Introduction
The migration of raptors constitutes one of the most spectacular movements of predators on earth (Bildstein and Zalles Reference Bildstein, Zalles, Greenberg and Marra2005). Because of their trophic status, raptors act as useful flagship species for conservation (Bildstein et al. Reference Bildstein, Zalles, Ottinger, McCarty, Chancellor and Meyburg2000). The greatest threat to migratory raptors is habitat loss, so understanding their migration patterns is important for effective conservation strategies (Alerstam Reference Alerstam1990, Bildstein et al. Reference Bildstein, Zalles, Ottinger, McCarty, Chancellor and Meyburg2000). Little is known about the migratory movements of many raptor species, particularly those moving between Asia and Africa (McClure Reference McClure1998) despite recent advances in satellite telemetry that have contributed to understanding movements of some species (Meyburg et al. Reference Meyburg, Mendelsohn, Ellis, Smith, Meyburg and Kemp1995, Kjellen et al. Reference Kjellen, Hake and Alerstam1997, Meyburg et al. Reference Meyburg, Ellis, Meyburg, Mendelsohn and Scheller2001, Trierweiler et al. Reference Trierweiler, Koks, Drent, Exo, Komdeur, Dijkstra and Bairlein2007). The application of the technique is limited by the lower limit of transmitter size, currently ~ 5 g (Strandberg et al. Reference Strandberg, Klaassen, Hake, Olofsson and Alerstam2009) which prevents similar studies on smaller migrants. The lightest species tracked to date has recently decreased from the Eurasian Hobby Falco subbuteo Linnaeus 1758 (mass = c. 230 g; Strandberg et al. Reference Strandberg, Klaassen, Hake, Olofsson and Alerstam2009) to the Red-footed Falcon F. vespertinus Linnaeus 1766 (mass = c. 135 g).
It is important in the conservation and long-term survival of migratory raptors, particularly in the context of global climatic changes and rapid habitat modification due to human activity, to determine their migratory origins. En route migratory stopover points may be critical for many species (Bildstein et al. Reference Bildstein, Zalles, Ottinger, McCarty, Chancellor and Meyburg2000), but equally important are the final “over-wintering” destinations where the build-up of resources for the coming breeding season may be crucial (Sanderson et al. Reference Sanderson, Donald, Pain, Burfield and van Bommel2006). In the southern African subregion there is a funnelling effect of migrants from an extensive longitudinal range in the northern hemisphere (Newton Reference Newton1995, Bildstein and Zalles Reference Bildstein, Zalles, Greenberg and Marra2005, Dingle Reference Dingle2008). This has implications for the conservation of many migrant species that “over-winter” in South Africa during the austral summer because the population is concentrated over a smaller area in their non-breeding African range.
Of particular concern is the Amur Falcon Falco amurensis Radde 1863, a small (male, 137.6 g, n = 18, female, 159.5 g, n = 26; Schäfer Reference Schäfer2003) insectivorous raptor that undergoes a one-way migration of ~13,000 km (Ferguson-Lees and Christie Reference Ferguson-Lees and Christie2001, Mendelsohn Reference Mendelsohn, Harrison, Allan, Underhill, Herremans, Tree, Parker and Brown1997, Jenkins Reference Jenkins, Hockey, Dean and Ryan2005). This includes the longest regular over-water passage of any raptor, flying over the Indian Ocean between south-western India and East Africa, a distance of c. 4,000 km (Cade Reference Cade1982, Ali and Ripley Reference Ali and Ripley1987, Orta Reference Orta, del Hoyo, Elliott and Sargatal1994, Mendelsohn Reference Mendelsohn, Harrison, Allan, Underhill, Herremans, Tree, Parker and Brown1997, Bildstein et al. Reference Bildstein, Zalles, Ottinger, McCarty, Chancellor and Meyburg2000, Birdlife International 2008). It breeds over a wide range in Mongolia, Siberia and northern China, although little has been documented on its distribution in the Palaearctic (Cheng Reference Cheng1987, Mendelsohn Reference Mendelsohn, Harrison, Allan, Underhill, Herremans, Tree, Parker and Brown1997, Ferguson-Lees and Christie Reference Ferguson-Lees and Christie2001, Bildstein and Zalles Reference Bildstein, Zalles, Greenberg and Marra2005, Global Raptor Information Network 2008). Birds arrive in southern Africa during November to early December and roost in colonies that number thousands of individuals (Benson Reference Benson1951, Cade Reference Cade1982, Tarboton and Allan Reference Tarboton and Allan1984). For a number of months they are a common sight on the eastern Highveld, before departing north again during April to May (Mendelsohn Reference Mendelsohn, Harrison, Allan, Underhill, Herremans, Tree, Parker and Brown1997, Jenkins Reference Jenkins, Hockey, Dean and Ryan2005).
Stable hydrogen isotopes have been used to determine migratory connectivity of numerous species in the Americas and Europe (e.g. Chamberlain et al. Reference Chamberlain, Blum, Holmes, Feng, Sherry and Graves1997, Hobson and Wassenaar Reference Hobson and Wassenaar1997, Chamberlain et al. Reference Chamberlain, Bensch, Feng, Åkesson and Andersson2000, Meehan et al. Reference Meehan, Lott, Sharp, Smith, Rosenfield, Stewart and Murphy2001, Hobson et al. Reference Hobson, Aubry and Wassenaar2004a,Reference Hobson, Bowen, Wassenaar, Ferrand and Lormeeb). On a global scale, the distribution of δDp is relatively predictable, with a general depletion (more negative δ values) in δDp with an increase in latitude (Bowen and Revenaugh Reference Bowen and Revenaugh2003). The assimilation of this water during feather growth, at different latitudes, can then be used to indicate the origins of birds (Hobson and Wassenaar Reference Hobson and Wassenaar2008). On a broad scale, the relationship between the isotope signature of precipitation (δDp) and that of feathers (δDf) is usually linear, with δDf values being 20–50‰ depleted (more negative) than that of δDp values (Meehan et al. Reference Meehan, Lott, Sharp, Smith, Rosenfield, Stewart and Murphy2001, Wassenaar and Hobson Reference Wassenaar and Hobson2001, Wassenaar and Hobson Reference Wassenaar and Hobson2003, Hobson and Wassenaar Reference Hobson and Wassenaar2008). This understanding of δD patterns has been particularly useful in determining the movement patterns and migratory origins of long distance migrants across the globe (e.g. Hobson and Wassenaar Reference Hobson and Wassenaar1997, Hobson and Wassenaar Reference Hobson and Wassenaar2008). Within Africa the hydrogen isotope gradient on either side of the equator is low, so movements of birds within the continent are more difficult to study using stable isotopes (but see Yohannes et al. Reference Yohannes, Hobson, Pearson and Wassenaar2005, Yohannes et al. Reference Yohannes, Hobson and Pearson2007, Wakelin et al. in press).
Distinct isotope fractionation processes occur during the C3 and C4 photosynthetic pathways, resulting in unique isotope signatures for each vegetation type that animals feed on; C3 plants are typically more depleted in 13C relative to C4 and CAM plants (Park and Epstein Reference Park and Epstein1960, Park and Epstein Reference Park and Epstein1961, Smith and Epstein Reference Smith and Epstein1971, Vogel et al. Reference Vogel, Fuls and Ellis1978, Ehleringer Reference Ehleringer, Rundel., Rundel and Nagy1991, Dawson et al. Reference Dawson, Mambelli, Plamboeck, Templer and Tu2002). Also, the distributions of C3 and C4 plants are associated with broad-scale global patterns of plant distribution (Stowe and Teeri Reference Stowe and Teeri1978). Studies of animal tissues that reflect diet can therefore be good indicators for interpreting the use of different resources (C3 or C4) on temporal and spatial scales (e.g. Cerling et al. Reference Cerling, Wittemyer, Rasmussen, Vollrath, Cerling, Robinson and Douglas-Hamilton2006). On the other hand, nitrogen isotopes are a useful tool in understanding trophic ecology because each step in a food chain is usually associated with an isotopic enrichment of 3–5‰ from diet to animal tissue (Peterson and Fry Reference Peterson and Fry1987, Mizutani et al. Reference Mizutani, Fukuda and Kabaya1992, Kelly Reference Kelly2000, Post Reference Post2002). Nitrogen and carbon are therefore useful elements in identifying carbon sources and delineating trophic levels of organisms (Minagawa and Wada Reference Minagawa and Wada1984, Peterson and Fry Reference Peterson and Fry1987, Hobson and Wassenaar Reference Hobson and Wassenaar1999).
Accordingly, stable isotope data from feathers of South African Amur Falcons were analysed to address specific questions concerning migratory connectivity and local movements in the over-wintering range of this species. Because Amur Falcons breed in the northern hemisphere we expected stable hydrogen isotopes in the feathers of juveniles to reflect their origins. Adults, on the other hand, undergo a complete flight feather moult in southern Africa (Northern Flagship Institution unpubl. data), so isotopes in feathers were expected to reflect the area in which the feathers grew. Some feathers (i.e. P4–6 and S5–7) appear to moult in the Palaearctic (Schäfer Reference Schäfer2003) and are not re-moulted in southern Africa. To complement analyses using hydrogen isotopes, we used stable carbon and nitrogen isotopes that provide further information on diet, to provide additional support for answering questions relating to movements of Amur Falcons. More specifically, we attempted to answer the following questions; 1) Can we determine the breeding origins of birds in the Palaearctic? 2) Do Amur Falcons display a leap-frog migration pattern, i.e. northernmost breeding birds overwinter furthest south? 3) Do members of roosting flocks originate from similar regions in the northern hemisphere? 4) Do populations exhibit roost fidelity in the South African subregion?
Materials and Methods
Feather collection
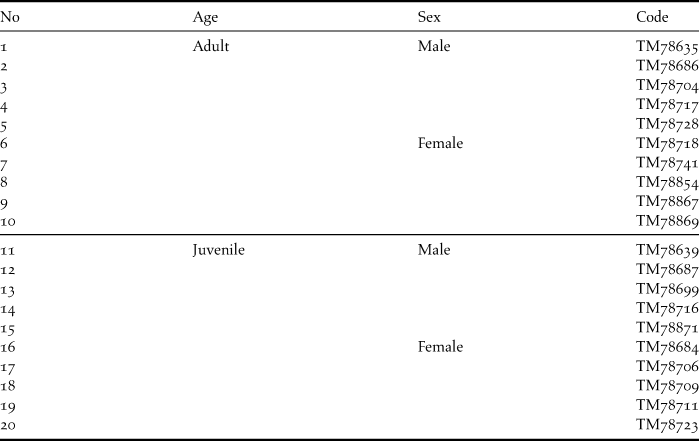
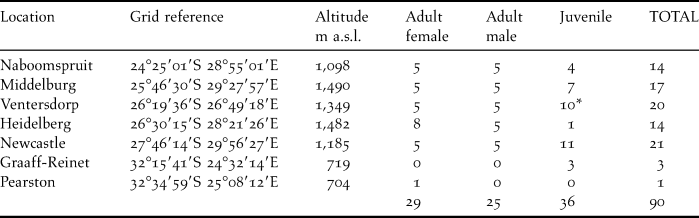
Specimens of individual adult male and female and juvenile male and female Amur Falcons that were killed during a thunderstorm on 25 December 2004 at a roost in Ventersdorp were collected for analysis (Bernitz Reference Bernitz2006). These specimens, housed in the Transvaal Museum, Pretoria, were surgically sexed and used as a feather identification reference for additional samples collected from beneath roosts throughout South Africa during December 2008–March 2009. The museum specimens were also sampled. Details of sites and number of samples collected at each site are given in Table 1.
Table 1. Amur Falcon feather collection sites in South Africa (ordered from northernmost to southernmost) and number of samples collected at each site.
* Five male and five female specimens. Details of Transvaal Museum specimens are given in Appendix 1.
Sample preparation and isotopes analysis
Feathers were washed in distilled water to remove faecal matter, followed by cleansing in a 2:1 chloroform:methanol solution to remove surface oils and contaminants (Hobson et al. Reference Hobson, Wassenaar, Milá, Lovette, Dingle and Smith2003, Reference Hobson, Aubry and Wassenaar2004a). 13C/12C isotope ratios are given relative to Vienna Pee Dee Belemnite (VPDB), 15N/14N ratios relative to Air and 2H/1H ratios relative to Vienna Standard Mean Ocean water (VSMOW), with values expressed in δ notation in parts per thousand (per mille, ‰).
For δ13C and δ15N analyses, representative samples (0.15–0.30 mg) were weighed in tin cups (pre-cleaned in toluene) and combusted at 1,020oC in an Elemental Analyser (Flash EA, 1112 Series, Thermo Electron Corporation, Bremen, Germany). The 13C/12C and 15N/14N isotope ratios were then determined using a Thermo Delta V Plus continuous-flow isotope ratio mass spectrometer (CFIRMS) (Thermo Electron Corporation) plumbed in-line with the elemental analyser via a ConFlo IV gas controller (Thermo Fisher Scientific, Bremen, Germany). Two aliquots of a laboratory standard (homogenised dried chicken blood; mean δ13C ± SD = -17.87 ± 0.15‰, n = 331) were used for every six to eight unknowns in sequence. In order to correct for equipment drift, duplicates were run for each sample. The laboratory standard was standardised against C652 ANU sucrose, 1577b bovine liver (National Institute of Standards and Technology) and SRM 1547 peach leaves (NIST).
In order to discount the exchangeable hydrogen fractionation we used a comparative equilibration approach where samples are equilibrated with the same ambient water vapour as working standards with known non-exchangeable δD values (Wassenaar and Hobson Reference Wassenaar and Hobson2003). Feather samples (0.15–0.30 mg) and keratin working standards (BWB-II = 108 ± 4‰, CFS = 138 ± 5‰, CHS = 187 ± 2‰; Wassenaar and Hobson Reference Wassenaar and Hobson2003) were weighed into silver cups and stored for 72 hr at room temperature prior to analysis. The 2H/1H ratios were measured using a high temperature TC/EA elemental analyzer (Thermo Fisher Scientific, Bremen, Germany) with pyrolysis at 1,450 °C that was coupled to the CFIRMS. A linear regression model fitted between measured vs actual δD values for the three keratin working standards was used to correct the δD values of the feather samples (Wassenaar and Hobson Reference Wassenaar and Hobson2003).
Data analyses
All mean values are presented ± SE, unless otherwise stated. Where data were normally distributed we conducted t-tests and where data were not normally distributed we conducted a non-parametric Mann-Whitney U-test, between adult and juveniles, and males and females. For comparisons between sites we used an ANOVA where data were normally distributed and a Kruskal-Wallis test where data were not normally distributed. Comparisons of δD in adult feathers with the predicted δD values of precipitation for the site where the feathers were collected (in South Africa) were made using a simple bivariate regression model. We calculated the δD (‰, VSMOW) value of precipitation for each site where feathers were collected in South Africa using The Online Isotopes in Precipitation Calculator (OIPC) (Bowen et al. Reference Bowen, Wassenaar and Hobson2005, Bowen Reference Bowen2009). To predict the origins of juveniles we calculated the δD values for precipitation (where the birds originated) by using a calibration curve derived for American Kestrels Falco sparverius (Hobson et al. Reference Hobson, deMent, Van Wilgenburg and Wassenaar2009). This calibration curve was derived from known-source F. sparverius feathers (from Lott and Smith Reference Lott and Smith2006), and predicted growing-season δD in precipitation (from Bowen et al. Reference Bowen, Wassenaar and Hobson2005). We cannot be sure of the proportion of prior precipitation that contributes to tail feather growth of juveniles. This is because a broad range of integration from the environment, representing a wider temporal period than simply the integration of D during the month of feather growth, is likely. We therefore used an annual mean δD value of precipitation throughout the breeding range to predict the D in precipitation that contributed to feather growth (Hobson et al. Reference Hobson, deMent, Van Wilgenburg and Wassenaar2009). In the northern Palaearctic the mean growing season average of precipitation δD values, as an estimate of mean hydrogen isotope input to the food webs, is typically used and is usually defined as those months where temperature is > 0oC. In temperate regions this may also include snow. However, until there is a good δD feather map for the Palaearctic this interpretation will remain an issue. Tail feathers of juveniles grow mostly during June (Schäfer 2002). We therefore expected the diet of Amur Falcons in their breeding range, which includes a wide range of invertebrate and small vertebrate prey (e.g. birds, rodents, amphibians; Schäfer Reference Schäfer2003); to represent precipitation many months prior to feather growth. The predicted δD values of precipitation that indicated the origins of juvenile feathers were then overlaid with the distribution range of Amur Falcons in the Palaearctic to indicate the likely origins of juveniles that we sampled in South Africa (Ferguson-Lees and Christie Reference Ferguson-Lees and Christie2001, S. Gombobaatar unpubl. data). We also overlaid this distribution with precipitation δD values (that indicated the origins of juvenile feathers) for the month (June) when juvenile feathers grew, but do not present the data.
Results
Shapiro-Wilk’s test for normality indicated that data were normally distributed for δD (W = 0.9859, P = 0.46) but not normally distributed for δ15N (W = 0.9673, P = 0.02) and δ13C (W = 0.9267, P < 0.01).
The δD values for juveniles were significantly depleted compared to adults (adults = -37.4 ± 1.8‰, range = -71.3 to -9.3‰; juveniles = -58.1 ± 2.5‰, range = -83.9 to -25.7‰, t = -6.87, df = 86, P < 0.001; Figure 1). There was no significant difference among sites for juveniles (ANOVA, F 5,29 = 0.984, P = 0.44) or adults (ANOVA, F 4,48 = 0.930, P = 0.45). The δD values for adult males and females were similar (male = -37.2 ± 2.5‰, female = -37.6 ± 2.6‰, t = 0.087, df = 51, P = 0.93). Also, δD values did not differ among sites for males (ANOVA, F 4,20 = 0.280, P = 0.887) or females (ANOVA, F 4,23 = 1.174, P = 0.348) (Figure 1).
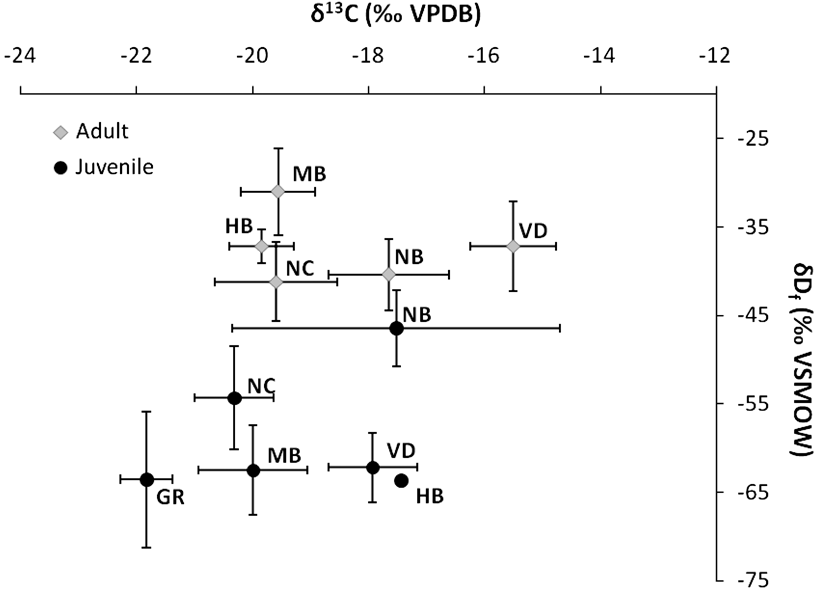
Figure 1. Mean δ13C (VPDB) and δD (VSMOW) (‰ ± SE) values of feathers for adult and juvenile Amur Falcons from different roost sites in South Africa. See Table 1 for details of sites and sample sizes. MB = Middelburg, GR = Graaff-Reinet, NB = Naboomspruit, NC = Newcastle, HB = Heidelberg, VD = Ventersdorp.
Values for δ13C between adults and juveniles were not significantly different, (Mann-Whitney U = 807.0, P = 0.174, adults -18.6 ± 0.4‰, juveniles = -19.3 ± 0.5‰). For adults there was a significant difference among sites (Kruskal-Wallis test: H 5,54 = 15.33, P = 0.009) with a post-hoc test determining Ventersdorp adults (δ13C = -15.5 ± 0.7‰) to be more enriched than Newcastle adults (δ13C = -19.6 ± 1.1‰) (P = 0.03). For juveniles, although there was no significant difference among sites (Kruskal-Wallis test: H 4,34 = 7.63, P = 0.11), although post-hoc tests revealed Ventersdorp (-17.9 ± 0.8‰) to be more enriched than Graaff-Reinet (-21.8 ± 0.4‰), Middelburg (-20.0 ± 0.9‰) and Naboomspruit (-17.5 ± 2.8‰), and Newcastle (-20.3 ± 0.7‰) more enriched than Graaff-Reinet (P < 0.05) (Figure 1).
Values for δ15N were similar between adults and juveniles (Mann-Whitney U = 911.0, P = 0.61, adults = 7.6 ± 0.3‰, juveniles = 7.3 ± 0.4‰). For adults and juveniles there was no difference among sites (Kruskal-Wallis test: H 5,54 = 4.33, P = 0.50 and H 5,36 = 5.33, P = 0.38, respectively; Table 2).
Table 2. Mean (‰ ± SE, AIR) δ15N values of adult and juvenile feathers for different roost sites in South Africa, mean predicted δD values (‰ ± SE, VSMOW) for precipitation during the moult period (December-February) of Amur Falcons at different feather collection sites and mean δD values (‰ ± SE, VSMOW) of feathers collected at respective sites during December 2008 – March 2009. Sample size in parentheses.
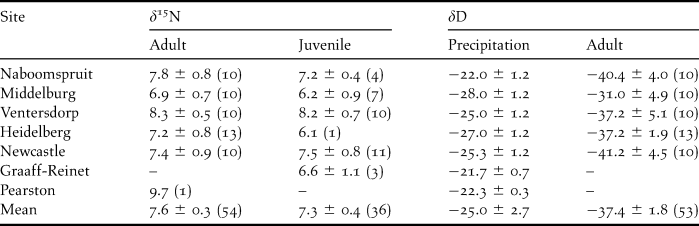
Using a global model of δD in precipitation we calculated predicted annual values of δD (-20.2 ± 0.9‰, n = 5 sites), for each site in South Africa where we collected adult feathers (Bowen et al. Reference Bowen, Wassenaar and Hobson2005, Bowen Reference Bowen2009). Adult δDf values ranged from -71.3‰ to -9.3‰ (mean = -37.4 ± 1.8‰) and mean site values were all more depleted (3.0 – 18.4‰) than predicted annual precipitation (δDp) values. The δDf values of adult feathers from the respective roost sites was not correlated with δDp values at each site (Bowen et al. Reference Bowen, Wassenaar and Hobson2005, Bowen Reference Bowen2009) (Df adults = -64.108 – 1.3213*Dp, F 1,3 = 2.51, P = 0.21) (Table 2).
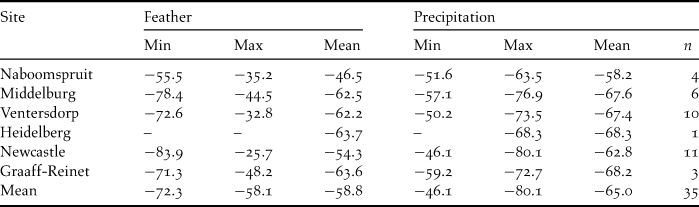
Predicting δDp values for sites where juveniles fledged was more difficult because we did not know their origins. Also, the Palaearctic distribution of Amur Falcons is poorly documented, with breeding recorded as far south as Bhutan (see Figure 2; Baker Reference Baker1935 in Feijen and Feijen Reference Feijen and Feijen2008). The mean value for juvenile feathers (δDf) was -58.8‰ which was well within expected values throughout the range of Amur Falcons in the Palaearctic (Figure 2; OIPC, Bowen Reference Bowen2009). Juvenile δDf values ranged from -83.9 to -25.7‰. We predicted δDp values within the range of Palaearctic Amur Falcons to range from -80.1‰ to -46.1‰ (Figure 2; Table 3; Hobson et al. Reference Hobson, deMent, Van Wilgenburg and Wassenaar2009). We therefore overlaid the range of δDp values from -85‰ to -40‰ to indicate the regions, within their known range, from where juvenile Amur Falcons originated (Figure 2; Table 3).

Figure 2. Distribution of Amur Falcon Falco amurensis in the eastern Palaearctic during the boreal summer (modified from Moreau Reference Moreau1972, Ferguson-Lees and Christie Reference Ferguson-Lees and Christie2001, S. Gombobaatar unpubl. data), and regions within the range (δD of precipitation, Bowen et al. Reference Bowen, Wassenaar and Hobson2005, Bowen Reference Bowen2009) that correspond to likely δDf values of juvenile Amur Falcons sampled in South Africa (Hobson et al. Reference Hobson, deMent, Van Wilgenburg and Wassenaar2009; see Table 3). The isotopic range of δDp, from where the birds are likely to have originated was determined using a calibration curve for American Kestrels Falco sparverius (Hobson et al. Reference Hobson, deMent, Van Wilgenburg and Wassenaar2009). Missing δD plots on the map do not fit within this range of predicted values, and become more depleted with an increase in latitude north.
Table 3. Minimum, maximum and mean (± SE) (‰, VSMOW) values for juvenile feathers collected in South Africa with predicted δD values (‰, VSMOW) of precipitation in Northern Hemisphere where feathers are likely to have grown. Predicted precipitation values calculated using calibration curve for American Kestrels (δDf = 53.06 + 1.71*δDp , n = 41; Hobson et al. Reference Hobson, deMent, Van Wilgenburg and Wassenaar2009).
Discussion
The Eastern Palaearctic to South Africa
Because juvenile flight feathers of birds sampled in South Africa were grown on the nest in the Palaearctic, they were expected to represent the breeding origin of birds (Hobson and Wassenaar Reference Hobson and Wassenaar1997, Meehan et al. Reference Meehan, Lott, Sharp, Smith, Rosenfield, Stewart and Murphy2001, Wassenaar and Hobson Reference Wassenaar and Hobson2001, Hobson et al. Reference Hobson, Bowen, Wassenaar, Ferrand and Lormee2004b). Most stable isotope studies on raptor species show a depletion in δD of feathers from precipitation of 37–52‰ (Lott et al. Reference Lott, Meehan and Heath2003, Meehan et al. Reference Meehan, Rosenfield, Atudore, Bielefeldt, Rosenfield, Stewart, Stout and Bozek2003). To predict the region where feathers of juveniles were grown we used a calibration curve determined for American Kestrels Falco sparverius that accounted for fractionation of D from local annual precipitation to feathers (Hobson et al. Reference Hobson, deMent, Van Wilgenburg and Wassenaar2009). We recorded a wide range (-83.9 to -25.7‰) of feather δD values of juveniles with all predicted values for juveniles falling well within the range of expected annual δDp values of their Palaearctic distribution range. Not surprisingly, the northern limit of where juveniles were predicted to have originated (i.e. predicted northern limit of breeding range for birds sampled in South Africa) correlates well with the known northern limit of the distribution of Amur Falcons in the Palaearctic. Rather than attempting to pinpoint the origins of individual birds, we focused on assigning the origins of juveniles to a broad geographic band within the known range of breeding Amur Falcons (Langin et al. Reference Langin, Reudink, Marra, Norris, Kyser and Ratcliffe2007). Our findings therefore suggest that juveniles in South Africa originated from throughout their distributional range in the Palaearctic (Figure 2, Wassenaar and Hobson Reference Wassenaar and Hobson2001, Bowen and Revenaugh Reference Bowen and Revenaugh2003, Hobson and Wassenaar Reference Hobson and Wassenaar2008). However, birds from a region west of Ulaanbaatar, Mongolia, appear not to have been sampled. This may reflect an absence of birds from this region in South Africa or simply inconsistencies in our method of analysis. Amur Falcons have been recorded breeding north of Ulaanbaatar and 378 individuals have been ringed in a study there (Schäfer and Stubbe Reference Schäfer and Stubbe2005). However, none of these individuals have been recovered in their “overwintering” grounds. This is more likely a reflection of the low numbers of recoveries and difficulties in catching birds in their overwintering range.
Because there is temporal variation in the δD values of precipitation we cannot be sure that hydrogen contributions to feather growth reflected values for an entire annual period, rather than δD values of precipitation for June, the month in which juveniles grew tail feathers (Schäfer Reference Schäfer and Stubbe2005). However, we consider the former scenario more likely because prey of Amur Falcons will probably reflect the signature of prey that had assimilated resources representing a broader time period prior to feather growth. It is, however, interesting to note that when precipitation values in the breeding range are considered for June, the origins of juveniles sampled in South Africa extends further north than their known distribution in the Palaearctic. Although plausible, this seems less likely.
On global and regional scales the distribution of C3 and C4 grasses has been well documented with C4 grasses largely restricted to warmer climates and (lower latitudes and altitudes) (e.g. Teeri and Stowe Reference Teeri and Stowe1976, Stowe and Teeri Reference Stowe and Teeri1978, Vogel et al. Reference Vogel, Fuls and Ellis1978, Hattersley Reference Hattersley1983). Also, there is a general decrease in the proportion of C4 grasses with an increase in latitude (cooler climates). We therefore expected enriched (more positive) δ13C values, representing carbon input from a C3 dominated system, to reflect a more northerly origin for juveniles (Kelly et al. Reference Kelly, Atudorei, Sharp and Finch2002). For juveniles sampled in South Africa there was a general trend of northern-most birds representing a stronger C4 dietary signature, and more southerly populations having a stronger C3 signature. In the northern hemisphere the diet of juveniles includes a greater proportion of mammals, birds and amphibians (Schäfer Reference Schäfer2003, Pietersen and Symes Reference Pietersen and Symes2010) so the link between δ13C values and latitude is less plausible for inferring breeding origins of South African birds. A more adequate explanation is that the wide range of δ13C values represents the variable ingestion of prey items from C3 and C4 sources.
Carbon input from the environment may therefore not be a good indicator of latitudinal origin because of diet switching between C3 and C4 resources at a site, or the broad spatial use of resources by adults feeding nestlings. This pattern is also reflected in the wide range of δ13C values of adult feathers in South Africa suggesting that birds within a roost are not all relying on the same food source, or food type, and that they are sourcing their diet from both C3 and C4 systems. Carbon sourced from both C3 and C4 in different adult individuals from the same roost may thus reflect, i) a wide feeding niche with reliance on C3 (strong broadleaved or high altitude and/or altitude grassland influence) to C4 (low altitude and/or altitude grassland) and/or crops (e.g. maize), and/or, ii) the sampling of birds whose feathers had grown with resources beyond that reflected in the vicinity of the roost. This latter point is supported by the lack of a positive correlation between the δDf values of adult feathers and the mean δDp of precipitation at the sites where feathers were collected, i.e. sites where feathers were presumed to have moulted if adult Amur Falcons were faithful to roost sites.
Roosting in colonies
The 2009 National Kestrel Count Day (24 January) recorded 111,291 Amur Falcons at 32 roosts in South Africa (www.kestreling.com). This annual national count has, over the past five years recorded significant changes in roost sizes, with some roosts being abandoned (www.kestreling.com). Also, it represents only a fraction of the estimated global population of 100,000–1,000,000 birds (Global Raptor Information Network 2008, BirdLife International 2008). Usually roosts are active over several consecutive years, although numbers within each roost are known to vary within and between seasons (www.kestreling.com). These changes may reflect a movement of birds across the subregion and real changes in Amur Falcon numbers at regularly counted roost sites. In addition they may reflect an absence of birds in the subregion, adding support to the idea that a large proportion of birds do not reach South Africa to overwinter. The proportion of the population not reaching South Africa may overwinter further north and we suggest that it comprises birds starting their migration later (Figure 2). These birds probably overwinter in Namibia, Zambia, Zimbabwe, Malawi and Mozambique, with some birds remaining as far north as Tanzania (Mendelsohn Reference Mendelsohn, Harrison, Allan, Underhill, Herremans, Tree, Parker and Brown1997, Stevenson and Fanshawe Reference Stevenson and Fanshawe2002, Jenkins Reference Jenkins, Hockey, Dean and Ryan2005). Other overwintering sites remain to be investigated and could likely include the Indian sub-continent (Mendelsohn Reference Mendelsohn, Harrison, Allan, Underhill, Herremans, Tree, Parker and Brown1997, Jenkins Reference Jenkins, Hockey, Dean and Ryan2005).
The δD values of adult feathers, as predicted, were more depleted relative to predicted δD values in precipitation. Adults undergo a near complete moult in South Africa (Schäfer Reference Schäfer2003) so we expected feathers to represent the site at which they were collected (see Hobson and Wassenaar Reference Hobson and Wassenaar2008). There was no correlation of δDf values with δDp suggesting that feathers grown in South Africa did not grow at the roost site where they were collected (OIPC, Bowen Reference Bowen2009). It is unknown whether roost site fidelity is high within a season, or whether the same birds return to the same roost site each year. Given these data, and large variation in roost counts between years, we suggest that roost site fidelity is low, with a prolific movement of birds across the southern African subregion both within and between years. Because individual birds are not fixed to a specific site by breeding they are able to wander, feeding on the episodic and patchy occurrence of food resources such as crop pests (e.g. maize pests), and grasshopper and termite alate irruptions (Brown Reference Brown1971, Brown et al. Reference Brown, Urban and Newman1982, Pietersen and Symes Reference Pietersen and Symes2010).
Most grasses within the range of Amur Falcon in South Africa are C4 although the diet of falcons is not confined to arthropods reliant on this vegetation type (Vogel et al. Reference Vogel, Fuls and Ellis1978, Mendelsohn Reference Mendelsohn1979, Reference Mendelsohn, Harrison, Allan, Underhill, Herremans, Tree, Parker and Brown1997, Jenkins Reference Jenkins, Hockey, Dean and Ryan2005, Pietersen and Symes Reference Pietersen and Symes2010). If all adult Amur Falcons at a roost site were feeding on a similar diet during the period of feather growth in South Africa we would expect them to have similar carbon isotope values in feathers. This was not the case and diet studies indicate they feed on wide range of invertebrates (Mendelsohn Reference Mendelsohn1979, Reference Mendelsohn, Harrison, Allan, Underhill, Herremans, Tree, Parker and Brown1997, Jenkins Reference Jenkins, Hockey, Dean and Ryan2005, Pietersen and Symes Reference Pietersen and Symes2010). The wide range of δ13C values is therefore associated with a variable diet and foraging over a wide area to access these food sources.
Rectrices were used because they can be readily aged and sexed and are freely available below roosts as part of the normal moult process. However, they may not be the best feathers for isotope studies because they are much more likely to be dropped and replaced irregularly and not as part of the normal physiological moult processes e.g. during escape from predation. The wide range of samples (and outliers) for adults and juveniles may therefore be the result of irregular moult patterns and not identification error. Remiges may have yielded better results but with the current sampling method, ageing and sexing of remiges would not have been possible.
Trophic position
In the open savanna environment where Amur Falcons were recorded, the δ15N values of invertebrates found in the grass (C4) and wooded vegetation (C3) over five months were sampled and mean values (± SD) of 4.7 ± 1.4‰ and 6.3 ± 1.9‰ recorded respectively (n = 15 each) (Symes et al. unpubl. data). This suggests that the values for adults (7.6 ± 0.3‰) are in line with a typical open savanna/grassland invertebrate diet, being an estimated one tropic position above the invertebrates in this system. Values of juveniles and adults were similar suggesting that in South Africa and the Palaearctic Amur Falcons feed at a similar trophic level in both regions. In South Africa the diet of Amur Falcons has been shown to include solifugids, beetles, termites but seldom rodents and birds (Mendelsohn Reference Mendelsohn, Harrison, Allan, Underhill, Herremans, Tree, Parker and Brown1997, Jenkins Reference Jenkins, Hockey, Dean and Ryan2005, Pietersen and Symes Reference Pietersen and Symes2010). However, dietary information on juveniles suggests otherwise; mammals, birds and amphibians appear to be more important dietary items for nestlings (Schäfer Reference Schäfer2003). En route diets are not known and difficult to interpret from stable isotope analysis and it is unknown whether Amur Falcons rely on stop-over feeding sites during migration. Their migration patterns may be timed to take advantage of prevailing weather conditions, moving ahead of the ITCZ with the shifting of seasons, much the same as dragonfly migrations between Africa and India (Anderson Reference Anderson2009). During the non-breeding period of Amur Falcons in South Africa, the irruption of termite alate and crop-pest species, important food sources, may often be unpredictable and patchy; in the Palaearctic this may be less so, although a wide range of δ15N values suggests a nitrogen input from a range of trophic levels for both adults in South Africa and juveniles in the Palaearctic.
Conclusions
The status of the Amur Falcon is currently unclear and there is little evidence for recent changes in its distribution and numbers in Africa (Mendelsohn Reference Mendelsohn, Harrison, Allan, Underhill, Herremans, Tree, Parker and Brown1997, Global Raptor Information Network 2008, BirdLife International 2008). In light of recent findings that the total number of birds recorded in South Africa alone is significantly below that estimated for the total world population, either a significant proportion of the population overwinter north of South Africa or the global estimate is significantly over-inflated (Global Raptor Information Network 2008, BirdLife International 2008, A. van Zyl pers. comm.). The grassland regions within their South African range, upon which they are reliant for foraging, is under threat from agriculture, afforestation and open-cast mining (Mendelsohn Reference Mendelsohn, Harrison, Allan, Underhill, Herremans, Tree, Parker and Brown1997, Jenkins Reference Jenkins, Hockey, Dean and Ryan2005). Stomach content analysis suggests that Amur falcons forage successfully over maize monoculture (Pietersen and Symes Reference Pietersen and Symes2010) indicating some flexible adaptation to anthropogenic changes. Also, the isotopic evidence suggests that the niche occupied by the Amur Falcons during their stay in South Africa is cosmopolitan, with a broad dietary base and opportunistic exploitation of wide spatial ranges. Before the conservation status of the species can be concluded, the niche bottlenecks associated with migration and breeding at other locations on their annual migration also need to be considered. For example reports of large scale capture of migrating birds at important stopover sites in northern India are cause for concern (Tan Reference Tan2009). In the northern hemisphere, the picture is less clear, and although the entire population may disperse over a breeding range far greater (~ 4,000,000 km2) than the South African range (~ 500,000 km2), similar factors may affect its conservation there. The species is categorised globally as a species of "Least Concern" by BirdLife International (2008). With rapid changes in human population growth and the associated impacts on the environment, more information is required to ensure the long term persistence of this long-distance migratory raptor.
Acknowledgements
The University of the Witwatersrand are thanked for providing funding for this project. Zephné Bernitz is thanked for assisting in age and sex identification of feathers, and for collecting feather samples from the Middelburg roost. Shirley Grindley and Roy Stauth (Graaff-Reinet), Saartjie Kidson (Naboomspruit), Rina Pretorius (Newcastle) and Ria Martins (Heidelberg) are thanked for collecting feather samples. Tamar Cassidy is thanked for access to skins in the Transvaal Museum (Pretoria), and the Northern Flagship Institution is thanked for permission to sample Amur Falcon feathers in the Transvaal Museum collection and for access to moult data. Dr. Sundev Gombobaatar (Gomboo) (National University of Mongolia) is also thanked for additional comments and providing Amur Falcon distribution maps. Tracy Symes and Jolene Fischer are thanked for assistance in map construction. Anthony van Zyl, Zephné Bernitz and an anonymous reviewer are thanked for comments to help improve the quality of the manuscript.
Appendix A. Specimen numbers of Amur Falcons from Ventersdorp sampled at the Transvaal Museum, Pretoria (Northern Flagship Institution).