Magnetic resonance imaging (MRI) studies have reported a wide range of brain abnormalities in patients with schizophrenia. Most of these are quantitative differences evident in statistical comparisons of group data, but some can be identified through radiological assessment of data from a single patient. Radiological findings associated with schizophrenia include reductions in global and regional grey matter volume, ventricular enlargement, cerebral atrophy and cavum septi pellucidi. Reference Lubman, Velakoulis, McGorry, Smith, Brewer and Stuart1,Reference Taylor and David2 Although some of these findings, such as cavum septi pellucidi, support neuro-developmental models of schizophrenia, their clinical relevance is generally low, as they do not require specific treatment. However, in a minority of patients presenting with acute psychosis, the patient may be diagnosed with ‘organic’ disorder underlying their symptoms. Reference Falkai3 Brain imaging techniques provide a means of detecting such ‘organic’ pathology in vivo, and may be used in the initial assessment of patients presenting with first-episode psychosis (FEP). However, although this may be regarded as good clinical practice, at present there is no agreement in national guidelines in terms of the use of neuroimaging as a standard part of the assessment of all patients with FEP. Reference Lehman, Lieberman, Dixon, McGlashan, Miller and Perkins4–6 The use of MRI in this group may also be less than might be expected because clinicians perceive that scanning people with FEP is too logistically difficult to be clinically worthwhile.
Although it is widely acknowledged that neuroimaging in patients presenting with FEP may identify individuals whose condition has an ‘organic’ aetiology, Reference Albon, Tsourapas, Frew, Davenport, Oyebode and Bayliss7 its routine use in the assessment of all patients with FEP would need to be justified on the basis of a cost (harm)–benefit analysis. This would entail knowing not just the prevalence of radiological abnormalities in patients with FEP, but particularly the prevalence of clinically meaningful findings. At present, the literature comprises prevalence estimates derived from patients who have been recruited to research studies, in which computed tomography (CT) or MRI scans were evaluated by radiologists as part of a quality control process to exclude incidental brain pathology. According to the most recent study using this approach, Reference Sommer, de Kort, Meijering, Dazzan, Hulshoff Pol and Kahn8 clinically relevant pathology is relatively frequent in patients with psychosis (11.1%), but not significantly more prevalent than in controls (11.8%, P = 0.45). Moreover, the studies are heterogeneous in terms of patients' age, treatment history, the inclusion of both patients with FEP and those with a chronic condition, and the imaging methodologies employed. Reference Sommer, de Kort, Meijering, Dazzan, Hulshoff Pol and Kahn8–Reference Goulet, Deschamps, Evoy and Trudel12 In addition, the acquisition protocols and radiological assessments that are typically used in research studies differ from those employed in clinical radiological studies, as they were designed for research purposes rather than being optimised for radiological examination.
In studies restricted to patients with FEP, the prevalence of radiological findings varies considerably (between 5 and 17% Reference Robert Williams, Yukio Koyanagi and Shigemi Hishinuma13,Reference Strahl, Cheung and Stuckey14 ). The majority of the findings can be interpreted as being ‘neurodevelopmental’ in origin Reference Falkai3 and are not thought to account for the psychotic disorder. On the other hand, it has been estimated, among the total population of patients with psychosis, that 5–25% have an organic disorder underlying their symptoms. Reference Falkai3,Reference Fladby, Schuster, Gr⊘nli, Sj⊘holm, L⊘seth and Sexton15 The aims of the present study were twofold. First, we sought to assess the feasibility of including MRI as part of the initial clinical assessment of patients with FEP. Our second aim was to quantify the prevalence, nature and clinical significance of radiological abnormalities in these patients. We addressed these objectives in two large samples of people with FEP who were examined at presentation with a standardised MRI protocol and had been recruited from the same geographical area. One sample (the research sample) comprised patients ascertained in an epidemiological research study and scanned using an MRI protocol designed for research purposes. The other (the clinical sample) involved patients scanned using an MRI protocol that was optimised for clinical radiological assessment. The clinical sample allowed us to examine the feasibility of routine MRI in a sample that is more representative of the population of patients with FEP typically presenting to clinical services. By contrast, the research sample was restricted to patients with FEP who were eligible for inclusion in a research study and had consented to take part, and excluded patients if there was a possibility that they would not be able to lie still or tolerate a confined environment. The comparison of two samples that had been studied using MRI protocols optimised for either a diagnostic assessment or for research purposes allowed us to investigate whether the type of protocol influenced the prevalence of radiological findings. We hypothesised that (a) MRI assessment of people with FEP is feasible in the majority of patients, and (b) radiological abnormalities are evident in a substantial proportion of people with FEP.
Method
Research sample
Participants in this sample (n = 206) were recruited as part of the Aetiology and Ethnicity in Schizophrenia and Other Psychoses (ÆSOP) study. The ÆSOP study was a multicentre, case–control epidemiological study that identified all people with FEP (ICD-10 F20–F29, F30–F33 16 ) living in a well-defined area in South London. Patients who presented for the first time to the local psychiatric services, operating within the same defined area, for a functional psychotic illness (ICD-10 F10–19, excluding F1x.0 for acute intoxication; F20–29 and F30–39, psychotic codings) over a 3-year period (September 1997 to August 2000) were consecutively approached for inclusion.
Inclusion criteria for patients (n = 108) were: (a) age between 16 and 64 years, (b) resident within the catchment area (boroughs of Lambeth and the southern two-thirds of Southwark in London), and (c) no previous contact with health services for psychosis. Importantly, patients in whom there was non-radiological evidence that their psychotic symptoms had an organic cause (such as substance use or other brain disorders) were excluded. Individuals with IQ ratings <50 were also excluded. Diagnoses were determined by consensus of senior psychiatrists using information obtained from the Schedules for Clinical Assessment in Neuropsychiatry (SCAN). 17
A random sample of population-based healthy comparison participants aged 16–64 years (n = 98) was selected using a procedure adapted from that used by the Office of Population Censuses and Surveys psychiatric morbidity survey. Reference Jenkins and Meltzer18 This generated 10 target addresses for each patient, from which controls were recruited. Each address was contacted three times (morning, afternoon, evening). The controls were thus broadly matched to the patients by area of residence. The study was approved by the South London and Maudsley Research Ethics Committee and all participants gave written informed consent.
Clinical sample
This sample (n = 307) comprised 241 patients with FEP who had been scanned as part of their initial clinical assessment at presentation to local mental health services between 2008 and 2012, plus 66 healthy volunteers scanned using the same MRI acquisition protocol over the same period. MRI in the patients was facilitated by clinically trained scanning coordinators, who served as a link between the neuroimaging centre and the referring clinical teams. The coordinator contacted the patient by phone, screened for contraindications for MRI, and provided the patient with information about the scanning procedure. The coordinator also helped the clinical team to arrange the scanning session, facilitated the transfer of the patient to and from the scanner, and reminded the patient about the scan by phone, 1 week and 1–2 days before the scan. After the scan, the coordinator ensured that the radiologist's report on the findings was delivered to the patient's clinical team.
All participants in both samples gave consent for their scans to be used in research studies, for them to be reviewed by a radiologist and for any abnormalities to be reported to their general practitioner.
MRI acquisition
Research sample
Images were acquired using a General Electric Signa 1.5-T system (GE Medical Systems, Milwaukee, WI, USA) at the Institute of Psychiatry, King's College London, UK. Contiguous, interleaved proton density- and T 2-weighted images, each 3 mm thick, were acquired in the coronal plane to provide whole-brain coverage. A repetition time (TR) of 4000 ms and effective echo times (TE) of 20 and 85 ms were used with an eight-echo train length. The matrix size was 256 × 192, collected from a rectangular field of view of 22 × 16.5 cm, giving an in-plane resolution of 0.859 mm. Image contrast for all data-sets was chosen with the aid of optimising software. Reference Simmons, Arridge, Barker and Williams19
Clinical sample
MRI data were acquired on a 3.0T GE Signa HDx MR system at the Centre for Neuroimaging Sciences, Institute of Psychiatry, King's College London. The body coil was used for radiofrequency transmission and an eight-channel head coil for radiofrequency reception. Following a three-plane localiser, a high-resolution sagittal 3D MPRAGE pulse sequence was acquired with 166 slices, 1.2 mm slice thickness, a 26 × 24 cm field of view, inversion time (TI) = 650 ms, TR = 6.97 ms, TE = 2.84 ms, flip angle 8 degrees. Full brain and skull coverage was required for each participant according to previously published criteria. Reference Simmons, Westman, Muehlboeck, Mecocci, Vellas and Tsolaki20 A motion-resistant T 2-weighted propeller (26 slices, 512 × 512 matrix, 24 × 24cm field of view, TR = 5000 ms, TE = 84.4 ms, 5.0 mm slice thickness with 1.0 mm gap, matrix, one data average) and a motion-resistant FLAIR propeller data-set (26 slices, 320 × 320 matrix, 24 × 24cm field of view, TR= 10 000 ms, TI = 2500 ms, TE= 162.2 ms, 5.0 mm slice thickness with 1.0 mm gap, matrix, one data average) were subsequently acquired.
Radiological assessments
All scans from both samples were evaluated by consultant neuroradiologists from the Neuroradiology Department, King's College Hospital, London. Their reports were then reviewed by clinically trained raters (I.F. and S.B.). Findings that did not involve brain tissue or related to features outside the cranial cavity (for example, abnormalities of the paranasal sinuses) were excluded. Reports were first categorised as containing normal or abnormal findings. All abnormalities were then subclassified according to the type of lesion Reference Sommer, de Kort, Meijering, Dazzan, Hulshoff Pol and Kahn8 (online Table DS1) and the recommended clinical response: Reference Katzman, Dagher and Patronas21 clinical responses were categorised as follows: (a) no further referral (incidental finding seen in healthy individuals); (b) routine referral (finding requires follow-up, but not urgent); (c) urgent referral (finding needs prompt evaluation); (d) immediate referral (finding requires immediate assessment).
Statistical analysis
All analyses were performed in IBM SPSS Statistics 21. Analysis of normality was performed with the Kolmogorov–Smirnov test. Comparisons of continuous variables between groups were performed using the Mann-Whitney U-test. A chi-square test and Fisher's exact test were employed for categorical variables, correcting for multiple comparisons with Bonferroni.
Results
Practicability of MRI in first-episode psychosis
All patients in the research sample and 97.5% of those in the clinical sample completed the scanning procedure. The six patients in the clinical sample who did not complete the scanning were excluded from further analysis. All of the controls in both samples tolerated the MRI procedure. Four of the radiological reports from patients in the clinical sample were not retrievable. The reports comprised printed sheets that were normally sent to the clinical team via the internal mail. All the reports for patients in the research sample and for the healthy controls from both samples were available.
Age and gender
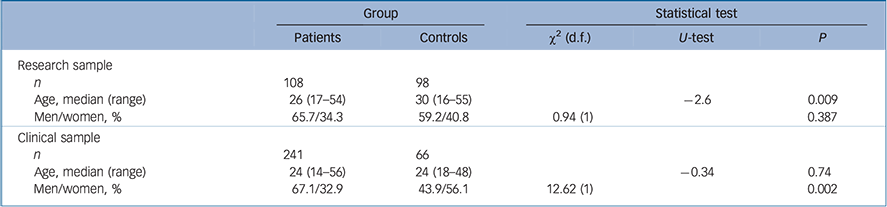
Significant age differences were found for controls in the research sample, who were significantly older than both patients in the research sample (U = − 2.6, P = 0.009; Table 1) and controls in the clinical sample (U = −3.35, P = 0.001; Table 2). The proportion of men was significantly higher in the patient group relative to the control group in the clinical sample (χ2(1) = 12.62, P = 0.002). No gender differences were found between patients and controls in the research sample or between the patients of the research and clinical samples (Table 1).
Table 1 Sample characteristics for the patient v. control groups in the research and clinical samples
| Group | Statistical test | ||||
|---|---|---|---|---|---|
| Patients | Controls | χ2 (d.f.) | U-test | P | |
| Research sample | |||||
| n | 108 | 98 | |||
| Age, median (range) | 26 (17–54) | 30 (16–55) | −2.6 | 0.009 | |
| Men/women, % | 65.7/34.3 | 59.2/40.8 | 0.94 (1) | 0.387 | |
| Clinical sample | |||||
| n | 241 | 66 | |||
| Age, median (range) | 24 (14–56) | 24 (18–48) | −0.34 | 0.74 | |
| Men/women, % | 67.1/32.9 | 43.9/56.1 | 12.62 (1) | 0.002 | |
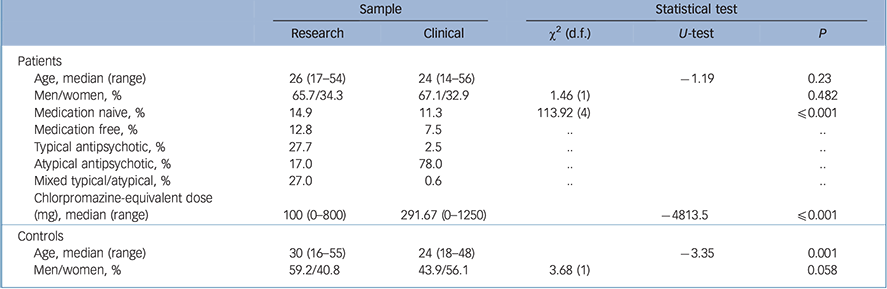
Table 2 Sample characteristics for the research v. clinical samples
| Sample | Statistical test | ||||
|---|---|---|---|---|---|
| Research | Clinical | χ2 (d.f.) | U-test | P | |
| Patients | |||||
| Age, median (range) | 26 (17–54) | 24 (14–56) | −1.19 | 0.23 | |
| Men/women, % | 65.7/34.3 | 67.1/32.9 | 1.46 (1) | 0.482 | |
| Medication naive, % | 14.9 | 11.3 | 113.92 (4) | ⩽0.001 | |
| Medication free, % | 12.8 | 7.5 | .. | .. | |
| Typical antipsychotic, % | 27.7 | 2.5 | .. | .. | |
| Atypical antipsychotic, % | 17.0 | 78.0 | .. | .. | |
| Mixed typical/atypical, % | 27.0 | 0.6 | .. | .. | |
| Chlorpromazine-equivalent dose (mg), median (range) |
100 (0–800) | 291.67 (0–1250) | −4813.5 | ⩽0.001 | |
| Controls | |||||
| Age, median (range) | 30 (16–55) | 24 (18–48) | −3.35 | 0.001 | |
| Men/women, % | 59.2/40.8 | 43.9/56.1 | 3.68 (1) | 0.058 | |
Medication
Significant differences were found for the treatment regimens of patients in the research v. the clinical samples. The proportion of patients who were medication naive or medication free (i.e. no antipsychotic medication for at least 3 weeks before the scan) was slightly higher in the research sample than in the clinical one (14.9% v. 11.3% and 12.8% v. 7.5%; Table 2). The majority of patients in the research sample were on a typical antipsychotic (27.7%), whereas the majority of patients in the clinical sample received atypicals (78%, χ2(4) = 113.92, P⩽ 0.001). This difference may have reflected differences in prescribing practice at the time that the respective samples were ascertained. The median chlorpromazine-equivalent dose in the research sample was 100mg/day v. 291.67mg/day in the clinical sample (U = −4813.5, P⩽0.001; Table 2).
Proportions of abnormal scans across samples and groups
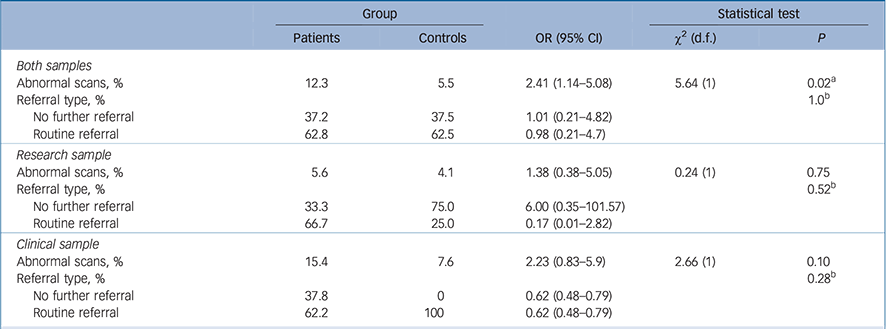
Across samples, 12.3% of patient scans and 5.5% of control scans were reported as abnormal (χ2(1) = 5.64, odds ratio (OR) = 2.41, 95% CI 1.14–5.08, P = 0.02; Table 3). No significant differences in rates of abnormal scans were found between the patients and controls of the research sample and the clinical sample, respectively.
Table 3 Rates of normal and abnormal findings In the patient v. control groups In the samples
| Group | Statistical test | ||||
|---|---|---|---|---|---|
| Patients | Controls | OR (95% CI) | χ2 (d.f.) | P | |
| Both samples | |||||
| Abnormal scans, % | 12.3 | 5.5 | 2.41 (1.14–5.08) | 5.64 (1) | 0.02 a |
| Referral type, % | 1.0 b | ||||
| No further referral | 37.2 | 37.5 | 1.01 (0.21–4.82) | ||
| Routine referral | 62.8 | 62.5 | 0.98 (0.21–4.7) | ||
| Research sample | |||||
| Abnormal scans, % | 5.6 | 4.1 | 1.38 (0.38–5.05) | 0.24 (1) | 0.75 |
| Referral type, % | 0.52 b | ||||
| No further referral | 33.3 | 75.0 | 6.00 (0.35–101.57) | ||
| Routine referral | 66.7 | 25.0 | 0.17 (0.01–2.82) | ||
| Clinical sample | |||||
| Abnormal scans, % | 15.4 | 7.6 | 2.23 (0.83–5.9) | 2.66 (1) | 0.10 |
| Referral type, % | 0.28 b | ||||
| No further referral | 37.8 | 0 | 0.62 (0.48–0.79) | ||
| Routine referral | 62.2 | 100 | 0.62 (0.48–0.79) | ||
a. Bonferroni-corrected.
b. Fisher's exact test (two sided).
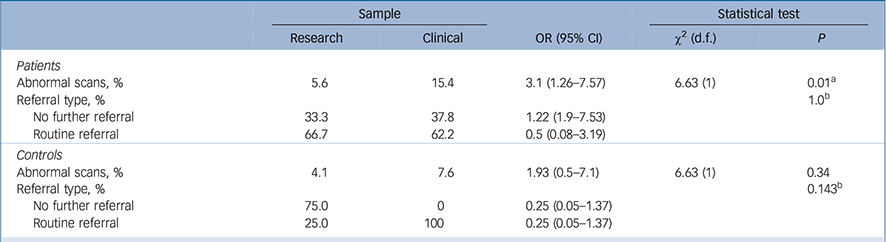
Patients in the clinical sample had a significantly greater proportion of abnormal scans than patients in the research sample (15.4% v. 5.6%, χ2(1) = 6.63, OR = 3.1, 95% CI 1.26–7.57, P = 0.01, Bonferroni-corrected; Table 4). Abnormality rates in the control groups did not differ between the two samples (clinical sample 7.6, research sample: 4.1%, χ2(1) = 6.63, OR = 1.93, 95% CI 0.5–7.1, P = 0.34; Table 4).
Table 4 Rates of normal and abnormal findings for the research v. clinical samples
| Sample | Statistical test | ||||
|---|---|---|---|---|---|
| Research | Clinical | OR (95% CI) | χ2 (d.f.) | P | |
| Patients | |||||
| Abnormal scans, % | 5.6 | 15.4 | 3.1 (1.26–7.57) | 6.63 (1) | 0.01 a |
| Referral type, % | 1.0 b | ||||
| No further referral | 33.3 | 37.8 | 1.22 (1.9–7.53) | ||
| Routine referral | 66.7 | 62.2 | 0.5 (0.08–3.19) | ||
| Controls | |||||
| Abnormal scans, % | 4.1 | 7.6 | 1.93 (0.5–7.1) | 6.63 (1) | 0.34 |
| Referral type, % | 0.143 b | ||||
| No further referral | 75.0 | 0 | 0.25 (0.05–1.37) | ||
| Routine referral | 25.0 | 100 | 0.25 (0.05–1.37) | ||
a. Bonferroni-corrected.
b. Fisher's exact test (two sided).
In addition to the quantitative differences between the patients in the research and clinical samples, there was also a qualitative difference in the nature of the abnormalities (Table 5). In the patients in the research sample, cysts and ventricular asymmetries were the most prevalent of all findings (1.9%), whereas white matter abnormalities (6%), cavum septi pellucidi (5.1%) and cysts (2.6%) were most prevalent in the patients in the clinical sample. Figure 1 shows an example of a normal MRI scan and a scan showing a pineal cyst.
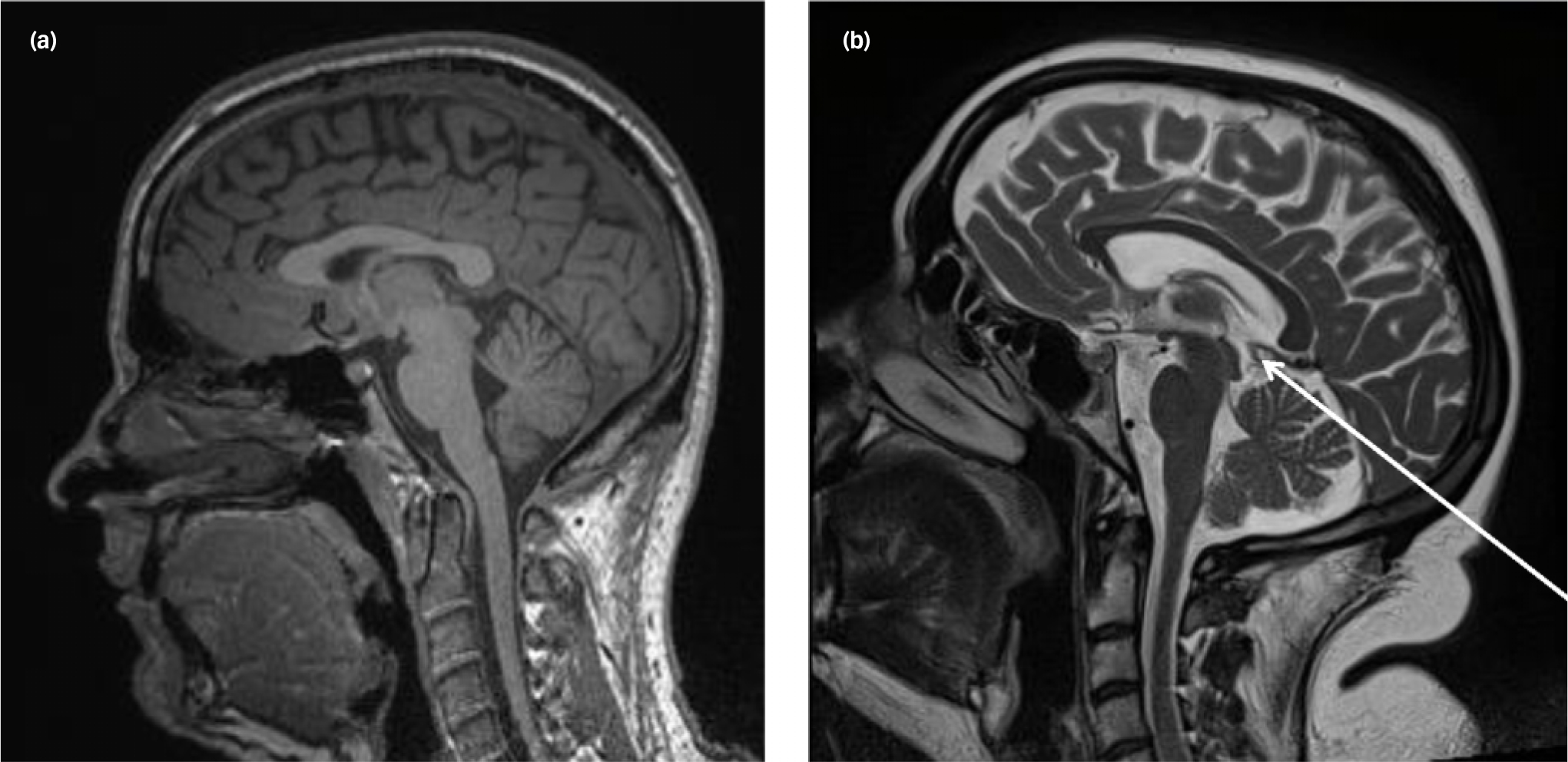
Fig. 1 Examples of (a) a normal scan, (b) a scan showing a small pineal cyst (arrow).
Table 5 Distribution of types of abnormalities and type of referral in patients between samples
| Sample, % | Statistical test | |||
|---|---|---|---|---|
| Research | Clinical | χ2 (d.f.) | P | |
| Cysts (routine) | 1.9 | 2.6 | 0.16 (1) | 0.69 |
| Mild asymmetry of ventricles, lobes or brain (no further referral) | 1.9 | 0.9 | 0.63 (1) | 0.59 |
| Cavum septum pellucidum (no further referral) | – | 5.1 | 5.74 (1) | 0.02 a |
| White matter abnormalities (routine) | 0.9 | 6.0 | 4.50 (1) | 0.03 a |
| Post-ischaemic lesions (routine) | – | 0.4 | 0.46 (1) | 0.50 |
| Sella (partially) empty (routine) | 0.9 | 0.9 | 0.04 (1) | 0.95 |
a. Bonferroni-corrected.
Impact on clinical management
Although abnormalities were more frequently reported in patients from the clinical sample than the research one, and there were differences in the types of abnormalities, there was no significant difference in the resulting referral type (i.e. routine referral or no further referral; χ2(1) = 0.05, P = 0.83). All abnormalities detected needed either no further referral or routine referral only.
Discussion
This study had two aims: (a) to evaluate the practicability of MRI as part of the initial clinical assessment of patients with FEP, (b) to determine the prevalence, nature and clinical significance of radiological abnormalities in patients with FEP.
Main findings regarding practicability of MRI
When performing MRI scans in patients who are acutely psychotic, one important consideration is anxiety induced by the procedure. Any individual may experience anxiety undergoing an MRI exam (4–30% Reference Meléndez and McCrank22 ) and this may be particularly likely in patients with psychosis, Reference Albon, Tsourapas, Frew, Davenport, Oyebode and Bayliss7,Reference Khandanpour, Hoggard and Connolly23 although this has not been studied systematically. Patients with psychosis may also be difficult to assess because they do not accept that they are unwell (and therefore require investigation), or because they have paranoid beliefs about clinical staff or the purpose of the MRI scan. They may also have been admitted for assessment or treatment against their will, which may make it logistically difficult to bring them to the scanning centre and manage them safely in that environment. All of these issues are especially relevant to patients who are presenting with psychosis for the first time and have no previous experience of psychiatric care. Awareness of these factors may lead clinicians to regard MRI as logistically difficult in this group.
However, we found that the great majority of patients were able to tolerate the scanning procedure very well, with scanning being interrupted in only 2.5% of the patients in the clinical sample, and none of those in the research one. We cannot exclude the possibility of some selection bias, with researchers in the research sample or clinicians in the clinical sample declining to refer or include patients that they thought would be too unwell to be scanned. Nevertheless, our data strongly suggest that an MRI assessment is practicable in most patients with FEP, including patients in whom scanning is being done for clinical purposes.
Main findings regarding prevalence of radiological abnormalities
Regarding the second aim of our study, we found that radiological abnormalities were relatively common in patients with FEP, although they were also evident in healthy controls. This is broadly consistent with data from previous studies, which have reported prevalence rates of up to 40% in patients with FEP Reference Borgwardt, Radue, Götz, Aston, Drewe and Gschwandtner24 and 3–19% in healthy controls. Reference Morris, Whiteley, Longstreth, Weber, Lee and Tsushima25,Reference Hartwigsen, Siebner, Deuschl, Jansen and Ulmer26 A previous study in people at increased clinical risk for developing psychosis indicated that radiological abnormalities are already present before the onset of the disorder, with similar prevalence rates in both individuals with FEP and those at high risk. Reference Borgwardt, Radue, Götz, Aston, Drewe and Gschwandtner24 They are therefore unlikely to be related to antipsychotic medication, as the majority of individuals at high risk and with FEP had never or only very briefly been treated with antipsychotics. Reference Borgwardt, Radue, Götz, Aston, Drewe and Gschwandtner24
Radiological abnormalities were significantly more frequent in the clinical than in the research sample. This may reflect the exclusion of patients from the research sample, either because they did not meet research inclusion criteria (for example, because of a suspected ‘organic’ cause), or because they were too unwell to provide informed consent for a research study or to tolerate the scanning procedure. Research scans usually take longer than clinical scans and therefore duration of the scan may have been less of an issue for the clinical sample. As we had not assessed current psychopathology in the clinical sample, we were not able to compare the degrees of severity of illness in the two groups. However, the findings on medication support the assumption that the patients in the clinical sample may have been more unwell than those in the research sample, as a larger proportion of patients in the research sample relative to the clinical one were medication naive or medication free. The majority of patients in the research sample were in fact on typical antipsychotics (with atypicals being more frequent in the clinical sample); however, this is probably related to the fact that the research sample data were collected about 10 years earlier than the clinical sample data (i.e. when atypicals were less widely available).
Higher field strength used in the clinical sample (3T v. 1.5 T) may also have allowed better delineation of subtle abnormalities, resulting in higher prevalence rates of abnormalities in the clinical sample. Nevertheless, the findings in the two patient samples were consistent in terms of clinical impact, as in both samples none of the abnormalities required any changes in the clinical management.
Previous estimates of the prevalence of radiological abnormalities in patients with psychosis have largely been based on data collected in research studies in which CT or MRI scans were evaluated by radiologists to exclude participants with incidental brain pathology that could have confounded interpretation of the research findings. The patients recruited to these studies are thus unlikely to be representative of the clinical population, and this issue may be better addressed using MRI data that were explicitly collected for clinical purposes. Furthermore, many of the patient samples in previous studies were small, they comprised patients with varying durations of illness and treatment, and some studies pooled data collected using different acquisition methods (for example, CT and MRI). Reference Sommer, de Kort, Meijering, Dazzan, Hulshoff Pol and Kahn8–Reference Goulet, Deschamps, Evoy and Trudel12 Finally, the acquisition protocols and radiological assessments used in these studies were designed for research purposes rather than clinical examination. In the present study, we sought to address these methodological issues by studying two large and homogenous samples of patients with FEP, with all those in each sample studied using a standardised MRI protocol. One sample was recruited through an epidemiological study of the local population, whereas the other comprised local patients who had been scanned as part of their initial clinical assessment. These samples may thus be more representative of the clinical population than those in previous studies.
Clinical implications
At present, although it is considered good practice to include a neuroimaging assessment in the initial clinical assessment of patients with FEP, Reference Gaebel27 this is not routinely carried out in all patients. A recent health economic analysis indicated that routine MRI of all patients with FEP under the age of 65 would be cost-saving, relative to the selective scanning of patients in whom there was a particular clinical indication, if the prevalence rate for serious abnormalities (such as brain tumour, large cyst) was 1% and the time between presentation and assessment was less than 3 months. Reference Albon, Tsourapas, Frew, Davenport, Oyebode and Bayliss7 In the present sample, none of the radiological abnormalities in the patient group was considered a possible substrate of organic psychosis. However, this may be related to the relatively young age of our patients. The prevalence of ‘organic’ causes of psychosis is much higher in older patients (up to 25% Reference Fladby, Schuster, Gr⊘nli, Sj⊘holm, L⊘seth and Sexton15 ). This is of particular relevance in the UK, in view of a recent directive to raise the maximum age for new referrals to early-intervention services from 35 to 65. 28
Given that in most patients the aetiology of psychosis is unknown, it is also possible that some patients have ‘organic’ aetiologies that have yet to be identified and that may be associated with MRI findings other than those known to cause psychosis. Diagnoses of such ‘organic’ psychoses may become possible with the development of new diagnostic techniques. For example, recent evidence suggests that in a proportion of patients with FEP, antibodies to central nervous system antigens may underlie the disorder. Reference Dalmau, Tüzün, Wu, Masjuan, Rossi and Voloschin29 These patients may not show neurological abnormalities, but MRI suggests that T 2 or FLAIR hyperintensities in the hippocampi, basal ganglia and insulae are detectable in around 50% of these patients. Reference Dalmau, Lancaster, Martinez-Hernandez, Rosenfeld and Balice-Gordon30 MRI may thus help to identify such individuals, who may respond to immunological treatment. Reference Dalmau, Lancaster, Martinez-Hernandez, Rosenfeld and Balice-Gordon30
Aside from health economic considerations, in clinical practice, failing to detect a psychosis with an ‘organic’ aetiology can have a disproportionate impact on the patient, their relatives and the clinical team, as the consequences can be so severe, especially when early detection (for example, of a brain tumour or encephalitis) could lead to a life-saving intervention. Thus, some clinicians take the view that even if such cases are rare, the impact of missing them is so great that it is worth assessing everyone.
Neuroimaging research has provided robust and replicable evidence of brain structural, functional and chemical abnormalities in psychotic disorders. Reference Ellison-Wright, Glahn, Laird, Thelen and Bullmore31–Reference Minzenberg, Laird, Thelen, Carter and Glahn34 Recent analytical advances in machine learning tools have demonstrated that their application to neuroimaging data from a single individual permits accurate classification of that individual as belonging to a psychotic patient or healthy volunteer group. Reference Orrù, Pettersson-Yeo, Marquand, Sartori and Mechelli35 The key advantage of these tools is that because they can be used to make predictions at the level of the individual they have high translational potential. Reference Brammer36 Neuroimaging markers may thus be used early in psychosis to predict prognosis in clinical settings Reference Palaniyappan, Marques, Taylor, Handley, Mondelli and Bonaccorso37 and stratify individual treatment plans, with appropriate resource allocation at the service level.
Yet brain scanning still has only a minor role in the clinical assessment of patients with psychosis. Clinical guidelines vary with regards to the use of neuroimaging in FEP. Most international guidelines recommend CT scanning in the initial assessment, 6,38,39 although a few recommend MRI rather than CT. Reference Gaebel27,38 Our data suggest that MRI is feasible in the majority of patients with FEP. MRI offers superior anatomic resolution and does not expose patients to ionising radiation, a particular consideration in young adults and adolescents. Moreover, it is now possible to acquire high-resolution magnetic resonance images in a relatively short scanning time, which is particularly useful in patients who may be acutely unwell. The main advantages of CT are that it is less expensive Reference Albon, Tsourapas, Frew, Davenport, Oyebode and Bayliss7 and that it is better than MRI at detecting certain types of lesions. Reference Morris, Whiteley, Longstreth, Weber, Lee and Tsushima25
Limitations
We cannot exclude the possibility that the rates of abnormal findings in the research sample were reduced by the exclusion of patients whose psychosis was thought to have had an ‘organic’ aetiology, or the possibility that the patients who are most likely to volunteer to participate in a research project may be less severely unwell. Even in the clinical sample, individuals who were severely ill may not have been referred for scanning because the clinician did not think it was logistically feasible, or the patient was being treated without their consent. The clinicians involved in the present study told the investigators that they would often defer a neuroimaging assessment if patients were acutely unwell. It is thus possible that the prevalence rates from both samples were underestimates.
In conclusion, MRI as part of the clinical assessment is feasible in most patients with FEP and although radiological abnormalities are quite common, most are incidental findings that do not require a change in clinical management. Abnormalities that could account for a psychosis are rare, but are likely be diagnosed more frequently in the future as diagnostic means improve. Nevertheless, these may result from conditions that can have fatal outcomes if not detected early, such as tumours or encephalitis. The consequences of failing to exclude such disorders in a young adult may be so grave that we suggest that an MRI scan is indicated in the clinical assessment of all patients presenting with a first episode of psychosis.
Funding
This work was supported by the G. A. Lienert Foundation, Adolf-Schmidtmann-Foundation, FAZIT-Foundation and German Academic Exchange Service (to I.F.). This work was funded by the NIHR Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust, and the Institute of Psychiatry, Psychology and Neuroscience, King's College London.









eLetters
No eLetters have been published for this article.