Introduction
Among pests, weeds are acknowledged as the primary contributor to economic loss in crop production (Pimentel et al. Reference Pimentel, Lach, Zuniga and Morrison2000) and other managed systems, including rangelands (Smith et al. Reference Smith, Strain and Sharkey1987) and forests (Webster et al. Reference Webster, Jenkins and Jose2006). Weeds are also known to have a wide variety of other effects on ecosystem dynamics (Mooney and Hobbs Reference Mooney and Hobbs2000), including negative impacts on species diversity and ecosystem services (Forseth and Innis Reference Forseth and Innis2004; Pejchar and Mooney Reference Pejchar and Mooney2009).
However, the definition of “weed” is always in the context of the plant system being impacted. As such, it is a human designation, not a biological one. For example, invasive weeds may be defined as plant species outside their native geographic ranges whose presence results in substantial economic (e.g., crop loss) or ecological (e.g., species diversity) consequences (Richardson et al. Reference Richardson, Pyšek, Rejmánek, Barbour, Panetta and West2000). But a strict definition of “invasive” can be elusive. For example, if we were to focus on North America, we would find that common lambsquarters (Chenopodium album L.) is from Eurasia, but is considered a native weed; kudzu [Pueraria montana (Lour.) Merr. var. lobata (Willd.) Maesen & S.M. Almeida ex Sanjappa & Predeep] is from East Asia and is generally deemed invasive; whereas native weeds such as ragweeds (Ambrosia spp.) are common, but if found beyond their geographic ranges, could be considered invasive.
Given the tremendous variety of agronomic and invasive weeds, as well as the differences among invaded ecosystems, generalizations regarding how weeds will evolve are unlikely. Yet there are some common themes in weed biology that are relevant to evolutionary responses. In this review, we want to explore those responses with the goal of identifying specific evolutionary consequences associated with a rapidly changing climate. We expect that such consequences will be of importance in defining and directing research for all weed biology, independent of context, for this century.
What aspects of a rapidly changing climate should concern weed scientists? Atmospheric CO2 concentrations have risen by ∼30% since 1957 and, at current levels of fossil fuel use and deforestation, may exceed 800 ppm by the end of the current century (Field et al. Reference Field, Barros, Dokken, Mach, Mastrandrea, Bilir, Chatterjee, Ebi, Estrada, Genova and Girma2014). Concomitant increases in average temperature between 0.15 and 0.3C per decade, with greater temperature extremes, are also expected by 2100. Predictions for altered precipitation are less certain, but include greater likelihood of drought at lower latitudes, increased precipitation at higher latitudes, and an increase in the frequency and intensity of extreme precipitation events (Dore Reference Dore2005; Qian et al. Reference Qian, Gregorich, Gameda, Hopkins and Wang2011; Rosenzweig et al. Reference Rosenzweig, Iglesias, Yang, Epstein and Chivian2001; Swain and Hayhoe Reference Swain and Hayhoe2015).
There are, in turn, two basic means whereby these global changes will impact weed biology. The first is related to physical changes in the environment. Both weeds and weed management are sensitive to climate, and changes in temperature and precipitation are likely to alter the range, composition, and competitiveness of any weed species (Bradley et al. Reference Bradley, Blumenthal, Wilcove and Ziska2010; Ziska and Dukes Reference Ziska and Dukes2011). A second impact is the “fertilization” effect of rising CO2 on plant photosynthesis. Because photosynthesis involves the conversion of CO2 to sugars and is limited by the current concentration of CO2, ongoing increases will stimulate photosynthesis and plant growth. Cool-season species that use C3 photosynthesis (~85% of plant species, including many weeds) are particularly responsive to increases in CO2 (Ogren and Chollet Reference Ogren and Chollet1982; Ziska Reference Ziska2003). In addition to its direct fertilization effect, CO2 can also increase plant water-use efficiency, with potentially strong effects on invasive plant species establishment (Belote et al. Reference Belote, Weltzin and Norby2003; Blumenthal et al. Reference Blumenthal, Resco, Morgan, Williams, LeCain, Hardy, Pendall and Bladyka2013; Smith et al. Reference Smith, Huxman, Zitzer, Charlet, Housman, Coleman, Fenstermaker, Seemann and Nowak2000).
Increasing CO2 and altered temperature and precipitation are therefore likely to affect all aspects of weed biology (Peters et al. Reference Peters, Breitsameter and Gerowitt2014; Ziska and Dukes Reference Ziska and Dukes2011), including establishment (Clements et al. Reference Clements, DiTommaso, Jordan, Booth, Cardina, Doohan, Mohler, Murphy and Swanton2004), competition (Valerio et al. Reference Valerio, Tomecek, Lovelli and Ziska2011), distribution (Bradley et al. Reference Bradley, Blumenthal, Wilcove and Ziska2010; Thuiller et al. Reference Thuiller, Albert, Araujo, Berry, Cabeza, Guisan, Hickler, Midgley, Paterson, Schurr and Sykes2008), and management (Waryszak et al. Reference Waryszak, Lenz, Leishman and Downey2018). Overall, our ability to characterize evolutionary adaptation of weeds to climate and CO2 has not been given adequate consideration (Moran and Alexander Reference Moran and Alexander2014). Yet such consideration may be particularly relevant, given that weeds are, in general, capable of rapid genetic change (Neve et al. Reference Neve, Vila-Aiub and Roux2009). The focus of the current review is to examine interactions between these impacts and adaptive evolution.
In examining how climate change can alter evolutionary aspects of weed biology, we acknowledge, given the eclectic nature of what constitutes a “weed,” the difficulty in developing conclusive evolutionary insights. However, we hope that a review of existing data can provide general trends related to evolutionary adaptation for three interrelated aspects of weed science: demographics, competition, and management. By examining these biological interactions, we also hope to gain insight into future research priorities that will help elucidate how elevated CO2 and/or climate change will alter selective pressures, fitness, and observed evolutionary responses that will be of fundamental importance in weed biology and weed science.
Weeds and Evolution
In examining evolution, it is important to distinguish between acclimation and adaptation, particularly for weeds. It is commonly accepted that weeds often have “general-purpose genotypes” (Baker Reference Baker1974) and could, potentially, respond to rapid environmental change primarily through plasticity or acclimation of traits such as phenological or morphological characteristics (Davidson et al. Reference Davidson, Jennions and Nicotra2011) with potential diminishment of the correlation between environmental and phenotypic variation. Conversely, weeds also have characteristics that may favor rapid adaptive evolution with climate shifts: large populations, short life cycles, strong dispersal abilities, and in the case of introduced or invasive weeds, novel selection pressures (Clements et al. Reference Clements, DiTommaso, Jordan, Booth, Cardina, Doohan, Mohler, Murphy and Swanton2004; Neve et al. Reference Neve, Vila-Aiub and Roux2009; Prentis et al. Reference Prentis, Wilson, Dormontt, Richardson and Lowe2008).
Any time that environmental conditions change, there is potential for concomitant shifts in natural selection and for adaptive evolution to occur. For weeds, this can take place when introduced to new locations with novel conditions or when existing environmental conditions (e.g., herbivores, competitors) change (Clements and DiTommaso Reference Clements and DiTommaso2011; Mooney and Cleland Reference Mooney and Cleland2001; Sakai et al. Reference Sakai, Allendorf, Holt, Lodge, Molofsky, With, Baughman, Cabin, Cohen, Ellstrand, McCauley, O’Neil, Parker, Thompson and Weller2001). For example, herbicide use can lead to selection for, and the evolution of, herbicide resistance (Heap Reference Heap2014; Powles and Yu Reference Powles and Yu2010). However, adaptive evolution will not always occur, as there are acknowledged limitations and constraints (Hoffmann et al. Reference Hoffmann, Donoghue, Levin, Mackay, Rieseberg, Travis, Wray, Losos, Baum, Futuyma, Hoekstra, Lenski, Moore, Peichel, Schluter and Whitlock2014). For example, many weedy species have failed to adapt to serpentine soils despite living in proximity to them, possibly due to lack of genetic variation for tolerance to serpentine soils (Brady et al. Reference Brady, Kruckeberg and Bradshaw2005).
A change in climate could also result in ecological sorting rather than adaptive evolution within populations. For example, earlier onset of spring due to global warming could select for earlier emergence within populations, or could favor species that already emerge earlier, or both (Willis et al. Reference Willis, Bailey, Bhagwat and Birks2010). Consequently, how and to what extent weeds will evolve in response to climatic and other environmental changes, the types of changes most likely to lead to evolution, and which species are most likely to adapt to climatic changes are empirical questions important to weed science.
A key prerequisite for adaptive evolution is genetic variation, because the rate of evolutionary response to selection is directly proportional to the amount of genetic variation in a population (Fisher Reference Fisher1958). Evidence is mixed regarding the level of genetic variation within weed populations. Founder effects, consistent selection pressures, and selfing may all reduce variation, while the presence of large seedbanks that maintain viability of previous biotypes and repeated introductions may enhance or restructure genetic variation over time (Clements et al. Reference Clements, DiTommaso, Jordan, Booth, Cardina, Doohan, Mohler, Murphy and Swanton2004; Dluglosch and Parker Reference Dluglosch and Parker2008). Measurements of genetic variation in weed populations include examples of weed species with ample variation and others in which variation is quite limited (Neve et al. Reference Neve, Vila-Aiub and Roux2009).
Despite potential limitations, there is increasing empirical evidence for rapid microevolutionary change within agronomic and invasive weed species (Maron et al. Reference Maron, Vilà, Bommarco, Elmendorf and Beardsley2004; Neve et al. Reference Neve, Vila-Aiub and Roux2009). In agronomic systems, herbicides represent extraordinarily strong selective pressures, and the evolutionary potential of weeds is perhaps best illustrated by the rapid and widespread documentation of herbicide resistance (Heap Reference Heap2014). In the study of invasive weeds, considerable effort has been devoted to understanding how species have evolved following their introduction to new ranges. Release from specialist herbivores in the introduced range has been hypothesized to allow evolution of reduced defense and increased growth or competitive ability (Blossey and Notzold Reference Blossey and Notzold1995). Common garden studies partially support this idea, suggesting that rapid evolution in both growth and defense is relatively common in species in introduced ranges. (Blossey and Notzold Reference Blossey and Notzold1995; Felker-Quinn et al. Reference Felker-Quinn, Schweitzer and Bailey2013; Zhang et al. Reference Zhang, Pan, Blumenthal, van Kleunen, Liu and Li2018). These examples suggest that the traditional paradigm of weed evolution as a very slow process is incomplete and that rapid evolutionary change (years or decades) can be pervasive within weed biology and could include evolution in response to climate (Clements et al. Reference Clements, DiTommaso, Jordan, Booth, Cardina, Doohan, Mohler, Murphy and Swanton2004; Ravet et al. Reference Ravet, Patterson, Krähmer, Hamouzová, Fan, Jasieniuk, Lawton-Rauh, Malone, McElroy, Merotto and Westra2018).
Overall, it is evident that weed populations can evolve quickly in response to intense selection pressures associated with novel environmental conditions arising from both introduction and management, in accord with the wider recognition that evolution can occur on ecological timescales (Neve et al. Reference Neve, Vila-Aiub and Roux2009; Thompson Reference Thompson1998). Consequently, weeds may often have the capacity to rapidly evolve in response to climatic changes. Further investigations into these evolutionary responses is likely to be a fruitful area of inquiry. Particularly useful may be studies using the resurrection approach (Franks et al. Reference Franks, Hamann and Weis2018) to study weed evolution, such as work done by Kuester et al. (Reference Kuester, Wilson, Chang and Baucom2016), who found evolutionary responses and genetic changes in an agronomic weed following the use of herbicides.
Observed Evolutionary Responses of Weeds to Climate and Climate Change
Much of what we know about how weeds evolve in response to climate comes from range expansions, where it is the weed that moves in relation to the climate, rather than the climate shifting around the weed. Clements et al. (Reference Clements, DiTommaso, Jordan, Booth, Cardina, Doohan, Mohler, Murphy and Swanton2004, Reference Clements, Feenstra, Jones and Staniforth2008) have summarized specific shifts in agronomic and invasive weed species and the adaptive traits associated with this type of northward expansion. For example, populations of the invasive forb common St. Johnswort (Hypericum perforatum L.) display clonal variation in its nonnative range that appears to have evolved since introduction (Maron et al. Reference Maron, Vilà, Bommarco, Elmendorf and Beardsley2004). Plants from more northern latitudes were found to have higher growth and seed production in four different common gardens. Latitudinal clines in phenology have been identified for an array of species, including tall goldenrod (Solidago altissima L.) and giant goldenrod (Solidago gigantea Alton) introduced to Europe and Japanese stiltgrass [Microstegium vimineum (Trin.) A. Camus] introduced into the eastern United States, saltcedar (Tamarix ramosissima Ledeb.) and Chinese tamarisk (Tamarix chinensis Lour.) introduced into the western United States, and jimsonseed (Datura stramonium L.) introduced into Canada (Friedman et al. Reference Friedman, Roelle and Cade2011; Novy et al. Reference Novy, Flory and Hartman2013; Weaver et al. Reference Weaver, Dirks and Warwick1985; Weber and Schmid Reference Weber and Schmid1998). In all cases, plants from northern populations grew, flowered, or set buds earlier in the season. Other traits displaying clonal variation included cold tolerance and plant and seed size.
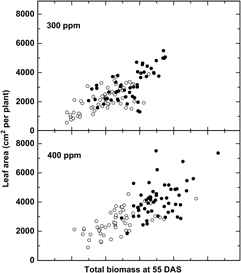
Additional evidence suggests that adaptation to recent changes, particularly the rapid increase in CO2 (+20% since 1980), may have already altered the relative fitness of crops and weeds. Bunce (Reference Bunce2001) studied the growth response of four annual weeds over a narrow CO2 range, from 90 ppm CO2 below to 90 ppm CO2 above ambient levels and demonstrated that the efficiency at which plants use CO2 declined significantly at CO2 concentrations above ambient, suggesting that weeds have been adapting to recent CO2 increases. Comparisons of six cultivated and six wild or weedy biotypes of rice (Oryza sativa L.) indicated a greater overall growth response among wild relative to cultivated rice (Oryza sativa L.) to recent (300 to 400 ppm) increases in CO2 (Ziska and McClung Reference Ziska and McClung2008) (Figure 1), suggesting that rapid evolution of weedy biotypes may have increased their fitness relative to the crop. Greater seed yields were also recorded for Stuttgart, a weedy biotype, relative to ClearfieldTM, a cultivated rice line for the same CO2 range (Ziska et al. Reference Ziska, Tomecek and Gealy2010). Similarly, using a resurrection approach (Franks et al. Reference Franks, Hamann and Weis2018), seed of two temporally distinct populations of wild oat (Avena fatua L.) from the same location, one from the 1960s and one from 2014 (a relative CO2 increase of 80 ppm, or 25% from 1960), demonstrated different competitive abilities against a cultivated oat (Avena sativa L.) line, with the more recent (2014) A. fatua population having greater growth and competitive ability at current CO2 levels (Ziska Reference Ziska2017).

Figure 1. Change in leaf area in response to biomass at 55 d after sowing (DAS) for six wild and six cultivated rice biotypes (closed and open circles, respectively). Differential changes to CO2 between weedy and cultivated rice may influence evolutionary selection and fitness. Adapted from Ziska and McClung (Reference Ziska and McClung2008).
Direct experimental evidence for weed evolution in response to climate change is rare, but there are a few examples. Experiments using seed of the annual weed birdsrape mustard (Brassica rapa L.) collected before and after a severe drought demonstrated that drought exerts strong selection pressure, that flowering time is heritable, and that B. rapa responded to selection by evolving earlier flowering and lower water-use efficiency (a drought escape strategy) within just a few generations (Franks Reference Franks2011; Franks et al. Reference Franks, Sim and Weis2007). Similarly, in a much wetter environment, the limestone grassland of Britain, 13 yr of experimental drought appear to have led to evolution of drought escape in the common weed buckhorn plantain (Plantago lanceolata L.) (Ravenscroft et al. Reference Ravenscroft, Fridley and Grime2014). When grown in a common garden, populations collected from plots subjected to drought displayed greater reproductive allocation. Further work demonstrated differences in genetic variation consistent with these phenotypic differences (Ravenscroft et al. Reference Ravenscroft, Whitlock and Fridley2015). Finally, the annual invasive grass foxtail brome (Bromus madritensis L.), was examined as part of the Mojave Desert CO2 enrichment experiment. The study found that within 7 yr, the grass populations subjected to increased CO2 had evolved reduced stomatal conductance, allowing them to lose less water but still obtain enough CO2 in the enriched environment, demonstrating rapid adaptive evolution to increased CO2 in this weed species (Grossman and Rice Reference Grossman and Rice2014).
There is also experimental evidence that climate change may be increasing gene flow between herbicide-resistant crops and weedy relatives. For many global rice systems, weedy or red rice is recognized as a major production constraint (Chauhan Reference Chauhan2013; Ziska et al. Reference Ziska, Gealy, Burgos, Caicedo, Gressel, Lawton-Rauh, Avila, Theisen, Norsworthy, Ferrero and Vidotto2015). A long-term USDA study comparing outcrossing rates between cultivated and weedy rice at three different CO2 concentrations (300, 400, and 600 ppm; or mid-20th-century, current, and mid-21st-century values, respectively) noted greater synchronicity in flowering times and enhanced outcrossing rates between a cultivated rice mutant that is resistant to a class of herbicides (imidazolinone, ClearfieldTM 161) and a weedy red rice accession (StgS) (Ziska et al. Reference Ziska, Gealy, Tomecek, Jackson and Black2012). Consequently, as CO2 increased, the number of weedy herbicide-resistant hybrid progeny also increased (Ziska et al. Reference Ziska, Gealy, Tomecek, Jackson and Black2012). While additional information on other environmental parameters (e.g., temperature) is needed, CO2 per se could alter floral synchrony and gene flow between crops and weeds, with subsequent consequences for hybridization, herbicide resistance, and evolution.
Climate Change, Selection, and Demography
Understanding factors influencing weed demography (population growth and spread) is of critical importance to weed biology. A changing climate may alter demography directly through differential selective pressures on weed species and indirectly through changes in the abiotic and biotic aspects of the ecosystems or through mediated changes in human management. Direct selection pressures are evident in how elevated CO2 and higher temperatures differentially alter weed growth, leaf production, plant height, and seed production (Liu et al. Reference Liu, Oduor, Zhang, Manea, Tooth, Leishman and van Kleunen2017; Patterson Reference Patterson1995; Walck et al. Reference Walck, Hidayati, Dixon, Thompson and Poschlod2011; Ziska Reference Ziska2011). For example, under elevated CO2 (500 to 800 ppm), flowers, fruits, seed production, and seed mass were all increased, but at different degrees, for a range of agronomic and invasive species (Jablonski et al. Reference Jablonski, Wang and Curtis2002). Variable stimulation of growth and seed production has also been noted for both recent and projected CO2 increases for agronomic and invasive weeds (Blumenthal et al. Reference Blumenthal, Resco, Morgan, Williams, LeCain, Hardy, Pendall and Bladyka2013; Dukes Reference Dukes2002; Dukes et al. Reference Dukes, Chiariello, Loarie and Field2011; Smith et al. Reference Smith, Huxman, Zitzer, Charlet, Housman, Coleman, Fenstermaker, Seemann and Nowak2000; Ziska Reference Ziska2003). CO2-induced stimulation of plant height (height is associated with greater seed dispersal; Thomson et al. Reference Thomson, Moles, Auld and Kingsford2011) has also been observed for red (weedy) rice (Gealy et al. Reference Gealy, Mitten and Rutger2003). Temperature can also influence the extent and timing of plant growth, as well as seed germination and emergence (Benech-Arnold et al. Reference Benech-Arnold, Sánchez, Forcella, Kruk and Ghersa2000). In warmer regions, increases in temperature are also likely to select for tolerance or avoidance of drought and heat (Franks et al. Reference Franks, Sim and Weis2007). Whether these initial responses are indirect (plastic) or direct (genetic), if CO2 and temperature elicit inter- or intraspecific responses that result in greater exploitation of additional carbon and/or longer growing seasons to increase seed production (Grossman and Rice Reference Grossman and Rice2014; Hovenden et al. Reference Hovenden, Miglietta, Zaldei, Vander Schoor, Wills and Newton2006), evolutionary selection will occur.
As weed managers adapt to a changing climate, changes in management may also alter selection pressures and weed demography. In cropping systems, producers are likely to shift to new crops better suited to new climates (Olesen et al. Reference Olesen, Trnka, Kersebaum, Skjelvåg, Seguin, Peltonen-Sainio, Rossi, Kozyra and Micale2011). In rangelands and forests, plant community changes may be driven by differential movement and local extinction of native species, as well as by changes in disturbance regimes (Thomas et al. Reference Thomas, Cameron and Green2004; Thuiller et al. Reference Thuiller, Lavorel, Araujo, Sykes and Prentice2005). For example, changes in fire regimes due to the introduction and spread of flammable weeds such as downy brome (Bromus tectorum L.) are expected to be widespread, leading to dramatic shifts in plant communities (Early et al. Reference Early, Bradley, Dukes, Lawler, Olden, Blumenthal, Gonzalez, Grosholz, Ibanez, Miller, Sorte and Tatem2016) and, presumably, strong selection pressures on extant species.
One of the most interesting forecasts regarding climate and weed demography was made almost 30 yr ago for P. montana, a well-established weed of the southeastern United States. Specifically, Tom Sasek and Boyd Strain at Duke University observed that the latitudinal distribution in 1990 was limited to regions in the southern United States where minimal winter temperatures were not below −15C (Sasek and Strain Reference Sasek and Strain1990: Figure 7), and they suggested that warming winter temperatures could result in the northward migration of this species. How much of this latitudinal migration is solely attributable to increasing minimum winter temperatures is unclear (see Coiner et al. Reference Coiner, Hayhoe, Ziska, Van Dorn and Sage2018), but the northward spread of P. montana is consistent with the Sasek and Strain hypothesis. Various models have since been developed for predicting invasive species movement with climate change (e.g., Bradley Reference Bradley2010; Bradley et al. Reference Bradley, Blumenthal, Wilcove and Ziska2010).
Rapid range shifts can lead to a variety of evolutionary responses. In addition to contributing to novel selection pressures, range shifts may also increase genetic variation, as previously separate populations interbreed, potentially increasing responses to selection and facilitating adaptation (Bell and Gonzalez Reference Bell and Gonzalez2011; Hufbauer et al. Reference Hufbauer, Szucs, Kayson, Youngberg, Koontz, Richards, Tuff and Melbourne2015). However, dispersal could also negatively affect adaptation in weeds if populations are locally adapted and dispersal introduces maladaptive alleles (Bourne et al. Reference Bourne, Bocedi, Travis, Pakeman, Brooker and Schiffers2014; Lenormand Reference Lenormand2002). Local adaptation was thought to be less common in introduced weeds than in other species; however, a recent review found that invasive plants were just as locally adapted as native species (Oduor et al. Reference Oduor, Leimu and van Kleunen2016).
While dispersal can influence rapid evolution positively, the reverse can also occur, with rapid evolution facilitating range expansion (Szűcs et al. Reference Szűcs, Melbourne, Tuff, Weiss-Lehman and Hufbauer2017). For example, in a common garden study of the invasive perennial weed Senecio inaequidens DC, populations collected farther from the initial site of invasion were found to have larger dispersal structures and therefore greater dispersal potential, suggesting that novel environments can select for greater dispersal ability (Mahy and Mahy Reference Mahy and Mahy2010). Interestingly, projected warming may be exceeding maximum rates of plant migration that were observed in postglacial time periods (Malcolm et al. Reference Malcolm, Markham, Neilson and Garaci2002), resulting in preferential evolutionary selection for the most mobile plants (Boeye et al. Reference Boeye, Travis, Stoks and Bonte2013). Characteristics associated with long-distance dispersal are commonly found among invasive plants (Rejmanek Reference Rejmanek1996), suggesting that, potentially, they may be among the fastest to migrate with warming temperatures (Dukes and Mooney Reference Dukes and Mooney2000). The ecological processes of dispersal and migration could be augmented by evolution if there is selection for increased dispersal or adaptation to novel conditions in the migrating invasive weed, meaning that current projections of future distributions of invasive weeds that do not take evolution into account may be overly conservative (Clements and DiTommaso Reference Clements and DiTommaso2011).
Competition and Fitness
Competition is a central aspect of weed biology, because it is through interspecific competition, or the reduction in fitness of two species over shared resources, that weeds reduce production in natural and managed systems. Yet competitive ability is not fixed, it reflects the environmental conditions under which competition occurs and can evolve as plant density or other factors influencing competitive advantage shift (Grace Reference Grace1990). Consequently, differential selection to climate and/or CO2 between weeds and the natural or managed plant community (e.g., forest plantations, rangelands, crops) may have significant economic and environmental repercussions.
Many weeds have the C4 pathway, which shows a minimal response to CO2, whereas crops often have the C3 pathway, which shows a stronger response. As such, it has been hypothesized that crops would outcompete weeds as CO2 rose (Ziska and Dukes Reference Ziska and Dukes2011). However, early studies did not capture the complexity of agroecosystems where, on average, each crop competes with 8 to 10 weed species (Bridges Reference Bridges1992). Moreover, a competitive advantage for C3 crops over C4 weeds is likely to occur only under rising CO2 without concomitant changes in climate. For example, at higher temperatures and increased drought, C4 weeds can still benefit (Alberto et al. Reference Alberto, Ziska, Cervancia and Manalo1996; Valerio et al. Reference Valerio, Tomecek, Lovelli and Ziska2011) relative to C3 crops.
For invasive weeds, data regarding the response of an individual invasive to rising CO2 can provide a sense of the growth or reproductive potential of that species relative to the community at large (Ziska Reference Ziska2003). In that regard, projected CO2 concentration value levels have been shown to preferentially select (within native plant communities) for weed species such as Japanese honeysuckle (Lonicera japonica Thunb.) (Belote et al. Reference Belote, Weltzin and Norby2003), cherry laurel (Prunus laurocerasus L.) (Hattenschwiler and Korner Reference Hattenschwiler and Korner2003), red brome (Bromus rubens L.) (Smith et al. Reference Smith, Huxman, Zitzer, Charlet, Housman, Coleman, Fenstermaker, Seemann and Nowak2000), mile-a-minute (Mikania micrantha Kunth.), Chinese wedelia (Wedelia chinensis L. Pruski.), beach morningglory [Ipomoea pes-caprae (L.) R. Br.] (Song et al. Reference Song, Wu, Changhan, Furong, Peng and Chen2009), and Dalmatian toadflax [Linaria dalmatica (L.) Mill.] (Blumenthal et al. Reference Blumenthal, Resco, Morgan, Williams, LeCain, Hardy, Pendall and Bladyka2013). Similarly, warming can favor invasive species relative to natives through the process of species sorting, but this effect appears to be inconsistent, perhaps because warming can increase water stress (Blumenthal et al. Reference Blumenthal, Resco, Morgan, Williams, LeCain, Hardy, Pendall and Bladyka2013; Compagnoni and Adler Reference Compagnoni and Adler2014; Sandel and Dangremond Reference Sandel and Dangremond2012; Walther et al. Reference Walther, Roques, Hulme, Sykes, Pyšek, Kühn, Zobel, Bacher, Botta-Dukat, Bugmann and Czucz2009; Williams et al. Reference Williams, Wills, Janes, Schoor, Newton and Hovenden2007).
Because of methodological difficulties, experimental manipulation of both CO2 and temperature for plant communities in situ are rare. The combination of these changes had no net effect on common catsear (Hypochaeris radicata L.) or lesser hawkbit (Leontodon saxatilis Lam.) in a Tasmanian grassland, but increased invasion of yellow starthistle (Centaurea solstitialis L.) in a California grassland (Dukes et al. Reference Dukes, Chiariello, Loarie and Field2011), and of L. dalmatica, B. tectorum, and diffuse knapweed (Centaurea diffusa Lam.) in a Wyoming mixed-grass prairie (Blumenthal et al. Reference Blumenthal, Resco, Morgan, Williams, LeCain, Hardy, Pendall and Bladyka2013, Reference Blumenthal, Kray, Ortmans, Ziska and Pendall2016; Reeves et al. Reference Reeves, Blumenthal, Kray and Derner2015). These data, while limited, suggest that ongoing increases in atmospheric CO2 and temperature could, potentially, lead to altered competition and relative increases in the abundance of invasive weeds relative to native plants within communities.
Given that both weeds and the desired or natural plant species will face novel selective pressures, another relevant question regarding competition is determining the differential growth and fecundity of weeds relative to the managed plant species within a given system. For managed plant communities, including pastures, forest plantations, and crops, genetic uniformity is utilized as a means of increasing productivity. Indeed, a great deal of effort by breeders is designed to identify and maintain desirable economic traits for a narrow selection of available germplasm. While selection to CO2 and/ or climate could, in the short term, reduce genetic diversity among weedy species, it seems likely that the difference in relative response between weeds and desired plant species (representing a narrow subset of genetic variation) will be enhanced, with greater negative impacts on the potential productivity of managed plant systems.
Weed Management and Herbicide Resistance
The ability to detect and respond to weed threats is of obvious importance, and there are several management strategies that are used globally to keep weed populations at acceptable levels (i.e., below an economic threshold). Such practices vary, but usually include cultural, mechanical, chemical, and biological options. For developed countries, chemical application of herbicides remains the most widely used means to control weed populations; indeed, herbicides are the most widely applied class of pesticides (Colborn and Short Reference Colborn and Short1999; Ziska and McConnell Reference Ziska and McConnell2015).
The evolutionary potential of weeds is perhaps best illustrated by the rapid and widespread documentation of herbicide resistance (Heap Reference Heap2014). The occurrence of resistance can vary and is a function of species, herbicide mode of action, and usage of the herbicide. Currently, the issue of herbicide resistance is recognized as a major issue in weed management and is the subject of ongoing research. Yet this research does not, in general, consider climate change and CO2 and how these factors could also affect the selection and evolution of herbicide resistance (Nguyen et al. Reference Nguyen, Malone, Boutsalis, Shirley and Preston2015; Ziska Reference Ziska2016).
Changes in climatic conditions such as wind speed, humidity, and soil/air temperature will influence herbicide coverage, persistence, and efficacy, thus altering patterns of selection on herbicide responses (Bailey Reference Bailey2003). Carbon dioxide or temperature changes could influence growth phenology, with less time spent in the seedling stage, which is the period of greatest herbicide sensitivity. Carbon dioxide-induced changes in leaf morphology or variation in root:shoot ratio can affect herbicide uptake and distribution. In Canada thistle [Cirsium arvense (L.) Scop.], for example, additional CO2 can stimulate root over shoot growth, diluting shoot-applied herbicide; failure to kill roots, in turn, results in regeneration of the whole plant (Ziska et al. Reference Ziska, Faulkner and Lydon2004). Interestingly, similar increases in root:shoot ratio have been observed for other invasive weeds in response to recent CO2 increases, although whether this allocation shift contributes to decreased herbicide efficacy has not been tested (Ziska et al. Reference Ziska, Blumenthal, Runion, Hunt and Diaz-Soltero2011: Figure 5).
The effects of climate change on herbicide efficacy may also depend on herbicide mode of action. Climate and/or CO2 could alter pigment production, photosynthesis, and overall metabolic activity. Herbicide modes of action are designed to disrupt these processes (e.g., atrazine is a photosystem II inhibitor; amitrole is a pigment inhibitor); consequently, where CO2 and/or climate change stimulate growth, these herbicides may become more effective. Conversely, there is general recognition that rising CO2 and/or rising temperatures could reduce protein levels in a wide range of plant tissues (e.g., Loladze Reference Loladze2014; Taub et al. Reference Taub, Miller and Allen2008). Less protein would result in less demand for aromatic and branched-chain amino acids, with potential declines in the efficacy of herbicides that act as enzyme inhibitors (e.g., glufosinate, glyphosate) (Varanasi et al. Reference Varanasi, Prasad and Jugulam2015).
At present, there is an emphasis on GMO-directed herbicide management. But long-term effectiveness of such a strategy is dependent on the absence of gene flow and transference of resistance between the GMO and associated weeds. Yet, depending on the degree of genetic similarity, climate and CO2 may alter gene flow, with consequences for herbicide efficacy.
As illustrated previously, for many global rice systems, weedy or red rice is recognized as a major production constraint (Chauhan Reference Chauhan2013; Ziska et al. Reference Ziska, Gealy, Burgos, Caicedo, Gressel, Lawton-Rauh, Avila, Theisen, Norsworthy, Ferrero and Vidotto2015). A long-term USDA study comparing outcrossing rates between cultivated and weedy rice at three different CO2 concentrations (300, 400, and 600 ppm; or mid-20th-century, current, and mid-21st-century values, respectively) noted greater synchronicity in flowering times and enhanced outcrossing rates between a cultivated rice mutant that is resistant to a class of herbicides (imidazolinone, ClearfieldTM 161) and a weedy red rice ascension accession (StgS) (Ziska et al. Reference Ziska, Gealy, Tomecek, Jackson and Black2012). Consequently, as CO2 increased, the number of weedy herbicide-resistant hybrid progeny also increased (Ziska et al. Reference Ziska, Gealy, Tomecek, Jackson and Black2012). While additional information on other environmental parameters (e.g., temperature) is needed, CO2 per se could alter floral synchrony and gene flow between crops and weeds, with subsequent consequences for hybridization, herbicide resistance, and evolution.
Evolutionary and Revolutionary Knowledge Gaps and Critical Needs
Overall, a review of current studies indicates that managing plant systems within the context of climate change will depend, in part, on related shifts in weed limitations to productivity, increased understanding and assessment of climate-induced evolutionary change, and related changes in management efficacy. Climate and CO2 will act directly (e.g., CO2 fertilization effects; Ziska Reference Ziska2001) and indirectly (e.g., biogeographical location; Bradley et al. Reference Bradley, Blumenthal, Wilcove and Ziska2010; McDonald et al. Reference McDonald, Riha, DiTommaso and DeGaetano2009) on selection, and weeds appear to have the requisite genetic variation to respond (Clements et al. Reference Clements, DiTommaso, Jordan, Booth, Cardina, Doohan, Mohler, Murphy and Swanton2004; Franks et al. Reference Franks, Sim and Weis2007; Ravenscroft et al. Reference Ravenscroft, Whitlock and Fridley2015).
In this context, we would highlight several research gaps that, if addressed, would improve our ability to understand and predict evolutionary responses of weeds to elevated CO2 and climate change. Evolutionary issues related to demographics, competition, and management, while presented independently here, should be considered in an integrative context specific to addressing and prioritizing research needs.
Differential Herbicide Resistance
One of the most practical research needs is an integrated assessment of how climate change and rising CO2 will affect the development and spread of herbicide resistance (Fernando et al. Reference Fernando, Manalil, Florentine, Chauhan and Seneweera2016; Waryszak et al. Reference Waryszak, Lenz, Leishman and Downey2018). As emphasized by Franks (Reference Franks2016), herbicide resistance remains a prosaic example of how rapid contemporary evolution functions in response to strong selection pressures. Indeed, recent data suggest that rising CO2 and/or temperature per se could select for resistant biotypes (Figure 2).
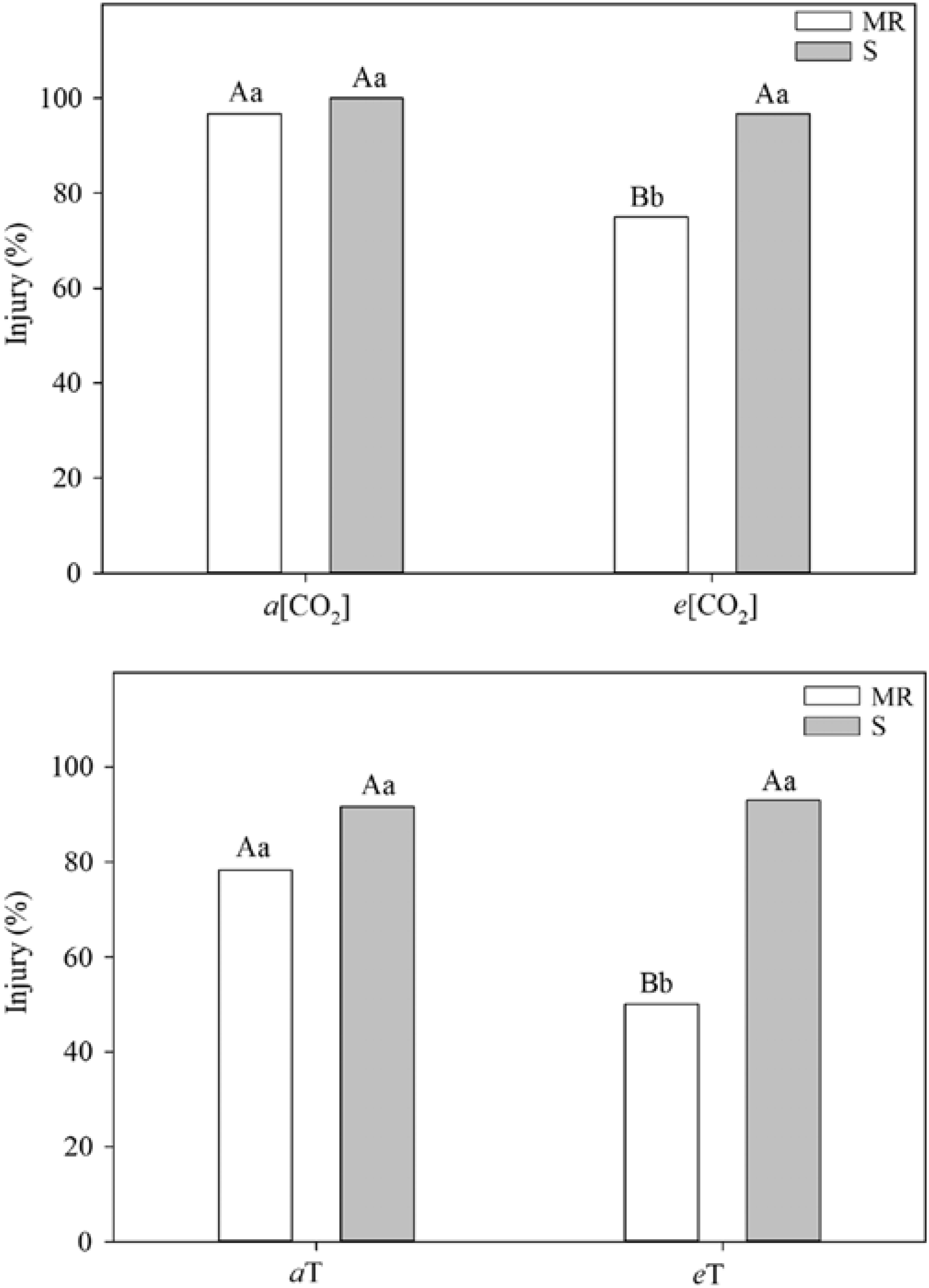
Figure 2. Differential effects and standard error of herbicide application on multiple-resistant (MR) and susceptible (S) biotypes of junglerice [Echinochloa colona (L.) Link]. Different letters above columns indicate a significant difference at the P<0.05 level; capital letters refer to treatment (CO2 and temperature) differences, and lowercase letters refer to MR and S biotypes. a and e refer to ambient and elevated treatment conditions for CO2 concentration [CO2] and temperature (T). Note the reduction in efficacy at warmer temperatures and higher CO2 levels for the MR biotype. Adapted from Refatti et al. (Reference Refatti, de Avila, Camargo, Ziska, Oliveira, Salas-Perez and Roma-Burgos2019).
Yet a host of issues specific to climate and CO2 require further elucidation in that regard: (1) potential changes in mutation rates that could alter herbicide mode of action; (2) morphological (leaf thickness, stomatal number) and phenological (root:shoot ratio) shifts with consequences for uptake and translocation of herbicides; (3) documentation of whether selection shifts for resistant and susceptible biotypes occurs (e.g., do resistant weedy biotypes show a stronger growth and yield response to rising CO2?) (see Refatti et al. Reference Refatti, de Avila, Camargo, Ziska, Oliveira, Salas-Perez and Roma-Burgos2019); and (4) observed increases in herbicide application rates associated with reduced efficacy and consequences for increased occurrence of resistance due to greater herbicide selection pressures.
Biological Constraints
In addition to chemical control, there is evidence that climate can influence other aspects of weed management; with subsequent consequences for selection and evolution. In perennial managed systems, including rangelands and forests, biological control can be the most efficient and effective method for controlling weeds (Clewey et al. Reference Clewey, Eschen, Shaw and Wright2012). However, climate change may alter the efficacy of weed biological control through changes in plant nutrient content, which often declines with elevated CO2; increases in insect activity with temperature; and shifts in phenology of both agents and host weeds (Reeves Reference Reeves2017; Reeves et al. Reference Reeves, Blumenthal, Kray and Derner2015). Adaptive responses to such changes are difficult to predict, given that both biological control agents and host weeds will have the potential to adapt to new selective pressures (Holt and Hochberg Reference Holt and Hochberg1997). However, the specificity of agent–host interactions suggests that differential adaptation and selection could also have important consequences for weed fitness and future management.
More broadly, a wide variety of interactions between weeds, pathogens, and pollinators may be influenced by climate change, with consequences for evolution. While not specific to weeds per se, it is of interest to note that in transgenic Bacillus thuringiensis (Bt) cotton (Gossypium hirsutum L.), elevated CO2 reduced Bt protein production relative to the ambient CO2 condition (Coviella et al. Reference Coviella, Morgan and Trumble2000). The impact of climate and CO2 suggests that ecological dynamics are likely to be affected (e.g., temporal shifts in pollen production and pollinators with warming temperature) and that there is a close coupling between ecological and evolutionary dynamics. To date, there has been little research on the role of climate and/or CO2 on biotic constraints to weed biology and the subsequent consequences for selection pressure. Yet interspecific checks and balances on populations may be altered by rapid evolutionary change imposed by climate/CO2.
Demography
Climate and CO2 are also likely to alter the evolutionary basis for ecotype differentiation and the ability of weeds to disperse and colonize quickly. There are several field-based studies indicating that elevated CO2 could select for more invasive weed species (reviewed in Ziska Reference Ziska2011). However, the basis for their selection is unclear. Specific factors related to demographics, including seed dormancy, germination, emergence, and dispersal, are acknowledged, but a comprehensive understanding of how climate/CO2 alters these selective factors is lacking. There is an immediate need to understand and document the role of climate/CO2 in changing demography and evolutionary potential (Ravet et al. Reference Ravet, Patterson, Krähmer, Hamouzová, Fan, Jasieniuk, Lawton-Rauh, Malone, McElroy, Merotto and Westra2018). The evolutionary potential specific to demographic change can be evaluated in terms of genetic variation; associated selection pressures, including hybridization; changes in life histories (e.g., annuals to perennials); acclimation capacity; photoperiodism (Saikkonen et al. Reference Saikkonen, Taulavuori, Hyvönen, Gundel, Hamilton, Vänninen, Nissinen and Helander2012); and human activities and agronomic shifts in cultivation (Clements and DiTommaso Reference Clements and DiTommaso2011). Information related to how evolution may influence population growth and spread associated with climate change would be of critical benefit in updated models of projected weed distribution and impact (e.g., Bradley et al. Reference Bradley, Blumenthal, Wilcove and Ziska2010; McDonald et al. Reference McDonald, Riha, DiTommaso and DeGaetano2009).
Epigenetics and Climate
Given the acknowledged acclimatory responses of weeds (e.g., Baker Reference Baker1974), differentiating between physiological acclimation and evolutionary adaptation to climate/CO2 is of obvious importance (Franks et al. Reference Franks, Weber and Aitken2014). At the crux of such differentiation is the role of epigenetics. However, the role of epigenetics, heritable phenotype changes that do not involve alterations in the DNA sequence, and the influence of climate/CO2 on their function is almost completely unknown. Yet epigenetic changes that are heritable could influence evolution.
Many of the most troublesome weeds are polyploids (Barrett Reference Barrett, Charudattan and Walker1982). For weeds, taxa with high chromosome numbers can potentially produce a variety of recombinant progeny and an enhanced degree of genetic variation. This variation, in turn, may be of benefit in adaptive evolution to a changing environment (Chen Reference Chen2007); consequently, understanding such an influence is key to determining suitable phenotypes, as well as adaptation and fitness in response to climate change. For example, a study of alligatorweed [Alternanthera philoxeroides (Mart.) Griseb.], an invasive weed of both terrestrial and aquatic systems, reported genome-wide epigenetic reprogramming in response to environmental variability (Gao et al. Reference Gao, Geng, Li, Chen and Yang2010). Given this degree of sensitivity, it seems essential to document and understand climate/CO2 effects on weed epigenetics and the consequences for evolutionary adaptation.
Conclusions
As emphasized by Neve et al. (Reference Neve, Vila-Aiub and Roux2009), other academic disciplines that study pests, such as entomology and pathology, are primarily concerned with biology, from the biochemical to the ecosystem, and the secondary application of this knowledge to management. Conversely, for weed science, we would argue that the success of chemical management has led to a primary technological and management focus with less emphasis on weed biology per se.
But now weed science faces twin challenges. The first is related to the rapid increase and spread of herbicide resistance; the second to the environmental uncertainty represented by climate change and rising levels of CO2. The evolutionary aspects of these two challenges are interrelated. Weed management is still paramount, but it is becoming clear that a more efficacious approach must include a renewed emphasis on fundamental research in weed biology, from the cellular to the ecosystem, for all circumstances in which unwanted plants pose an environmental or economic constraint. And in that context, a greater understanding of weed evolution is essential to maintaining and improving future productivity in managed plant systems (Harker Reference Harker2013)
The overview presented here emphasizes this point for environmental change, provides a review of weed evolution, and tries to assess the evolutionary consequences specific to three research areas: demographics, competition, and management. Demographic traits, including seed biology, germination, life span, and fecundity will be influenced by climate/CO2, with consequences for selection and adaptation. Similar influences on crop–weed interactions can be expected, with initial evidence suggesting that differences in genetic variation between crops and weeds may already be leading to differential responses to recent CO2 increases. Management, in turn, is perhaps exemplified by herbicide resistance, the selection role that climate and CO2 would play in that regard, and the unknown consequences of CO2/climate influences on gene flow between crops and weeds. Finally, any effort to review a subject provides a tempting platform for new ideas and future direction, and some suggestions are offered. However, it should be kept in mind that these are by no means exhaustive, and other perspectives from different disciplines are welcome.
Author ORCID
Lewis H. Ziska, 0000-0003-2980-3985
Acknowledgments
SJF was funded by a grant from the National Science Foundation (IOS 1546218). No conflicts of interest have been declared.