitivity against PAF and ADP in healthy subjects. No other effect was observed on inflammatory or endothelial function markers with the exception of intracellular PAF catabolic enzyme, namely, PAF acetylhydrolase, where an increase was detected.
Acknowledgements
The authors are grateful to the participants of this study. Also the authors would like to thank Antigoni Tsiafitsa for her technical assistance in blood sample collection and Chrysa Argyrou and Ioanni Malagari for their contribution in handling of biological samples.
The study was partly funded from LR Healthy and Beauty. The founder was not involved in the study design, the collection, the analysis, the data interpretation, the writing of the manuscript as well as in the decision to submit the article for publication.
E. F. designed and supervised the implementation of the study; L. G. contributed to the handling of biological samples and measured endothelial/inflammation markers, platelet aggregation and PAF enzyme activity; M. D. and F. P. contributed to the measurement of PAF enzyme activity; S. A. critically revised the manuscript.
The authors declare that there are no conflicts of interest.










