Diet may play important causal roles in myriad diseases and conditions, including obesity( Reference Malik, Pan and Willett 1 – Reference Dror 4 ), dental caries( Reference Nakayama and Mori 5 , Reference Peltzer, Mongkolchati and Satchaiyan 6 ), diabetes( Reference Malik, Popkin and Bray 7 ) and the metabolic syndrome( Reference Malik, Popkin and Bray 7 ). Diet holds considerable potential for both clinical and population benefit, because it is a modifiable factor that people of all ages can theoretically change. A better understanding of diet and its malleability could point the way to more effective interventions targeting obesity( Reference Wang, Lau and Wang 8 ) and other diseases( Reference Emmett and Jones 9 ). More specifically, studying dietary stability could help to determine which foods or diets are amenable to change and when( Reference Ambrosini, Emmett and Northstone 10 )- particularly important if certain diets are associated with health outcomes or diseases( Reference Ambrosini, Emmett and Northstone 10 ). This understanding may be especially important for children( Reference Todd, Street and Ziviani 11 ), because lifestyle habits and conditions such as obesity may be harder to change from adolescence onwards( Reference Todd, Street and Ziviani 11 , Reference Venn, Thomson and Schmidt 12 ).
Unfortunately, studying dietary change is no easy task. First, diet is not a single event but a cumulative and evolving exposure over many years, yet most studies measure it only once, twice or infrequently. Dietary-related diseases, such as obesity, probably reflect cumulative risk over time from multiple interacting factors, including diet( Reference Gillman 13 , Reference Northstone, Smith and Newby 14 ). It is therefore likely that frequently repeated reporting over an extended time frame will give a longer-term picture of children’s diets over time. Moreover, although intakes of multiple foods and nutrients correlate with one another( Reference Ambrosini 15 – Reference Perry, Keane and Layte 17 ), many studies consider individual foods or nutrients distinctly. Two main possibilities arise: studying dietary scores and/or dietary patterns over time.
Dietary scores or indices are often based on prior research and sum the frequency or number of foods eaten that are believed to have health benefits or detriments, or measure the extent to which a person’s diet aligns with dietary guidelines or recommendations( Reference Michels and Schulze 18 ). Previous research( Reference Meyerkort, Oddy and O’Sullivan 19 – Reference Barnes, Crandell and Bell 24 ) has assessed the tracking of diet, measured by dietary scores or indices, in children and/or adolescents. Of the studies that have measured diet across at least three time points( Reference Meyerkort, Oddy and O’Sullivan 19 , Reference Boddy, Abayomi and Johnson 21 , Reference Barnes, Crandell and Bell 24 ), most have observed dietary stability over time( Reference Meyerkort, Oddy and O’Sullivan 19 , Reference Barnes, Crandell and Bell 24 ). One such study observed that dietary scores in early childhood were strongly associated with diet at 14 years of age( Reference Meyerkort, Oddy and O’Sullivan 19 ). Other research identified consistency of the Dietary Approaches to Stop Hypertension (DASH) dietary score at three time points in children aged 10 years or older( Reference Barnes, Crandell and Bell 24 ).
Dietary patterns present an alternative for assessing overall diet. Studying dietary patterns recognises that there is clustering between food groups in children’s diets( Reference Ambrosini 15 , Reference Perry, Keane and Layte 17 ). Techniques such as factor analysis and principal component analysis can empirically uncover the natural ways into which the foods group into dietary patterns within populations, independently of disease and unconstrained by dietary guidelines( Reference Michels and Schulze 18 ). Many studies have tracked the stability of or change in empirically derived dietary patterns( Reference Ambrosini, Emmett and Northstone 10 , Reference Northstone, Smith and Newby 14 , Reference Mikkilä, Räsänen and Raitakari 25 – Reference Okubo, Crozier and Harvey 40 ), with those measuring diet at multiple time points( Reference Mikkilä, Räsänen and Raitakari 25 , Reference Brazionis, Golley and Mittinty 31 , Reference Lioret, Betoko and Forhan 33 , Reference Okubo, Crozier and Harvey 40 ) generally observing strong stability of patterns over time. However, as with the literature surrounding dietary scores, none has followed children over a substantial period of childhood (at least 10 years) and at frequent time intervals, precluding a longer-term understanding of trajectories and pinpointing specific time point(s) at which intervention may be most promising.
The Longitudinal Study of Australian Children provides the opportunity to examine stability and change in both dietary scores and patterns within two parallel population cohorts of children of different ages( Reference Sanson, Nicholson and Ungerer 41 ). Such a design supports validation and confirmation of results, and increases their broader applicability and generalisability( Reference Sanson, Nicholson and Ungerer 41 ). It can also help disentangle the effects of cohort, time and age on dietary trajectories or stability( Reference Sanson, Nicholson and Ungerer 41 ). Therefore, the aims of this study were to: (a) cross-sectionally derive dietary scores and patterns; and (b) derive and compare dietary score and pattern trajectories from 2–3 to 10–11 years and from 4–5 to 14–15 years of age, in parallel population-based cohorts.
Methods
Recruitment and sampling
This study used data collected up to 2014 from the Longitudinal Study of Australian Children (LSAC)( Reference Soloff, Lawrence and Johnstone 42 ). LSAC began in 2004 and involves the biennial collection of data from two cohorts, the Baby or ‘B’ Cohort (initially aged 0–1 years) and the Kindergarten or ‘K’ Cohort (initially aged 4–5 years)( Reference Norton and Monahan 43 , 44 ). Participants were sampled from the Medicare enrolment database, with 5107 and 4983 children recruited for the B and K Cohorts, respectively( Reference Soloff, Lawrence and Johnstone 42 ). The sample of children included in LSAC was broadly population representative( Reference Soloff, Lawrence and Johnstone 42 ), although the most remote areas of Australia were excluded( Reference Soloff, Lawrence and Johnstone 42 ). LSAC used a postcode-based two-stage clustered design, as previously outlined in detail( Reference Soloff, Lawrence and Johnstone 42 ). In brief, a number of postcodes were randomly selected and then several children within each postcode were randomly selected for the study( Reference Soloff, Lawrence and Johnstone 42 ). Fig. 1 shows the numbers and percentages of children who responded to each of the waves of LSAC. Families provided their consent to participate and the Australian Institute of Family Studies Ethics Committee approved each wave of LSAC.

Fig. 1 Flow and retention through Longitudinal Study of Australian Children (LSAC), described in Soloff et al. ( Reference Soloff, Lawrence and Johnstone 42 ), and Norton & Monahan( Reference Norton and Monahan 43 ). B Cohort, Baby Cohort; K Cohort, Kindergarten Cohort.
Procedures and measures
Relevant to the current analyses, LSAC’s data collection methods have included face-to-face interviews with parents of children and audio computer-assisted self-interviews, completed by children( 45 ).
This study used dietary data collected for children from waves two to six (ages: 2–11 years) of the B Cohort and all waves (ages: 4–15 years) of the K Cohort at interview. Parents (at children ages 2–9 years) or children (from age 10 years onwards) were asked a standard set of twelve to sixteen questions relating to the study child’s consumption of individual or grouped food or drink items (online Supplementary Table S1). Each question asked how often the study child ate a particular food or drink item or group of foods or drinks in the last 24 h or yesterday (online Supplementary Table S1). These dietary questions were not previously validated.
To report baseline demographic characteristics, we used variables describing age, sex, socioeconomic position, disadvantage and remoteness area, which were all taken from the baseline wave for this paper (wave two of the B Cohort and wave one of the K Cohort). Age was measured in months and converted to years. Socioeconomic position, a composite measure of socioeconomic status, combined and averaged information on annual family income, parental educational attainment and parental occupational status, standardised to a mean of 0 (sd 1)( Reference Blakemore, Strazdins and Gibbings 46 ). Annual family income was calculated by combining both parents’ weekly incomes from all sources, then transformed using natural logarithms( Reference Blakemore, Strazdins and Gibbings 46 ). Parental educational attainment was measured by the number of years of education completed by each parent, using data from three variables (‘highest level of schooling completed, ‘completed other qualification’ and ‘highest level of qualification obtained’)( Reference Blakemore, Strazdins and Gibbings 46 ). Occupational status was measured for parents’ current or last main occupations using scores that group occupations by both occupation type and skill level, indirectly accounting for income and education( Reference Blakemore, Strazdins and Gibbings 46 ). If there were two parents in the home, socioeconomic position included data related to both parents( Reference Blakemore, Strazdins and Gibbings 46 ). Otherwise, it included information on the one resident parent( Reference Blakemore, Strazdins and Gibbings 46 ). The census-based Australian Bureau of Statistics Index of Relative Socio-economic Disadvantage, one of the four Socio-Economic Indexes for Areas, summarises information about social and economic resources of households and people in an area( 47 ), in this case postcode. The national standardised mean is 1000 (sd 100), with higher scores representing lower disadvantage( 47 ). Remoteness area, based on the Australian Standard Geographical Classification remoteness structure( 48 ), comprises five categories: major cities, inner regional, outer regional, remote and very remote.
Statistical analysis
All statistical analyses were performed using Stata/IC 14.1 (StataCorp LP).
Baseline characteristics
We summarised baseline characteristics of the sample using mean values and standard deviations for continuous variables, and percentages for categorical variables.
Dietary scores and patterns
First, we derived overall dietary scores and patterns by wave, for each cohort. Second, we summarised both dietary scores and patterns over time using trajectories.
Derivation of dietary scores by wave
We developed a dietary scoring system that aligned as closely as possible with the 2013 Australian Dietary Guidelines( 49 ). First, participants were assigned an individual score, ranging from 0 to 2, for each of seven categories of foods: fruit, vegetables, water, fatty foods, sugary foods, sweetened drinks and milk products or alternatives. The scores for each individual category were then summed to give an overall score, ranging from 0 to 14, with 14 being healthiest. The derivation of the dietary scoring system is outlined in detail in Table 1. Each category comprised one or more questions. For the purposes of scoring, ‘the last 24 h’ and ‘yesterday’ were considered to be equivalent, as were ‘more than once’ and ‘twice or more’. In order to have a dietary score for a particular wave, an individual needed complete dietary data for all of the questions required for the calculation of the score. We then compared the distribution of dietary scores by wave, for each cohort, using mean values and standard deviations, and medians and 25th and 75th percentiles and ranges.
Table 1 Derivation of the dietary scoring system

B Cohort, Baby Cohort; K Cohort, Kindergarten Cohort.
* Although one serve of fruit per day is recommended for 2–3-year-olds( 49 ), we used the same scoring system for wave two of the B Cohort as for all other waves of the B and K Cohorts, in order to keep the scoring system for all waves consistent for fruit consumption.
† Points were allocated assuming that a frequency of ‘once’ corresponds to the consumption of approximately one serve of fruit, vegetables or milk products/alternatives.
‡ In order to give approximately equal weighting to healthier and less healthy foods in the overall scoring system, we separated discretionary foods into fatty foods and sugary foods.
Trajectories of dietary scores
To examine trajectories of dietary scores across waves, we conducted group-based trajectory modelling using the ‘traj’ plug-in in Stata/IC 14.1( Reference Jones and Nagin 54 ). Group-based trajectory modelling examines how developmental courses of particular variables (in this case, dietary scores) vary between groups of individuals in the population( Reference Jones and Nagin 54 ). Overall dietary scores from each individual wave were used as dependent variables and the study child’s ages (in years) at each wave were used as independent variables. In order to be included in the trajectories, individuals were required to have dietary scores from at least two waves. For trajectory modelling, dietary scores were modelled with the normal distribution.
For both cohorts, we fitted and compared models with one trajectory for all children, up to eight trajectories, and dropped non-significant (i.e. P>0·05) quadratic or cubic parameters for each number of trajectories( Reference Helgeson, Snyder and Seltman 55 ). Once we obtained a model with no non-significant cubic or quadratic parameters for each number of trajectories (i.e. from one up to eight trajectories), we decided upon the number of trajectories to retain by considering Bayesian information criterion values( Reference Andruff, Carraro and Thompson 56 ), the log Bayes Factor( Reference Andruff, Carraro and Thompson 56 ), average posterior probabilities( Reference Andruff, Carraro and Thompson 56 ), the proportion of the sample in each trajectory and visual graphs of trajectories, in order to extract the most meaningful and distinct trajectories. For consistency in our analyses, we strove to retain the same number of trajectories from both the B and K Cohorts. Individuals were assigned to the trajectory for which their posterior probability of membership (the probability that each individual belongs to a particular trajectory) was highest( Reference Andruff, Carraro and Thompson 56 ).
Derivation of dietary patterns by wave
In addition to deriving dietary scores, we also derived dietary patterns for the separate waves, by performing exploratory factor analyses( 57 ), using all twelve to sixteen healthy and unhealthy food or drink items. Factor analysis is a data-driven technique that reveals how particular variables (in this case, foods or drinks) group together( Reference Michels and Schulze 18 ). Factor analysis is based on the assumption that the observed variables are linear composites of latent or unobserved factors( Reference Michels and Schulze 18 ). As all dietary variables were ordinal, we used polychoric correlation matrices for these factor analyses( 57 ). In order to determine how many factors to retain for each wave (the factors described dietary patterns at each wave), we considered the following criteria, collectively: eigenvalues above 1, a visual inspection of the scree plots and the interpretability of the factors obtained( Reference Costello and Osborne 58 ). We used an orthogonal rotation, in order to force the factors to be uncorrelated( Reference Mikkilä, Räsänen and Raitakari 25 , Reference Costello and Osborne 58 ). For the naming of factors, we only considered particular foods with factor loadings of a magnitude of at least 0·3( Reference Costello and Osborne 58 ). The Kaiser–Meyer–Olkin (KMO) statistic( Reference de Vaus 59 ) was used to determine if factor analysis was appropriate for our group of dietary variables from each wave. All KMO values obtained for the factor analyses for each wave were above 0·5, meaning that factor analysis was appropriate for the group of dietary variables from each individual wave( Reference de Vaus 59 ). Finally, dietary pattern scores for each participant, for each of the factors, were calculated using regression scoring, using the means and standard deviations of each dietary variable.
Trajectories of dietary patterns
For those patterns that showed reasonably high consistency between waves for both cohorts (‘healthy’ and ‘unhealthy’ factors – see the ‘Results’ section), we conducted group-based trajectory modelling to examine their trajectories over the study period. For these analyses, we used a very similar approach to that for trajectories of dietary scores, but with factor scores from each individual wave as the dependent variables. In order to be included in the trajectories, individuals were required to have the relevant factor scores from at least two waves. For trajectory modelling, ‘healthy’ and ‘unhealthy’ factor scores were modelled with the normal and censored normal distributions, respectively.
Concordance between overall score and pattern trajectories
We used cross-tabulations with percentages to measure the agreement between all combinations of score and pattern trajectories from each cohort.
Results
Baseline characteristics
Table 2 shows the baseline characteristics of the total sample, and children included in and excluded from the trajectories, by wave and cohort. The mean ages of children included in and excluded from the trajectories (2·8 and 4·7 years for the B and K Cohorts, respectively) were similar at baseline. Approximately equal proportions of males and females were included in the study sample, with slightly higher proportions of males included in than excluded from the trajectories. The mean disadvantage index of the total sample was close to the national average of 1000( 60 ). Compared with excluded children, those included in all trajectories had a higher mean socioeconomic position (0·0 v. −0·5 for both cohorts) and were relatively less disadvantaged (disadvantage indices of 1011 v. 989 for the B Cohort and 1012 v. 1001 for the K Cohort) (Table 2).
Table 2 Baseline characteristics of the sample, by wave and cohort (Mean values and standard deviations)

2B, wave two of the Baby Cohort; 1K, wave one of the Kindergarten Cohort.
* Higher=less disadvantaged.
Dietary scores and patterns
Dietary scores by wave
The distribution of dietary scores was consistent across waves for both cohorts (Table 3). For the B Cohort, the mean dietary score was 10·4 at baseline (wave two), compared with a mean dietary score of 9·6 for the final wave (wave six). Similarly, for the K Cohort, the mean dietary score was 9·8 at baseline (wave one), compared with a mean dietary score of 9·6 at wave six. The interquartile range of dietary scores for each wave was three. Dietary scores had similar medians and ranges in each wave (Table 3).
Table 3 Distribution of overall dietary scores, by wave and cohortFootnote * (Mean values and standard deviations; Medians and 25th and 75th percentiles and ranges)
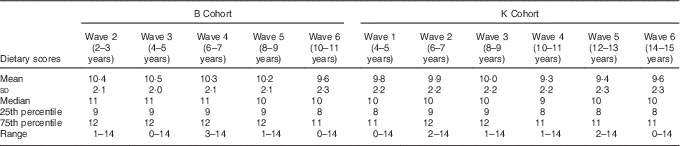
B Cohort, Baby Cohort; K Cohort, Kindergarten Cohort.
* n ranged from 3344 to 4855.
Trajectories of dietary scores
Overall, tracking of dietary scores was moderately high in the B Cohort and moderate in the K Cohort (Fig. 2). We obtained four trajectories of overall dietary scores for both cohorts that we labelled ‘never healthy’, ‘moderately healthy’, ‘becoming less healthy’ and ‘always healthy’. Similar proportions of B and K Cohort children were in the ‘never healthy’ (8·8 %; 95 % CI 7·9, 9·6 and 11·9 %; 95 % CI 10·9, 12·8, respectively), ‘moderately healthy’ (24·0 %; 95 % CI 22·8, 25·3 and 20·7 %; 95 % CI 19·5, 21·9, respectively) and ‘always healthy’ (50·7 %; 95 % CI 49·2, 52·2 and 40·2 %; 95 % CI 38·8, 41·6, respectively) trajectories. A higher percentage of children (27·3 %; 95 % CI 26·0, 28·6) belonged to the ‘becoming less healthy’ trajectory in the K Cohort than in the younger B Cohort (16·6 %; 95 % CI 15·5, 17·7). For these ‘becoming less healthy’ trajectories, dietary scores began to decrease rapidly at the age of 7 years in both cohorts. For the B Cohort, scores decreased even more rapidly from approximately 9 years. For ‘moderately healthy’ trajectories, dietary scores showed an improving trend over the study period in both cohorts.

Fig. 2 Overall dietary score trajectories for the Baby (B) Cohort, aged 2–11 years (n 4505) (a), and the Kindergarten (K) Cohort, aged 4–15 years (n 4640) (b), obtained using group-based trajectory modelling. ![]() , 95 % CI. a:
, 95 % CI. a: ![]() , Always healthy 50·7 %;
, Always healthy 50·7 %; ![]() , becoming less healthy 16·6 %;
, becoming less healthy 16·6 %; ![]() , moderately healthy 24·0 %;
, moderately healthy 24·0 %; ![]() , never healthy 8·8 %; b:
, never healthy 8·8 %; b: ![]() , always healthy 40·2 %;
, always healthy 40·2 %; ![]() , becoming less healthy 27·3 %;
, becoming less healthy 27·3 %; ![]() , moderately healthy 20·7 %;
, moderately healthy 20·7 %; ![]() , never healthy 11.9 %.
, never healthy 11.9 %.
Dietary patterns by wave
In all eleven waves (five for the B and six for the K Cohort), we identified reasonably similar ‘healthy’ and ‘unhealthy’ patterns; in some waves, we additionally identified a ‘dairy’ pattern (online Supplementary Table S2). Table 4 and the online Supplementary Table S2 show the factor loadings for food and drink items in the dietary patterns from each wave, for each cohort. The ‘healthy’ pattern was characterised by a high consumption of fresh fruit, cooked vegetables and raw vegetables or salad in all waves; water in most waves; and dairy products and bread or toast in a few waves (Table 4; online Supplementary Table S2). The ‘unhealthy’ pattern consisted of a high consumption of savoury snacks and sweetened drinks in all waves; meat pies, hamburgers, hot dogs, sausages or sausage rolls, hot chips and fruit juice in most waves; sugary foods, diet drinks, energy drinks, coffee and soya milk products mainly in the later waves; and low water consumption in six of the eleven waves (Table 4; online Supplementary Table S2). Finally, the ‘dairy’ pattern, obtained in seven waves, was characterised by a high consumption of full-cream milk products and a low consumption of low-fat milk products in most waves, except in wave three of the K Cohort, where it was characterised by a low consumption of full-cream milk products and a high consumption of low-fat milk products (online Supplementary Table S2).
Table 4 Factor loadings for items from the factor analyses of each wave separately, by age, wave and cohort, for the ‘healthy’ and ‘unhealthy’ patternsFootnote *

2B, wave two of the Baby Cohort; 3B, wave three of the Baby Cohort; 1K, wave one of the Kindergarten Cohort; 4B, wave four of the Baby Cohort; 2K, wave two of the Kindergarten Cohort; 5B, wave five of the Baby Cohort; 3K, wave three of the Kindergarten Cohort; 6B, wave six of the Baby Cohort; 4K, wave four of the Kindergarten Cohort; 5K, wave five of the Kindergarten Cohort; 6K, wave six of the Kindergarten Cohort.
* n ranged from 3344 to 4850.
† Food/drink items with loadings ≤−0·3 and ≥0·3.
Dietary pattern trajectories
Trajectories were created for ‘healthy’ and ‘unhealthy’ patterns, but not for the ‘dairy’ pattern.
‘Healthy’ pattern trajectories
Similar to dietary scores, we also observed moderately high stability of ‘healthy’ patterns in both cohorts, with higher scores indicating a healthier diet (Fig. 3). As for dietary scores, four trajectories of ‘healthy’ patterns emerged for both cohorts: ‘never healthy’, ‘moderately healthy’, ‘becoming less healthy’ and ‘always healthy’ (Fig. 3). Proportions of B and K Cohort children in each ‘healthy’ pattern trajectory were similar to those in each of the homonymous overall score trajectories (Fig. 3). Moreover, similar proportions of B and K Cohort children were in the ‘never healthy’ (10·0 %; 95 % CI 9·1, 10·9 and 10·1 %; 95 % CI 9·3, 11·0, respectively) and ‘always healthy’ (44·0 %; 95 % CI 42·5, 45·4 and 43·1 %; 95 % CI 41·7, 44·5, respectively) trajectories. However, the proportions differed for the remaining two trajectories, with 35·5 %; 95 % CI 34·1, 36·9 and 17·2 %; 95 % CI 16·1, 18·3 of B and K children in the ‘moderately healthy’ trajectories, respectively, and 10·6 %; 95 % CI 9·7, 11·5 and 29·6 %; 95 % CI 28·3, 30·9 of B and K children in the ‘becoming less healthy’ trajectories, respectively.

Fig. 3 ‘Healthy’ pattern trajectories for the Baby (B) Cohort, aged 2–11 years (n 4504) (a), and the Kindergarten (K) Cohort, aged 4–15 years (n 4640) (b), obtained using group-based trajectory modelling. ![]() , 95 % CI. a:
, 95 % CI. a: ![]() , Always healthy 44·0 %;
, Always healthy 44·0 %; ![]() , becoming less healthy 10·6 %;
, becoming less healthy 10·6 %; ![]() , moderately healthy 35·5 %;
, moderately healthy 35·5 %; ![]() , never healthy 10·0 %; b:
, never healthy 10·0 %; b: ![]() , always healthy 43·1 %;
, always healthy 43·1 %; ![]() , becoming less healthy 29·6 %;
, becoming less healthy 29·6 %; ![]() , moderately healthy 17·2 %;
, moderately healthy 17·2 %; ![]() , never healthy 10·1 %.
, never healthy 10·1 %.
Again similar to overall score trajectories, the ‘healthy’ factor scores for the ‘becoming less healthy’ trajectories began to worsen rapidly from around the age of 7 years for both cohorts, and steepened further from approximately 9 years for the B Cohort (Fig. 3). Moreover, ‘healthy’ factor scores for the ‘moderately healthy’ trajectories showed an improving trend over the study period in both cohorts. However, by contrast to the homonymous overall score trajectory, ‘healthy’ factor scores for the ‘moderately healthy’ trajectory began to increase rapidly at the age of 7 years for the K but not B Cohort (Fig. 3).
‘Unhealthy’ pattern trajectories
Again, we observed high stability of ‘unhealthy’ patterns in both cohorts, with four trajectories emerging for both cohorts: ‘never unhealthy’, ‘becoming unhealthy’, ‘moderately unhealthy’ and ‘always unhealthy’, with the highest proportions of children from both cohorts belonging to the ‘never unhealthy’ trajectory (Fig. 4). Moreover, the proportion of B Cohort children in the ‘moderately unhealthy’ pattern trajectory (21·9 %; 95 % CI 20·6, 23·1) was similar to that in the ‘moderately healthy’ score trajectory (24·0 %; 95 % CI 22·8, 25·3), and the proportion of K Cohort children in the ‘moderately unhealthy’ pattern trajectory (17·8 %; 95 % CI 16·7, 18·9) was similar to those in the ‘moderately healthy’ score and ‘healthy’ pattern trajectories (20·7 %; 95 % CI 19·5, 21·9 and 17·2 %; 95 % CI 16·1, 18·3, respectively). However, the proportions of B and K Cohort children in other ‘unhealthy’ pattern trajectories differed from those in similar dietary score and ‘healthy’ pattern trajectories. Similar proportions of B and K Cohort children were in the ‘never unhealthy’ (70·6 %; 95 % CI 69·2, 71·9 and 67·6 %; 95 % CI 66·2, 69·0, respectively), ‘moderately unhealthy’ (21·9 %; 95 % CI 20·6, 23·1 and 17·8 %; 95 % CI 16·7, 18·9, respectively) and ‘always unhealthy’ (4·0 %; 95 % CI 3·4, 4·6 and 3·4 %; 95 % CI 2·9, 4·0, respectively) trajectories. However, a higher percentage of children (11·2 %; 95 % CI 10·3, 12·1) belonged to the ‘becoming unhealthy’ trajectory in the K Cohort than in the B Cohort (3·6 %; 95 % CI 3·0, 4·1).

Fig. 4 ‘Unhealthy’ pattern trajectories for the Baby (B) Cohort, aged 2–11 years (n 4504) (a), and the Kindergarten (K) Cohort, aged 4–15 years (n 4640) (b), obtained using group-based trajectory modelling. ![]() , 95 % CI. a:
, 95 % CI. a: ![]() , Always unhealthy 4·0 %;
, Always unhealthy 4·0 %; ![]() , moderately unhealthy 21·9 %;
, moderately unhealthy 21·9 %; ![]() , becoming unhealthy 3·6 %;
, becoming unhealthy 3·6 %; ![]() , never unhealthy 70·6 %; b:
, never unhealthy 70·6 %; b: ![]() , always unhealthy 3·4 %;
, always unhealthy 3·4 %; ![]() , moderately unhealthy 17·8 %;
, moderately unhealthy 17·8 %; ![]() , becoming unhealthy 11·2 %;
, becoming unhealthy 11·2 %; ![]() , never unhealthy 67·6 %.
, never unhealthy 67·6 %.
Reverse mirroring the earlier findings, ‘unhealthy’ factor scores for the ‘becoming unhealthy’ trajectories began to increase rapidly at the age of 7 years for both cohorts, and even more rapidly from approximately 9 years onwards for the B Cohort (Fig. 4). However, for the ‘becoming unhealthy’ trajectory for the K Cohort, ‘unhealthy’ factor scores decreased from approximately 13 years onwards (Fig. 4). For the ‘always unhealthy’ trajectory for the K Cohort, ‘unhealthy’ factor scores began to increase rapidly at the age of 9 years, and even more rapidly from 11–13 years (Fig. 4). Moreover, similar to the ‘moderately healthy’ trajectory of ‘healthy’ patterns, for the ‘moderately unhealthy’ trajectory for the K Cohort, ‘unhealthy’ factor scores began to decrease at approximately 7 years of age (Fig. 4).
Concordance between overall score and pattern trajectories
The percentage concordance between overall score and ‘healthy’ pattern trajectories was moderately high, with high proportions of individuals belonging to homonymous score and pattern trajectories (Table 5). For example, 80·0 and 70·6 % of children in the ‘always healthy’ pattern trajectories from the B and K Cohorts, respectively, were also in the ‘always healthy’ score trajectories. Agreement between overall score and ‘unhealthy’ pattern trajectories was also moderately high, with high proportions of children belonging to similar score and pattern trajectories (Table 5). For example, 66·5 and 55·3 % of children in the ‘never unhealthy’ pattern trajectories from the B and K Cohorts, respectively, were also in the ‘always healthy’ score trajectories.
Table 5 Cross-tabulations, showing the percentage concordance between overall score, and ‘healthy’ and ‘unhealthy’ pattern trajectories for both cohortsFootnote *

B Cohort, Baby Cohort; K Cohort, Kindergarten Cohort.
* Cell values denote percentages of children in both trajectories.
By contrast, the percentage concordance between ‘healthy’ and ‘unhealthy’ pattern trajectories was moderately low (Table 6), suggesting that these trajectories provide different information. Although 49·6 and 48·7 % of children in the ‘never unhealthy’ pattern trajectories from the B and K Cohorts, respectively, were also in the ‘always healthy’ pattern trajectories, percentage concordance was substantially lower for other combinations of similar trajectories. For example, for the B Cohort, only 6·9 % of children in the ‘becoming unhealthy’ trajectory were also in the ‘becoming less healthy’ pattern trajectory. Moreover, for the K Cohort, only 13·8 % of children in the ‘always unhealthy’ trajectory were also in the ‘never healthy’ pattern trajectory.
Table 6 Cross-tabulations, showing the percentage concordance between ‘healthy’ and ‘unhealthy’ pattern trajectories for both cohortsFootnote *

B Cohort, Baby Cohort; K Cohort, Kindergarten Cohort.
* Cell values denote percentages of children in both trajectories.
Discussion
Statement of principal findings
Overall, we observed moderately high tracking of dietary scores in both cohorts of children across four trajectories labelled ‘never healthy’, ‘moderately healthy’, ‘becoming less healthy’ and ‘always healthy’. Moderate to high stability of dietary patterns in both cohorts provided further confidence regarding these findings. The same four trajectories emerged for ‘healthy’ patterns in both cohorts, with all containing similar proportions of children to homonymous score trajectories, and similar trajectories also emerged for ‘unhealthy’ patterns in both cohorts. For the trajectories characterised by changing levels of healthiness or unhealthiness with age, diet tended to change at approximately 7 years of age for both cohorts.
Comparison with prior literature
Like other studies( Reference Meyerkort, Oddy and O’Sullivan 19 , Reference Rauber, Hoffman and Vitolo 22 , Reference Barnes, Crandell and Bell 24 , Reference Mikkilä, Räsänen and Raitakari 25 , Reference Brazionis, Golley and Mittinty 31 , Reference Okubo, Crozier and Harvey 40 ), we observed moderate to strong stability of dietary scores and patterns over time. For instance, regarding dietary scores, Meyerkort et al.( Reference Meyerkort, Oddy and O’Sullivan 19 ) observed that higher dietary scores (indicating healthier diets) at 1, 2 and 3 years were strongly associated with a higher intake of fibre at 14 years of age (P<0·01 at all ages)( Reference Meyerkort, Oddy and O’Sullivan 19 ). However, because they did not consider time points between 3 and 14 years of age( Reference Meyerkort, Oddy and O’Sullivan 19 ), their results preclude the generation of accurate trajectories over childhood that are characterised by specific inflection points. Barnes et al.( Reference Barnes, Crandell and Bell 24 ) observed high stability in the DASH dietary score over time in older children (≥10 years of age) with type 1 diabetes at baseline, 12- and 60-month follow-up( Reference Barnes, Crandell and Bell 24 ). The mean DASH score (which ranged from 0 to 80) changed very little, decreasing by approximately 0·4 units after 60 months of follow-up( Reference Barnes, Crandell and Bell 24 ). Furthermore, Rauber et al.( Reference Rauber, Hoffman and Vitolo 22 ) observed high stability of the Healthy Eating Index score at 3–4 and 7–8 years of age in a control group( Reference Rauber, Hoffman and Vitolo 22 ). The Healthy Eating Index score, which ranged from 0 to 100, averaged 63·8 at 3–4 years and 64·9 at 7–8 years of age( Reference Rauber, Hoffman and Vitolo 22 ).
In relation to dietary patterns, we were able to show consistency of diet over a substantial period of childhood to preadolescence/adolescence. Previous studies have observed consistency of diet during infancy and/or early childhood( Reference Brazionis, Golley and Mittinty 31 , Reference Lioret, Betoko and Forhan 33 , Reference Okubo, Crozier and Harvey 40 ). For example, one study, which assessed diet at 6, 15 and 24 months of age, observed similarity in the healthier and unhealthier foods consumed at each of these ages( Reference Brazionis, Golley and Mittinty 31 ). Further research showed consistency in dietary quality scores, derived from principal component analysis, at 6 and 12 months, and 3 and 6 years of age( Reference Okubo, Crozier and Harvey 40 ). The Young Finns Study, which tracked dietary patterns at baseline and then 6 and 21 years later( Reference Mikkilä, Räsänen and Raitakari 25 ), identified two distinct dietary patterns that both showed reasonably high stability, with approximately one-third of participants who were initially 3–12 years of age remaining in the lowest quintile of each of these patterns 6 years after baseline( Reference Mikkilä, Räsänen and Raitakari 25 ). We observed even higher dietary stability, with over 50 % of participants from both cohorts remaining in either ‘healthy’ or ‘unhealthy’ pattern trajectories during the study period. However, we acknowledge that these findings could partly reflect the fact that our item pool was smaller than that of the Young Finns Study( Reference Mikkilä, Räsänen and Raitakari 25 ). The Avon Longitudinal Study of Pregnancy and Childhood( Reference Northstone and Emmett 27 ) identified ‘processed’, ‘traditional’ and ‘health conscious’/‘health conscious/vegetarian’ patterns at 3, 4, 7 and 9 years of age, and an additional ‘snack’ pattern at 3 years of age( Reference Northstone and Emmett 27 ). However, in contrast to other studies( Reference Mikkilä, Räsänen and Raitakari 25 , Reference Brazionis, Golley and Mittinty 31 , Reference Okubo, Crozier and Harvey 40 ) and ours, the Avon Longitudinal Study of Pregnancy and Childhood only observed moderate stability of dietary patterns, with consistency in dietary patterns from 4–7 years but not from 3–4 or 7–9 years of age, which may also be attributed to the larger number of food items included in their analyses( Reference Northstone and Emmett 27 ). Similarly, in the Étude des Déterminants pré- et postnatals précoces du développement et de la santé de l’ENfant (EDEN) mother–child cohort, there were only moderate correlations between similar dietary patterns at 2, 3 and 5 years of age( Reference Lioret, Betoko and Forhan 33 ).
Strengths and limitations
A major strength of this study was that it repeated nearly identical questions on participants’ diets from multiple evenly spaced time intervals over a period of >10 years. As the foods that individuals consume may vary on a daily basis and evolve over multiple years, the repeated sampling of diet is likely to provide a more accurate representation of dietary exposures over time than single reports or measures. A further advantage is the replication across two cohorts of children that overlapped in age. This cross-sequential design helps to validate and confirm the study findings, increases their broader applicability and generalisability, and can help to separate the effects of cohort, time and age on dietary stability( Reference Sanson, Nicholson and Ungerer 41 ). Our sample size was large, with over 4500 (approximately 90 %) participants included in the trajectory analyses, providing power and precision even for the trajectories with the lowest membership. LSAC’s population basis( Reference Soloff, Lawrence and Johnstone 42 ) provides confidence that the study findings can be applied to the broader Australian population. Finally, and in contrast to previous research( Reference Meyerkort, Oddy and O’Sullivan 19 , Reference Boddy, Abayomi and Johnson 21 , Reference Mikkilä, Räsänen and Raitakari 25 , Reference Northstone and Emmett 27 , Reference Brazionis, Golley and Mittinty 31 , Reference Okubo, Crozier and Harvey 40 ), we considered empirically generated dietary patterns as well as dietary scores, each of which has specific advantages( Reference Michels and Schulze 18 ). Studying dietary patterns with factor analysis allows us to gain an insight into which foods or drinks are typically consumed within the same diet, and is independent of disease and unrestrained by the recommendations of dietary guidelines( Reference Michels and Schulze 18 ). On the other hand, dietary scores or indices may be easier for the general public to understand and are developed a priori, often based on previous research( Reference Michels and Schulze 18 ).
We also consider the implications of the study’s limitations. First, dietary intake was self- and parent-reported, which is limited by participants under-reporting( Reference Garaulet, Martínez and Victoria 61 ) and misremembering( Reference Dwyer and Coleman 62 ) dietary intake. Implausible measurements may not support plausible associations between diet and disease( Reference Ioannidis 63 ). However, importantly these self-report biases should not have differed by wave or by cohort in the current study. Second, the dietary tool, though widely used, has not been validated, so it remains unclear how accurately this tool measures children’s diets. For example, without validation we do not know the extent to which social desirability bias might have lead to an overestimation of the healthiness of the trajectories. Nevertheless, the tool spans typical healthy and unhealthy food and drink choices that children consume at all ages, and it generates highly replicable trajectories across cohorts and techniques.
A further potential limitation is that at each wave the dietary measure was short (only twelve to sixteen questions, depending on wave) so could not capture all dietary elements. For example, there was limited information on some protein-rich foods, such as meat, chicken and fish, along with some carbohydrate-based foods, such as cereals and breads, precluding us from gaining a more detailed picture of children’s total diets. More dietary questions might have yielded more or different dietary patterns at each wave and/or a greater dietary scoring range. However, because our scoring system weighted healthy and unhealthy foods approximately equally, more questions would probably not have greatly altered the overall distributions of dietary scores and score trajectories. On the upside, the tool’s brevity translated into speed and high completion, such that this major national longitudinal study, unlike others, measured diet with virtually the same tool every two years. The resulting low rates of missing data at each time point should have in turn enhanced the accuracy of the dietary trajectories.
Fourth, our diet measure only contained three categories for consumption. Therefore, our scoring system (which ranged from 0 to 2 for each category) did not allow us to separate out the highest frequencies of food and drink consumption. Nevertheless, we were able to gain an insight into the stability of dietary scores and patterns for higher, compared with lower, consumption of a number of food and drink items. Similarly, this study assessed frequency of dietary intake, rather than quantity, which is likely to vary within and between individuals. Furthermore, although dietary intake in the previous 24 h may not necessarily represent a child’s regular intake, including dietary intake from up to six waves in the trajectories is likely to have reduced the error associated with this issue. Moreover, a limitation of using data-driven techniques, such as factor analysis, is that they may not be generalisable to other populations( Reference Michels and Schulze 18 ). For this reason, we also considered non data-driven dietary scores and obtained similar results, giving further validity to our overall findings.
A further potential limitation is the slight discrepancies between the ‘healthy’ and ‘unhealthy’ dietary patterns and therefore, meaning of the factor scores, from each individual wave, which were used to derive the trajectories. However, the close similarity in both ‘healthy’ and ‘unhealthy’ dietary patterns at each wave still allows an overall picture of ‘healthy’ and ‘unhealthy’ dietary pattern trajectories throughout the study period. Importantly, this approach also allows flexibility to reflect variability in the definition of ‘healthy’ and ‘unhealthy’ diets, according to the stage of childhood or adolescence. Finally, a further drawback is that children included in all trajectories had a higher mean socioeconomic position than those excluded. As higher socioeconomic status is associated with adherence to a healthier diet( Reference Northstone, Smith and Newby 14 , Reference Brazionis, Golley and Mittinty 31 , Reference Lioret, Betoko and Forhan 33 ), we may have therefore underestimated the proportions of children in the least healthy or unhealthiest dietary trajectories.
Meaning of the study: possible mechanisms and implications for clinicians or policymakers
Because we observed moderately high stability in diet from an early age (including children with consistently non-healthy dietary trajectories from as early as the age of 2 years), the findings of this study illustrate that, for children with less healthy diets, it might be beneficial to introduce interventions that aim to improve dietary habits earlier, rather than later, in childhood. These early interventions would be particularly valuable if further research demonstrates that these dietary patterns or scores are associated with adverse or prevalent outcomes (e.g. obesity), especially if these outcomes are apparent in early childhood. The concerning dietary deterioration in the reasonably large ‘becoming less healthy’ trajectories and smaller ‘becoming unhealthy’ trajectories from the age of 7 years onwards occurred after children had attended primary school for about 2 years. Among possible explanations, by the 3rd year of primary school (i.e. the age 8 years assessment), some children may experience greater exposure to less healthy foods, either from other children or the school environment; they might also be more effective in persuading their parents to purchase these types of foods. They may also have greater autonomy of choice and/or greater susceptibility to the palatability of less healthy foods. These findings illustrate the importance of governments and policymakers introducing dietary-modifying interventions before or during early primary school years – further research is needed.
Unanswered questions and future research
This study points to several avenues for future research. First, it is important to thoroughly investigate the associations between a wide range of demographic and environmental factors in children and parents, and dietary trajectories. This investigation could allow differentiation of specific population subgroups that may receive more benefit from targeted dietary-modifying interventions and provide a further understanding into the types of interventions that may be effective. Second, it will now be possible to quantify the extent to which these trajectories differentiate early health phenotypes that might precede or protect against future non-communicable diseases. Studying these associations would allow us to determine which adverse and prevalent health outcomes are most strongly associated with dietary exposures and by what age. Finally, further follow-up of this and other cohorts will enable extension of dietary trajectories into later adolescence and adulthood. Future research should also consider a larger number of food and drink items, including the quantities consumed.
Conclusion
In conclusion, a brief dietary measure administered repeatedly across childhood generated robust, nuanced dietary trajectories that were replicable across parallel cohorts and two methodologies. These rigorously derived dietary trajectories are now available to interrogate the demographic/environmental determinants and health outcomes (including obesity, cardiovascular health, mental health and educational outcomes) of dietary inequity within the rich LSAC data set. More broadly, these analytic methods could inform other cohorts with repeated dietary measures, and the identified inflection points could guide life course timing of future interventions.
Acknowledgements
This paper uses unit record data from Growing Up in Australia, the Longitudinal Study of Australian Children. The study is conducted in partnership between the Department of Social Services (DSS), the Australian Institute of Family Studies (AIFS) and the Australian Bureau of Statistics (ABS). The findings and views reported in this paper are those of the authors and should not be attributed to DSS, AIFS or the ABS. The authors thank all the parents and children who took part in the Longitudinal Study of Australian Children. The authors would also like to thank Professor John Carlin (Murdoch Childrens Research Institute) for his advice on the statistical methods for this paper.
Authors of this work were supported by the Australian National Health and Medical Research Council (F. K. M., Early Career Fellowship – 1037449, Career Development Fellowship 1111160), (M. W., Senior Research Fellowship – 1046518); and an Australian Government Research Training Program Scholarship (C. E. G.). Research at the Murdoch Childrens Research Institute is supported by the Victorian Government’s Operational Infrastructure Support Program. The Australian National Health and Medical Research Council, Australian Government and Victorian Government had no role in the design, analysis or writing of this article.
The authors’ contributions are as follows: C. E. G., J. A. K., F. K. M. and M. W. formulated the research questions; C. E. G. designed the research, with input from J. A. K., F. K. M. and M. W.; C. E. G. conducted the research and wrote the article; C. E. G. analysed the data, with supervision from J. A. K., F. K. M. and M. W. All authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517000897













