Kawasaki disease is a febrile disease that usually occurs in children aged 0–5 years old and has an unclear aetiology. The main pathological processes of Kawasaki disease involve small and medium-vessel necrotising arteritis, subacute or chronic vasculitis, intraluminal myofibroblast proliferation, etc. Reference McCrindle, Rowley and Newburger1 According to the guidelines of the Japanese Circulation Society, about 2.3% of Kawasaki disease patients will have cardiovascular sequelae, among which coronary artery aneurysm-based cardiac sequelae are an important cause of death and disability in Kawasaki disease patients. Reference Fukazawa, Kobayashi and Ayusawa2 In recent years, studies have found that endothelial dysfunction is also one of the sequelae of Kawasaki disease, especially in the convalescence of Kawasaki disease. Reference Koibuchi, Kotani and Minami3,Reference Routhu, Singhal and Jindal4 However, the mechanism of endothelial dysfunction is still unclear, so there is a lack of preventive measures for this sequela.
Circulating microRNAs (miRNAs) are usually defined as miRNAs that are secreted from donor cells by extracellular vesicles and released into the blood circulation. Reference Creemers, Tijsen and Pinto5,Reference Makarova, Shkurnikov and Turchinovich6 Circulating miRNAs are remarkably stable due to their normal encapsulation in extracellular vesicles. Reference Mitchell, Parkin and Kroh7 In a variety of diseases, such as tumours and cardiovascular diseases, specific circulating miRNA expression profiles are found in the blood circulation of patients. Reference Creemers, Tijsen and Pinto5,Reference Cui, Wang and Yao8,Reference Min and Chan9 These specifically elevated circulating miRNAs were found to promote disease progression by being taken up by recipient cells and targeting their gene expression. In the past few years, multiple reports have suggested that various circulating miRNAs are upregulated in the blood of Kawasaki disease patients, such as miR-92a-3p, let-7i-p and miR-24-3p. Reference Rong, Jia and Hong10–Reference Weng, Cheng and Chien13 In particular, Jone et al. found that circulating miR-210-3p, miR-184 and miR-19a-3p are upregulated in Kawasaki disease patients as compared to non-Kawasaki disease febrile controls. Reference Jone, Korst and Karimpour-Fard12 However, the role and potential mechanism of these three circulating miRNAs in Kawasaki disease sequelae have not been investigated.
Here, we investigated the role and potential mechanism of miR-210-3p, miR-184 and miR-19a-3p in the endothelial dysfunction. We determined that overexpression of miR-184 and miR-19a-3p, but not miR-210-3p, induced endothelial dysfunction. Further, we revealed that both miR-184 and miR-19a-3p could target AGO2 by binding to its 3’untranslated regions and thus impede the AGO2/PTEN/VEGF axis. This study identified the precise mechanisms by which two upregulated mRNAs in the Kawasaki disease circulation induce endothelial dysfunction.
Materials and methods
Cell culture
We obtained human umbilical vein endothelial cells from American Type Culture Collection. We cultured human umbilical vein endothelial cells in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12; Thermo Fisher Scientific, MA, USA) containing 10% foetal bovine serum (Thermo Fisher Scientific) and supplemented with 100 U of penicillin/mL and 0.1 mg of streptomycin/mL at 37°C in a 5% CO2 humidified atmosphere.
MiRNA mimics, plasmids and transfection
The mimics of miR-184, miR-19a-3p, miR-210-3p and control mimics were commercially synthesised by Synbio Technologies (Suzhou, China). The AGO2 expression plasmid was cloned by RiboBio (Guangzhou, China). Human umbilical vein endothelial cells were seeded to culture dishes and allowed to adhere overnight. The next day, the mimics and/or plasmids were transfected using Lipofectamine 3000 (Invitrogen, Fisher Scientific) per the manufacturer’s instructions. The targeting efficiency of miRNA mimics was determined by qRT-PCR 48 hours after transfection. The expression of exogenous AGO2 protein was evaluated by Western blot 48 after transfection.
Tube formation assay
We performed the tube formation assay as described previously. Reference Zheng, Lu and He14 We added the BD Matrigel™ Basement Membrane Matrix to a μ-slide (#81506; Ibidi, Germany) and allowed it to solidify for 1 hour at 37°C. Then, 1 × 105 of human umbilical vein endothelial cells suspended in 50 µL of Dulbecco’s Modified Eagle Medium F-12 containing 10% foetal bovine serum were added to each well. Subsequently, the slide was incubated for 4 hours at 37°C in a 5% CO2 humidified atmosphere. Tube formation capacity of the closed networks of vessel-like tubes was analysed by using AngioTool. Reference Zudaire, Gambardella and Kurcz15
Prediction of miRNA target genes
We predicted target genes of miR-184 and miR-19a-3p using TargetScan (https://www.targetscan.org/vert_80/), an online algorithm for the prediction of miRNA target genes for vertebrates. Reference Agarwal, Bell and Nam16 The common target genes of these two miRNAs were generated by Venn diagram analysis (http://bioinformatics.psb.ugent.be/webtools/Venn/). The binding site of these two miRNAs on the 3’untranslated regions of AGO was downloaded and the binding site mutant was created accordingly.
Dual luciferase reporter assay
The wild-type or mutant binding sequences of miR-184 and miR-19a-3p on the 3’untranslated regions of AGO were cloned into the pGL3 vector, respectively. Human umbilical vein endothelial cells were transfected with these wild-type or mutant reporter vectors and miR-184 and miR-19a-3p or control mimics. The activities of these two reporters were measured using the Promega dual luciferase reporter assay system (#E1910; Promega, WI, USA) after 48 hours transfection as per the manufacturer’s instructions. The relative luciferase activities were analysed from three repeated assays.
Western blot
Cells were lysed in the RIPA lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1% Triton X-100, protease and phosphatase inhibitor mixture) on ice for 15 minutes, and lysates were centrifuged at 12,000 × g for 15 minutes. The denatured protein samples were separated using sodium dodecyl sulphate-polyacrylamide gel and transferred to polyvinylidene difluoride membrane. The membrane was then blocked in 5% skim milk for 1 hour at room temperature, followed by incubation of primary antibodies overnight at 4°C. The membrane was incubated with secondary antibodies for 1 hour at room temperature. Signals were detected using Western ECL Substrate (Bio-Rad). Primary antibodies against the following proteins were used: AGO2 (#2897, Cell Signaling Technology, USA), PTEN (#9188, Cell Signaling Technology, USA), VEGF (AF0312, Beyotime, China), and GAPDH (10494-1-AP, ProteinTech, China).
Statistical analysis
We created graphs and performed statistical analyses using GraphPad Prism (Version 8.0). All data are presented as mean ± SD from at least three independent assays. Statistical significance was calculated by one-way ANOVA or two-way ANOVA. Differences were considered significant when the p value was <0.05.
Results
Overexpression of miR-184 and miR-19a-3p induced endothelial dysfunction
To determine whether miR-184, miR-19a-3p and miR-210-3p affected the function of endothelial cells, we transfected their mimics in human umbilical vein endothelial cells. Then, the function of transfected human umbilical vein endothelial cells was evaluated by tube formation assay. Surprisingly, overexpression of miR-184 and miR-19a-3p but not miR-210-3p remarkably impeded the tube formation ability of human umbilical vein endothelial cells (Fig 1 a and b). To confirm this data, we simultaneously transfected miR-184 and miR-19a-3p in human umbilical vein endothelial cells and found that overexpression of both miRNAs led to a more substantial inhibitory effect on tube formation ability (Fig 1 a and b). These results demonstrated that overexpression of miR-184 and miR-19a-3p induced endothelial dysfunction.
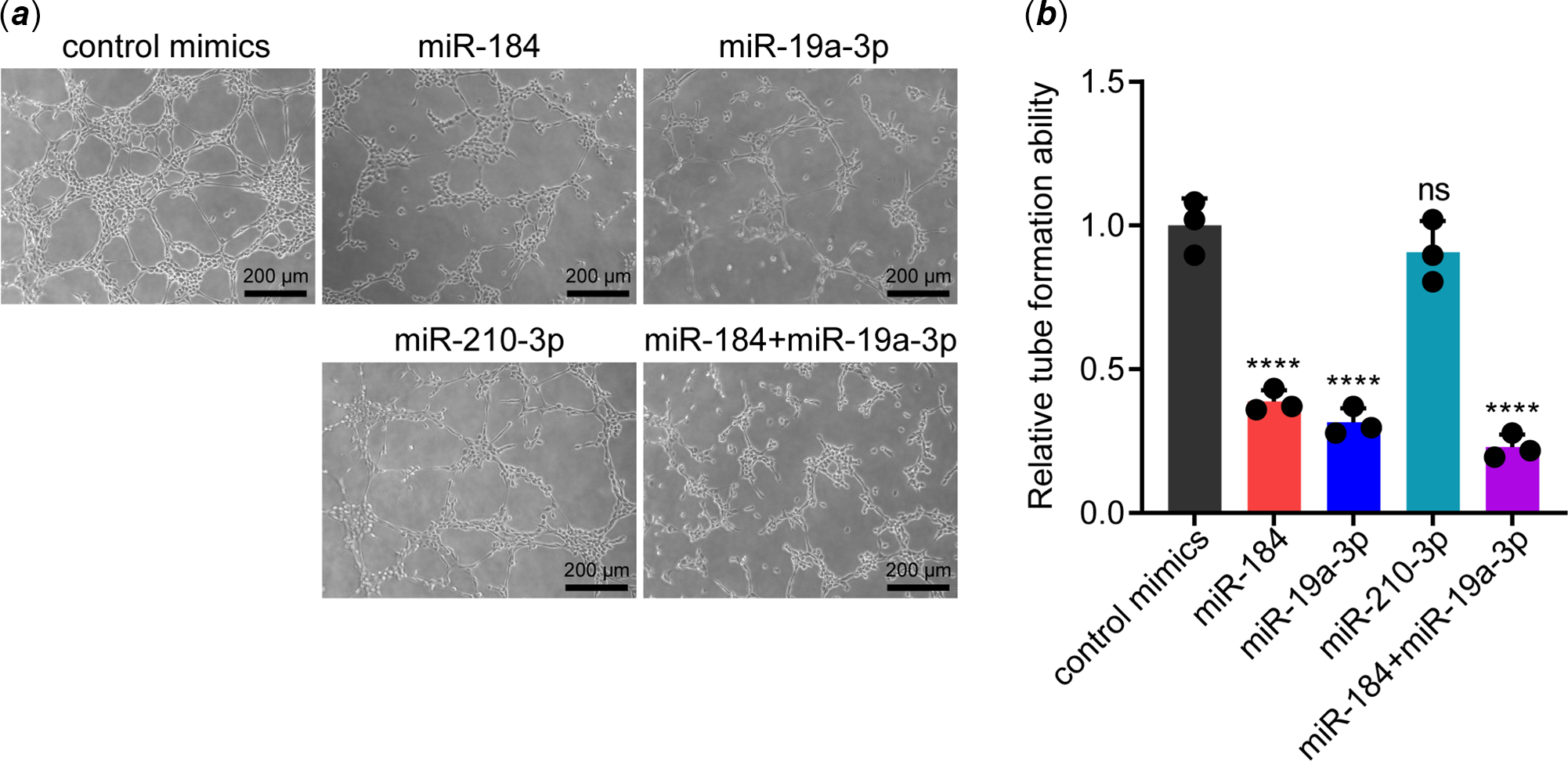
Figure 1. Effects of miR-184, miR-19a-3p and miR-210-3p on the tube formation ability of vascular endothelial cells. ( a ) The effects of miR-184, miR-19a-3p and miR-210-3p on the tube formation ability of HUVECs were detected by tube formation assay. Scale bars, 200 µm. ( b ) The tube formation of HUEVCs in each group was analysed. The means of relative tube formation ability are shown. One-way ANOVA was used to analyse and compare the data. ****p < 0.0001.
miR-184 and miR-19a-3p target AGO2 by binding to its 3’untranslated regions in endothelial cells
We then investigated how miR-184 and miR-19a-3p induce endothelial dysfunction. By accessing TargetScan, we determined that the 3’untranslated regions of AGO2 have potential binding sites for both these two miRNAs; that 5'UCCGUCC3' for miR-184 and 5'UUUGCAC3' for miR-19a-3p (Fig 2a). To further identify whether the binding of miR-184 and miR-19a-3p on the 3’untranslated regions of AGO2 was dependent on these two sites, we constructed luciferase reporter vectors with either the wild-type binding sites or mutant binding sites (Fig 2a). Results from the dual luciferase reporter assay showed that overexpression of miR-184 and miR-19a-3p in human umbilical vein endothelial cells significantly decreased the luciferase activities of both the wild-type reporters but not the mutant reporters (Fig 2b and c). These results indicated that miR-184 and miR-19a-3p target AGO2 by binding to its 3’untranslated regions in human umbilical vein endothelial cells.
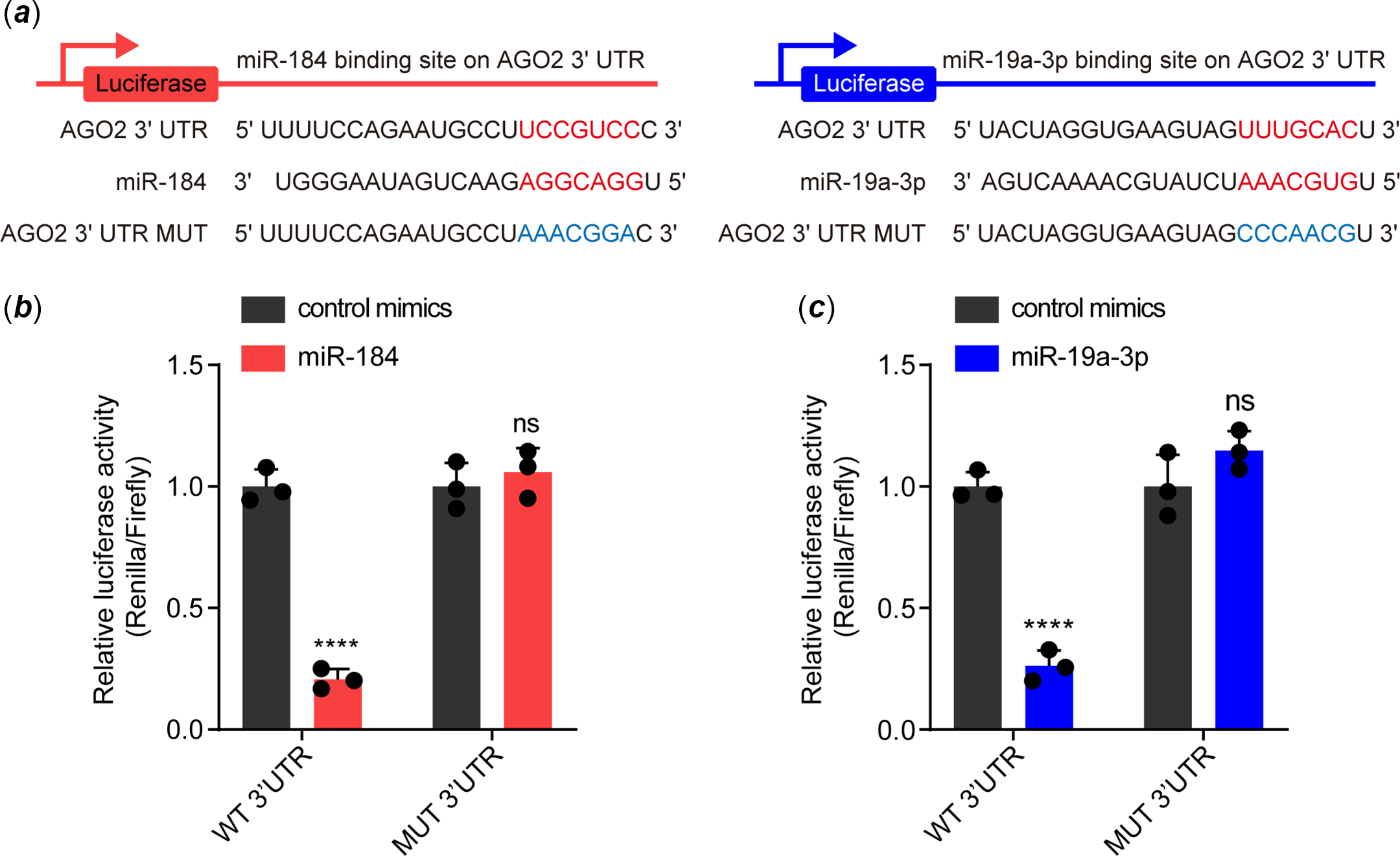
Figure 2. Both miR-184 and miR-19a-3p target the 3'UTR of AGO2 mRNA. ( a ) The binding sites of miR-184 and miR-19a-3p with the 3'UTR of AGO2, and the mutation of the corresponding binding sites of AGO2 3'UTR. ( b and c ) The binding ability of miR-184 and miR-19a-3p to wild-type and mutant AGO2 3'UTR was evaluated by dual luciferase reporter assay. The means of relative luciferase activities are shown. Two-way ANOVA was used to analyse and compare the data. ****p < 0.0001.
miR-184 and miR-19a-3p impeded the AGO2/PTEN/VEGF axis in endothelial cells
It has been widely reported that AGO2 is crucial for maintaining endothelial function, although through various potential mechanisms. Reference Leonov, Shah and Yee17–Reference Ye, Huang and Li20 In particular, AGO2 was shown to maintain the expression of VEGF via suppressing PTEN in endothelial cells. Reference Ye, Huang and Li20 As VEGF is a canonical effector in maintaining endothelial function, we speculated that whether miR-184 and miR-19a-3p induced endothelial dysfunction via suppression of AGO2/PTEN/VEGF pathway. To this end, we transfected the mimics of miR-184 and/or miR-19a-3p in human umbilical vein endothelial cells, followed by detection of the expression of AGO2, PTEN and VEGF by Western blot. GAPDH was assessed as an internal control. The expression of these proteins was normalised based on GAPDH. Results from Western blot showed that both miR-184 and miR-19a-3p downregulated the expression of AGO2 and VEGF, while upregulated the expression of PTEN, in human umbilical vein endothelial cells (Fig 3a and b). These results indicated that both miR-184 and miR-19a-3p inhibited the AGO2/PTEN/VEGF axis in endothelial cells.
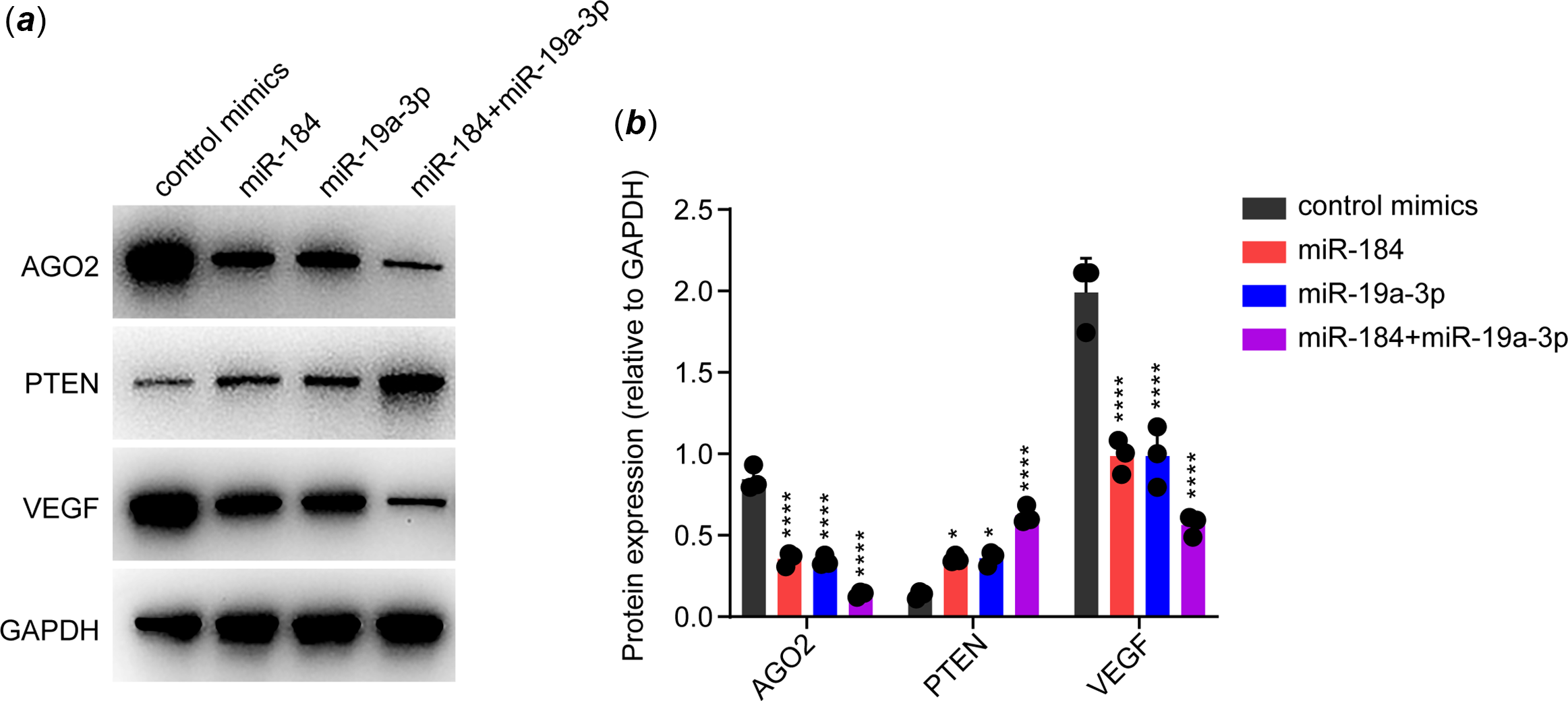
Figure 3. Effects of miR-184 and miR-19a-3p on AGO2/PTEN/VEGF signalling pathway. ( a ) The effects of miR-184 and miR-19a-3p on the expression of AGO2, PTEN and VEGF proteins in HUVECs were detected by Western blot. GAPDH was used as a loading control. ( b ) Relative protein levels in each group from ( a ) were quantified. The means of protein levels of AGO2, PTEN and VEGF are shown. One-way ANOVA was used to analyse and compare the data. *p < 0.05, ****p < 0.0001.
Overexpression of AGO2 rescued the endothelial function that inhibited by miR-184 and miR-19a-3p
As a miRNA could target more than one gene, Reference Krek, Grün and Poy21 we warranted to verify whether AGO2 was the key target of miR-184 and miR-19a-3p on inducing endothelial dysfunction. For elucidation, we transfected mimics of both miR-184 and miR-19a-3p to human umbilical vein endothelial cells with or without transfection of AGO2 expression vector. Data from tube formation assay showed that transfection of AGO2 plasmid notably recovered the tube formation ability that was inhibited by miR-184 and miR-19a-3p in human umbilical vein endothelial cells (Fig 4a and b). Collectively, these results demonstrated that both miR-184 and miR-19a-3p induce endothelial dysfunction by targeting AGO2 and inhibiting AGO2/PTEN/VEGF axis.
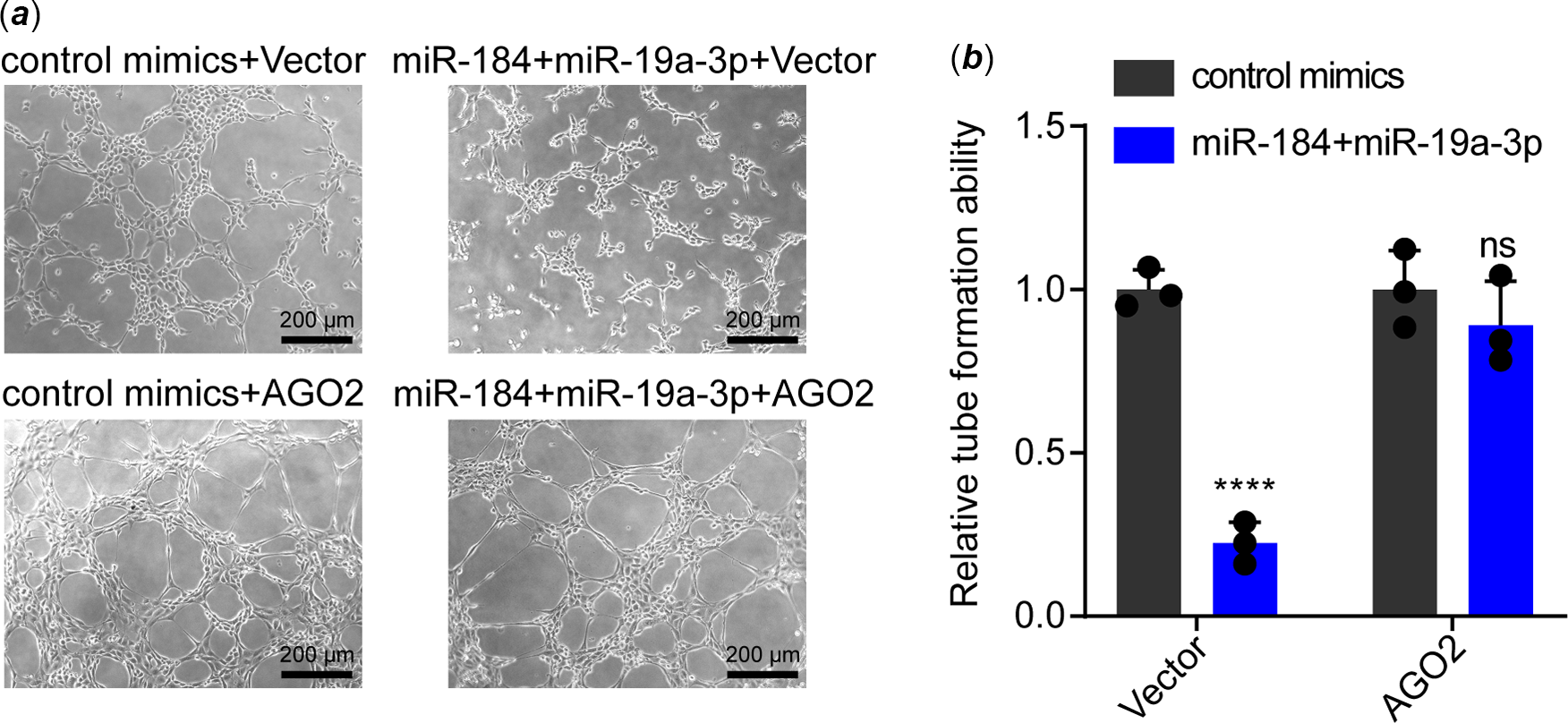
Figure 4. Effect of AGO2 overexpression on the inhibition of tube formation that mediated by miR-184 and miR-19a-3p. ( a ) HUVECs transfected with miR-184 and miR-19a-3p mimics were transfected with AGO2 plasmids or control plasmids. The tube formation of HUVECs in these groups was examined. Scale bar, 200 µm. ( b ) The tube formation of HUEVCs in each group was analysed. The means of relative tube formation ability are shown. Two-way ANOVA was used to analyse and compare the data. ****p < 0.0001.
Discussion
Endothelial dysfunction has been considered as a sequela of Kawasaki disease, especially during Kawasaki disease convalescence. Reference Koibuchi, Kotani and Minami3,Reference Routhu, Singhal and Jindal4 However, the mechanism of endothelial dysfunction caused by Kawasaki disease is unclear. Recently, Jone et al. found that circulating miR-210-3p, miR-184 and miR-19a-3p are upregulated in Kawasaki disease patients, Reference Jone, Korst and Karimpour-Fard12 but these miRNAs are with unknown mechanism in endothelial dysfunction. Our study found that miR-184 and miR-19a-3p, two circulating miRNAs that are upregulated in Kawasaki disease, could induce endothelial dysfunction by targeting AGO2.
In recent years, accumulation of studies has reported the correlation between various circulating miRNAs and Kawasaki disease, Reference Rong, Jia and Hong10–Reference Weng, Cheng and Chien13 despite the potential role and mechanism of the differential miRNAs in Kawasaki disease progression being largely unknown. In this study, we focused on the effects of three miRNAs (miR-184, miR-19a-3p and miR-210-3p) in endothelial dysfunction, as they were recently found to be upregulated in the serum of Kawasaki disease patients. Reference Jone, Korst and Karimpour-Fard12 Previous studies have shown that miR-184, miR-210-3p and miR-19a-3p play a role in inhibiting angiogenesis in corneal avascularity, sepsis and myocardial ischaemia/reperfusion injury, respectively, Reference Park and Peng22–Reference Liu, Yang and Fu24 but have not been studied in Kawasaki disease. To our surprise, we found that overexpression of both miR-184 and miR-19a-3p could induce endothelial dysfunction in vitro, despite it being difficult to verify this result in vivo. Unmodified RNAs are extremely unstable and vulnerable to degradation by RNAase. Reference Boo and Kim25 In general, the circulating miRNAs are remarkably stable, Reference Mitchell, Parkin and Kroh7 as they are usually encapsulated in extracellular vesicles with a lipid bilayer membrane. Reference Théry, Zitvogel and Amigorena26 Thus, it is reasonable that the upregulated circulating miRNAs would be likely up-taken by endothelial cells in Kawasaki disease patients. If possible, investigating whether the circulating miR-184 and miR-19a-3p were positively associated with endothelial dysfunction in Kawasaki disease patients would better demonstrate this finding.
Although a miRNA has the potential to bind more than one gene via its motif, Reference Krek, Grün and Poy21 we determined that AGO2 is the key downstream of miR-184 and miR-19a-3p in inducing endothelial dysfunction, as overexpression of AGO2 rescued the endothelial function that inhibited by miR-184 and miR-19a-3p. This result is not surprising as AGO2 is essential for maintaining endothelial function. AGO2 is a member of the Argonaute family of proteins which is central to miRNA-mediated gene silencing and miRNA biogenesis. Reference Matranga, Tomari and Shin27 In human dermal lymphatic endothelial cells, knockdown of AGO2 miR-132 decreased the expression of miR-221, Reference Leonov, Shah and Yee17 a miRNA crucial for angiogenesis. Reference Li, Song and Li28 In addition, lipopolysaccharide-induced AGO2 downregulation was found to correlate with the dysfunction of brain endothelial cells. Reference Machado-Pereira, Saraiva and Bernardino18 Moreover, suppression of AGO2 led to angiogenesis disorder in human umbilical vein endothelial cells and in the xenograft of human hepatocellular carcinoma by inhibiting PTEN/VEGF axis. Reference Yang and Chen19,Reference Ye, Huang and Li20 Considering that VEGF is a key effector for maintaining endothelial function, Reference Kliche and Waltenberger29 we validated that miR-184 and miR-19a-3p ultimately inhibited VEGF expression via AGO2/PTEN axis in endothelial cells.
This study has limitation. Our results suggest that miR-184 and miR-19a-3p upregulated in Kawasaki disease can inhibit endothelial function by targeting AGO2 in vitro. However, whether these two microRNAs can target AGO2 to induce endothelial dysfunction in vivo requires further investigation, for example, using Kawasaki disease animal models to fulfil this notion.
In conclusion, our study reveals a novel mechanism that upregulation of miR-184 and miR-19a-3p targets AGO2 and inhibits AGO2/PTEN/VEGF axis to induce endothelial dysfunction. The findings of this study may contribute to the development of potential prevention or treatment strategies for endothelial dysfunction in Kawasaki disease patients.
Acknowledgements
The authors would like to thank the Department of Pediatric of The Third People’s Hospital of Longgang District and the Department of Pediatrics of Longgang District Maternity & Child Healthcare Hospital of Shenzhen City.
Financial support
This work was supported by grants from the National Nature Science Foundation of China [81870364], GuangDong Basic and Applied Basic Research Foundation [2022A1515012468], Shenzhen Scientific Plan [JCYJ20190809164004023], Shenzhen Longgang District medical and health science and technology plan project [LGKCYLWS2021000020] and [LGWJ2021- (61)].
Conflicts of interest
None.







