Worldwide, the most effective strategy to control iodine deficiency is universal salt iodisation(Reference Zimmermann1, Reference Mannar and Hetzel2). In industrialised countries, where the main proportion of salt (about 80 %) is consumed through processed foods(Reference Andersson, de Benoist and Darnton-Hill3), the use of iodised salt particularly in the food industry and food trade (e.g. bakeries, butchers) should be an important prophylactic measure to combat iodine deficiency. The impact of the latter can be assessed by urinary iodine measurements, a suitable method to monitor iodine status in large cohorts(Reference Ristic-Medic, Piskackova and Hooper4, Reference Vejbjerg, Knudsen and Perrild5). In Germany, for example, one of the first steps towards effective iodine deficiency prophylaxis was taken in 1989 by permitting the addition of iodised salt to industrially processed foods. In 1993, the repeal of legislative restrictions (i.e. allowing separate labelling of foods containing iodised salt) followed and markedly helped to improve the iodine status of the German population(Reference Remer and Neubert6, Reference Scriba, Heseker and Fischer7). Concomitant measurements of urinary iodine concentrations in children and adolescents allowed direct monitoring of the efficiency of these prophylaxis measures: until 1992, median urinary iodine concentration in 24-h urine samples was lower than 70 μg/l; since 1993, it had been increasing steadily (to 95 μg/l until 1997(Reference Remer and Neubert6), and up to 117 μg/l until 2003(Reference Remer, Fonteyn and Alexy8)). The German Health Interview and Examination Survey for Children and Adolescents (KiGGS) during 2003–6 affirmed this positive development of iodine status by a measured median urinary iodine concentration of 117 μg/l in 0–17-year-olds(Reference Thamm, Ellert and Thierfelder9). Despite the fact that this median urinary iodine concentration was above the WHO cut-off mark for iodine sufficiency of 100 μg/l, it exceeded the cut-off level only by a narrow margin, suggesting that some fringe areas or population subgroups still may be at risk of inadequate iodine intakes. Especially in children, this picture is not satisfactory, as even mild iodine deficiency increases the risk of cognitive impairment(Reference Gordon, Rose and Skeaff10–Reference Santiago-Fernandez, Torres-Barahona and Muela-Martinez12).
In the coming years, according to scheduled public health measures in the European community and worldwide(Reference Klaus, Hoyer and Middeke13–16), a general reduction of salt intake is expected. Therefore, the proportion of iodine intake that is provided by iodised salt will also decrease. To date, only one study evaluated the association between salt restriction and iodine deficiency in adults and provided a first hint that in persons who are consuming low levels of iodine, salt restriction may cause iodine deficiency(Reference Tayie and Jourdan17).
In Germany, actual observations already show a slow but constant decrease in the use of iodised salt in processed foods since 2004(Reference Scriba, Heseker and Fischer7, 18). We intended to investigate whether this development has already affected the iodine status of German schoolchildren, to depict possible consequences of a general salt reduction (and therefore also iodine reduction) in processed foods. For this purpose, we studied 24-h urinary iodine excretion (UIE) of 6–12-year-old children from 2004 to 2009, to monitor the current development of iodine status of children in Germany. Concomitant investigations of the children's intakes of the most important dietary iodine sources (e.g. iodised salt, milk, fish) provided the basis to calculate the contributions of the latter to iodine excretion and to study related changes over time; with special regard to salt intake.
Methods
Subjects and study design
The present investigation was carried out in healthy children selected from the ongoing Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) Study, an open cohort study, since 1985 gathering information about diet, development and metabolism between infancy and adulthood in healthy participants(Reference Remer, Fonteyn and Alexy8, Reference Remer, Neubert and Maser-Gluth19). The children visit the Research Institute of Child Nutrition, Dortmund, once a year for examinations and assessments. The assessments include 3-d weighed dietary records, anthropometric measurements, collection of 24-h urine samples, and interviews on lifestyle and medical assessments. The DONALD Study is exclusively observational and non-invasive (until the age of 18 years). The study was conducted according to the guidelines laid down in the Declaration of Helsinki and was approved by the Ethics Committee of the University of Bonn (Germany). All examinations are performed with parental, and later on with the children's written consent.
For the present evaluation, all children who had ≥ 1 completely collected 24-h urine sample along with a 3-d weighed dietary record (encompassing the day of urine collection) during 2004–9 at the age of 6–12 years were enrolled. Only those examinations performed at ages 6–12 were included for each child. Exclusion criterion for an incompletely collected urine sample was a body weight-related 24-h-creatinine excretion rate < 0·1 mmol/kg per d(Reference Remer, Neubert and Maser-Gluth19). A total of 287 children met the criteria, providing 723 urine samples and corresponding dietary records. In order to obtain a preferably homogeneous study sample, urine samples and corresponding dietary records were excluded in case of no or irregular use of iodised table salt, iodine-containing drug use, or intake of iodine-containing supplements during the time of the respective urine collection. This was true for sixteen observations of fifteen children. As for six children, further repeated observations were available, and only nine children had to be completely excluded from the study sample. Therefore, 278 children with 707 repeatedly collected 24-h urine samples and related dietary records remained for the present investigations. Overall, 188 children (68 %) had at least two out of a maximum of six possible measures during the 5-year period.
Dietary records
To estimate the individual food and nutrient intake, 3-d weighed dietary records were used. On three consecutive days, the weight of all foods and beverages consumed was recorded using a digital, regularly calibrated food scale to the nearest 1 g. Out-of-home consumed food was estimated by semi-quantitative recording (e.g. numbers of glasses, cups). Intakes of energy, nutrients (including food fortification and nutritional supplements) and food groups were calculated as individual means of the three recorded days by using our in-house nutrient database LEBTAB(Reference Sichert-Hellert, Kersting and Chahda20), which contains detailed data on the energy and nutrient content of all recorded food items and is continuously updated. The following food groups were relevant for the present analysis: milk and whey-based milk products, salt-water fish, eggs and egg products, and meat and meat products. Because salt intake could not be quantitatively recorded in the dietary records, it was estimated from 24-h urinary Na measurements (g/d).
Urine collection and urinary measurements
The urine collection of the DONALD participants was generally carried out on the third day of the 3-d weighed dietary records. The procedures for the 24-h urine collections have been described in detail previously(Reference Remer, Fonteyn and Alexy8).
Iodine concentration was determined in the 24-h urine samples by a modified Sandell-Kolthoff method after acidic wet-ashing of the samples(Reference Lorenz-Wawschinek, Tiran and Eber21); and 24-h Na excretion was measured by flame atomic absorption spectrometry with a Perkin Elmer 1100 Spectrometer (Perkin Elmer, Überlingen, Germany). For determining completeness of the urine collection, creatinine concentration was quantified in all samples by the Jaffé method with the use of a creatinine analyser (Beckman-2; Beckman Instruments, Inc., Fullerton, CA, USA).
Anthropometric measurements
Anthropometric measurements of the DONALD participants were performed at each annual visit by nurses who had been trained according to standard procedures(Reference Lohman, Roche and Martorell22), with the children dressed in underwear only and barefoot. Standing height was measured with a stadiometer (Harpenden, Crymych, UK) to the nearest 0·1 cm, and weight was measured on an electronic scale (Seca 753E; Seca Weighing and Measuring System, Hamburg, Germany) to the nearest 0·1 kg. From these measurements, BMI and body surface area were calculated, the latter according to the formula of Du Bois & Du Bois(Reference Du Bois and Du Bois23). Sex- and age-independent BMI-standard deviation scores were calculated by using the German national reference data(Reference Kromeyer-Hauschild, Wabitsch and Kunze24).
Statistical analysis
All calculations were performed with SAS procedures (version 9.1.3, SAS Institute, Cary, NC, USA). Significance was defined as P < 0·05.
Anthropometric, dietary and urinary characteristics are presented sex-stratified as well as stratified by time period (2004–6 v. 2007–9), given as means and standard deviations or medians with interquartile ranges when appropriate. Sex differences were evaluated by unpaired t test or Wilcoxon rank sum test, differences between the two time periods were tested by a linear mixed-effects regression model (PROC MIXED in SAS, adjusted for sex and age) to account for the dependency between repeated measurements on the same child. A random statement accounted for variation between the individuals; the structure of the variance covariance matrix was set to compound symmetry.
Linear mixed-effects regression models (PROC MIXED) were also used to analyse time trends in iodine excretion (2004–9). In contrast to other models, e.g. linear regression for balanced longitudinal data (PROC GLM in SAS), the mixed linear regression model considers all available measurements rather than using only participants with complete follow-up data(Reference Verbeke, Molenberghs, Verbeke and Molenberghs25). A random statement was included to allow for variation between the individuals, with the variance covariance structure set to compound symmetry. Since no interaction between sex and the relationship of UIE to time was observed, data from girls and boys were pooled.
The basic model included the predictor variable time (continuously in years since 2004), sex, age and urine volume(Reference Johner, Shi and Remer26). Furthermore, we added creatinine excretion (standardised to a body surface area of 1·73 m2) to account for correlated measurement errors potentially introduced by the fact that iodine excretion and urine volume were measured in the same urine sample(Reference Barr, Wilder and Caudill27).
Subsequently, the dietary iodine sources, such as salt intake (estimated by Na excretion), milk, fish, egg and meat intake (g/d), were included as further fixed effects in a second model. Energy intake level, BMI-standard deviation scores and season were considered as potential confounders but were not included since none of them considerably modified the effect of time on iodine excretion or significantly predicted the outcome variable or improved the fit of the model (Akaike's information criterion). Finally, analyses were repeated time-stratified (2004–6 v. 2007–9) to address potential changes of the contributions of the different dietary iodine sources to iodine excretion over time.
Results
In Tables 1 and 2, anthropometric, dietary and urinary characteristics of the study sample are presented. UIE (μg/d) and concentration (μg/l) were significantly lower in girls than in boys (P < 0·0005). However, when corrected for individual energy intake (which was higher in boys), statistical differences disappeared (P = 0·13). Urinary iodine concentration significantly decreased from 2004–6 to 2007–9 (P < 0·0005) and fell below the WHO recommendation of 100 μg/l in the second time period (2007–9).
Table 1 Study sample characteristics at the time of first 24-h urine collection within 2004–9 of n 278 participants (6 to 12 year olds) of the Dortmund Nutritional and Anthropometric Longitudinally Designed Study, stratified by sex
(Mean values and standard deviations; median values and 25th (P25) and 75th (P75) percentiles)

SDS, standard deviation score.
* Sex differences were tested with an unpaired t test or Wilcoxon rank sum test, respectively.
† Only whey-based milk products.
‡ Only salt-water fish products.
§ Eggs and egg products.
‖ Meat and meat products.
Table 2 Anthropometric, dietary and urinary parameters, stratified by time period (total measurements n 707 of 278 children (6 to 12 years old) participating in the Dortmund Nutritional and Anthropometric Longitudinally Designed Study)
(Mean values and standard deviations; median values and 25th (P25) and 75th (P75) percentiles)

SDS, standard deviation score.
* Differences between the two time periods were tested with a linear mixed-effects regression model (PROC MIXED in SAS) to account for the dependency between repeated measurements on the same child. Regression models were adjusted for sex and age (for the comparison of mean age, only adjustment for sex was conducted).
† Only whey-based milk products.
‡ Only salt-water fish products.
§ Eggs and egg products.
‖ Meat and meat products.
Longitudinal trend analysis (i.e. the basic linear mixed-effects regression model adjusted for sex, age, urine volume and creatinine) of 24-h UIE showed a trend of a decline over the 6-year period under study (2004–9; β = − 1·05, P = 0·08). When this analysis was stratified by time (2004–6 v. 2007–9), a significant decline of urinary iodine was only observable in the second time interval (2007–9; β = − 3·86, P = 0·01); whereas from 2004 to 2006, UIE remained constant (β = 0·92, P = 0·58; Fig. 1). In Fig. 1, this trend is visualised by presenting the median daily UIE/year (2004–9). To obtain an adapted reference value for 24-h UIE that reflects an adequate iodine intake, we corrected the recommended dietary iodine intakes for an average portion of 15 % of non-urinary iodine losses (i.e. intake recommendation × 0·85). Compared to the estimated average requirement (EAR) of iodine set by the Institute of Medicine (65 μg/d for 4–8-year-olds, 73 μg/d for 9–13-year-olds(28), translating to UIE of 55 and 62 μg/d, respectively), 12·75 % of the children had an iodine intake below the EAR in 2004–6; this proportion increased to 15·5 % in 2007–9. When using the RDA as reference (90 μg/d for 4–8-year-olds, 120 μg/d for 9–13-year-olds(28), translating to UIE of 77 and 102 μg/d, respectively), 51·0 % of the population in the first time period and 57·2 % in the second time period did not reach the reference.
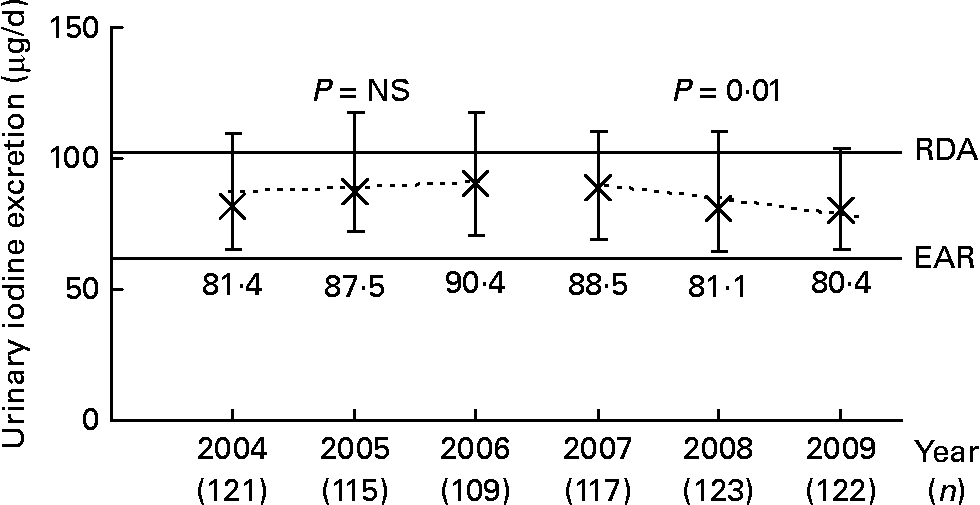
Fig. 1 Median ( × ) and interquartile range (![]() ) of urinary 24-h iodine excretion in 6–12-year-old schoolchildren, participating in the Dortmund Nutritional and Anthropometric Longitudinally Designed Study, between 2004 and 2009. Dashed lines plot the predicted values based on the time-stratified longitudinal regression model adjusted for sex, age, urine volume and creatinine. For comparison, development of urinary iodine concentration (μg/l): 2004: 113·1 (81·9–144·2) μg/l; 2005: 114·8 (81·0–155·1) μg/l; 2006: 101·8 (81·5–143·6) μg/l; 2007: 101·2 (81·5–139·3) μg/l; 2008: 92·9 (78·4–120·3) μg/l; 2009: 96·9 (72·7–127·1) μg/l. RDA and estimated average requirement (EAR) were derived from the iodine intake recommendations of the Institute of Medicine (IoM)(28) for 9 year olds (according to the mean age of the children, Table 2). The recommended iodine intakes were corrected for non-urinary iodine losses, assuming an average 15 % loss through the faeces and sweat, to obtain the corresponding adapted 24-h urinary iodine excretion. This yielded the approximate excretion reference values of 102 (IoM RDA) and 62 μg iodine/d (IoM EAR), respectively.
) of urinary 24-h iodine excretion in 6–12-year-old schoolchildren, participating in the Dortmund Nutritional and Anthropometric Longitudinally Designed Study, between 2004 and 2009. Dashed lines plot the predicted values based on the time-stratified longitudinal regression model adjusted for sex, age, urine volume and creatinine. For comparison, development of urinary iodine concentration (μg/l): 2004: 113·1 (81·9–144·2) μg/l; 2005: 114·8 (81·0–155·1) μg/l; 2006: 101·8 (81·5–143·6) μg/l; 2007: 101·2 (81·5–139·3) μg/l; 2008: 92·9 (78·4–120·3) μg/l; 2009: 96·9 (72·7–127·1) μg/l. RDA and estimated average requirement (EAR) were derived from the iodine intake recommendations of the Institute of Medicine (IoM)(28) for 9 year olds (according to the mean age of the children, Table 2). The recommended iodine intakes were corrected for non-urinary iodine losses, assuming an average 15 % loss through the faeces and sweat, to obtain the corresponding adapted 24-h urinary iodine excretion. This yielded the approximate excretion reference values of 102 (IoM RDA) and 62 μg iodine/d (IoM EAR), respectively.
In a second longitudinal regression model, the dietary iodine sources of milk, fish, meat and egg intake, and Na excretion as a marker of salt intake were included (Table 3). All were significant predictors of UIE (P < 0·05), except meat intake in the second time period (2007–9: P = 0·58). The contributions of Na excretion and fish intake to UIE decreased substantially from the first to the second time period (time-stratified models), as indicated by lower regression coefficients. However, inclusion of Na-by-time or fish-by-time interactions in the basic longitudinal regression model could not confirm significance for these changes (Na × time, P = 0·3, fish × time, P = 0·2; here, time was treated as categorical variable, categories: 2004–6 and 2007–9).
Table 3 Trend and predictors of 24-h urinary iodine excretion (μg/d) in 278 children (6 to 12 years old) participating in the Dortmund Nutritional and Anthropometric Longitudinally Designed Study with urine samples collected repeatedly between 2004 and 2009 (707 urine samples in total)*
(β-Coefficients with their standard errors)
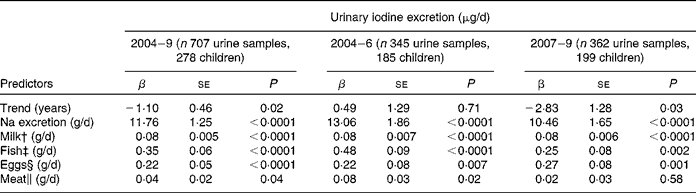
* Results of the linear mixed-effects regression model (PROC MIXED in SAS); adjusted for sex, age, creatinine excretion (mmol/d) and urine volume (l/d).
† Only whey-based milk products.
‡ Only salt-water fish products.
§ Eggs and egg products.
‖ Meat and meat products.
The quantitatively most important iodine sources in the children's diet were salt and milk (salt: 48 %, milk: 38 %; Fig. 2).
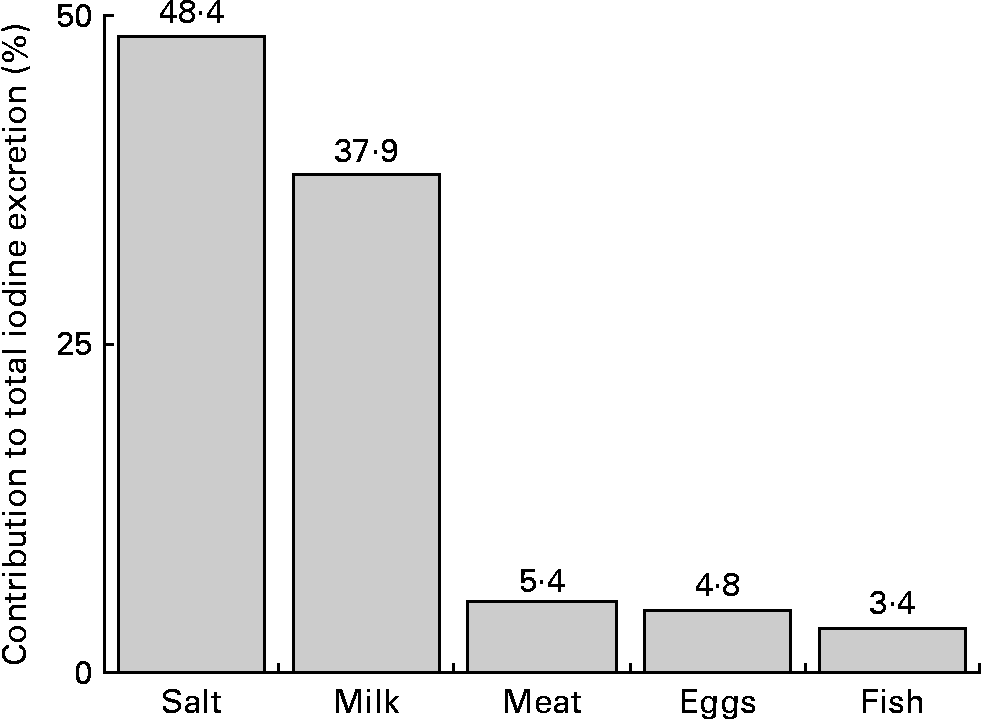
Fig. 2 Percentage contribution of the investigated food groups to estimated urinary iodine excretion, calculated by median daily dietary intakes (g/d) and respective regression coefficients (μg/g) of the mixed linear regression model (2004–9; Table 3).
Discussion
In the last decades, the German population has experienced a clear improvement in their iodine status, indicated by constantly increasing 24-h UIE rates(Reference Remer, Fonteyn and Alexy8, Reference Thamm, Ellert and Thierfelder9, Reference Meng and Scriba29–Reference Gärtner, Manz and Grossklaus31). The present analysis of UIE trends in children from 2004 to 2009, however, suggests that this former increase did not continue after 2003. From 2007 onwards, it has rather reversed into a decline. In 2009, median UIE/d was about 5·1 μg (6 %) lower than in the plateau phase 2004–6. The parallel investigation of the children's dietary intakes suggested a decreased use of iodised salt as a proportion of total salt in processed foods to be one possible reason for the decreasing of UIE.
The WHO recommendation of a median urinary iodine concentration of >100 μg/l in a population(Reference Andersson, de Benoist and Darnton-Hill3) was not achieved after 2007 (for data, see legend of Fig. 1). As this reference marker can substantially be confounded by the hydration status of the population investigated(Reference Remer, Fonteyn and Alexy8), we compared the children's absolute 24-h UIE rates (μg/d) (that nearly reflect total daily iodine intakes(Reference Riggs32)) with the recommended dietary iodine intakes (Fig. 1). Depending on the selected reference value, the classification of iodine nutritional status of the children differed markedly. When compared to the RDA(28), the average daily nutrient intake that is estimated to meet the needs of 97·5 % of the healthy population, more than 50 % of the children did not reach the reference value. When applying the EAR(28), which is estimated to meet the requirements of half of the healthy individuals in a group, only about 15 % of the children can be categorised to have an inadequate iodine intake. As stated by the Institute of Medicine, the RDA – in contrast to the EAR – is an inappropriate approach to assess the proportion of nutrient inadequacy in a group(33). It leads to an overestimation of the true prevalence, given that, by definition, the RDA is the intake level that exceeds the requirement of a large proportion of individuals in the group(33). Especially, when drawing public health conclusions, it is important to consider the meaning and interpretation of the different reference values for the decision on which value is the most appropriate. According to our data, using the EAR as reference, only a moderate proportion of the examined children (about 15 %) are at risk of dietary iodine inadequacy. However, of concern is that the proportion of the population below the EAR is increasing, thus lending strong evidence that the iodine supply in the German population is deteriorating.
To identify possible dietary reasons for the declining iodine excretion rates, we studied the development of the respective regression coefficients (i.e. β of Na excretion, milk, fish, egg and meat intake) over the two time periods investigated (2004–6 v. 2007–9; Table 3). A decrease in the second time period existed for Na excretion and for fish intake; although these changes did not reach statistical significance. Regression coefficients describe the expected change of the outcome variable (here: UIE) for a one-unit increase in the respective covariate (here: Na excretion and food groups). Consequently, they directly reflect the iodine content of the investigated food groups. With respect to Na excretion, a decreased β-value indicates a decreased proportion of iodised salt consumed among total salt consumed. Iodine intake from iodised salt at the household level is very stable (the proportion of households using iodised salt was 79 % in 2004, and about 80 % in 2009(Reference Gärtner34), mandatory iodine content ranging from 15 to 25 μg/g salt in Germany(35)). Table salt amounts to only a small proportion of the total daily salt consumption, i.e. about 80 % of the total salt intake stems from processed foods(Reference Andersson, de Benoist and Darnton-Hill3). As we included only those children who regularly used iodised table salt at home, the observed decrease in the respective regression coefficient should be primarily attributable to changes in the use of iodised salt in processed foods. These results are in good agreement with actual statistics about the developments in the German salt industry: since 2004, the market shares pertaining to the use of iodised salt in industrial food production have decreased from 35 % in 2004(Reference Scriba, Heseker and Fischer7) to 29 % currently(18, Reference Gärtner34). Reasons for this may be low-price imports of non-iodised salt and non-iodised processed foods, price differences for conventional and iodised salt, and until a few years ago trade barriers at the level of the European Union for food industry(Reference Gärtner34). As salt is the main contributor to UIE (48 %; Fig. 2), our results suggest this development to be one possible reason for the declining UIE rates starting in 2007. However, because of the lack of statistical confirmation, these results should only be seen as a first hint that has to be verified by further analysis.
Besides a decreased use of iodised salt in the previous years, our analysis also indicates a lower iodine content of consumed fish (observable by a decreased regression coefficient of fish in the second time period) to be one possible reason for the decreasing UIE rates. This result is somewhat striking, as we could not find any changes in the consumed fish species in our children over time (data not shown; in both time periods the fish most consumed were coalfish, salmon and redfish). One possible explanation could be the worldwide rapid expansion of aquacultures for fish production(36), as first studies indicate a decreased iodine content of fish from aquaculture compared to wild fish(Reference Karl and Meyer37). However, due to the low fish consumption in Germany, its quantitative contribution to iodine supply is low compared to iodised salt (3 % v. 48 %, Fig. 2), and the observed decrease should not be very important for the development of iodine nutritional status.
The impact of milk on UIE remained constant over the investigated time period and was at a similar level as in 2000–3 (an ingestion of 100 g milk resulted in an iodine excretion of about 70(Reference Remer, Fonteyn and Alexy8) and 80 μg (Table 3), respectively). Therefore, the strong increase in the iodine content of milk from 1996 to 2003 did obviously not continue after 2003. Compared to other European countries, like the UK(Reference Wenlock, Buss and Moxon38), in Germany milk and milk products are of lower importance for iodine supply, because of the lower iodine concentrations (about 100–120 μg/l)(Reference Hampel, Kairies and Below39, 40).
Apart from the characterisation of iodine status, the data of our investigation can also be used to estimate the current amounts of salt intake in children. Na is excreted almost quantitatively in the urine and 24-h urine collections are deemed to be the ‘gold standard’ for determining the daily Na and salt intake in a population(Reference Dyer, Elliott and Chee41, Reference Vandevijvere, De Keyzer and Chapelle42). According to the upper level for Na intake given by the US Food and Nutrition Board of 1·9 g/d for 4–8-year-olds and 2·2 g/d for 9–13-year-olds(43), the observed median Na excretion in our population of 1·7–1·8 g/d (which equals a total salt intake of about 4·5 g/d) indicates that nearly 50 % of the children met this upper level and a substantial proportion even exceeded it. As our DONALD Study population in general shows a rather higher socio-economic status, compared to the German population(Reference Kroke, Manz and Kersting44), it can be assumed that the actual average amounts of salt intake in German children are somewhat higher. Against the background of possible programming effects of an inappropriate salt intake in childhood with respect to blood pressure elevations in later life(Reference Cutler and Roccella45), these data indicate a need for future public health measures in children.
The strength of the present investigation is the availability of 24-h urine collections. It enabled us to assess the prevalence of children who are at risk of dietary iodine inadequacy more precisely than the use of the urinary iodine concentration of 100 μg/l as the reference median for a population. Also for the comparison of iodine status between subgroups, the use of 24-h UIE seems more suitable. Seeming sex differences in iodine status, indicated by significantly lower iodine concentrations in girls (Table 1), disappeared when relating 24-h UIE to energy intake. The sex differences in iodine concentration can be explained by the frequently more favourable beverage intake of German girls compared to boys(Reference Ebner and Manz46). The lower 24-h UIE in girls is physiologically based on their lower total food and energy intake on average, which can be accounted for by considering individual energy intake. Accordingly, the collection of 24-h urine samples allowed us to perform a much more sensitive and hydration status-independent investigation of the children's iodine status.
A limitation of our study was that not all of the children had repeated collections of 24-h urine samples and dietary records. This, for example, impedes the estimation of customary salt intake from 24-h Na excretion. However, the calculated regression coefficients that estimate the contribution of salt intake to iodine supply and allowed thus to assess the proportion of iodised to total salt consumption (and not the amount consumed) should not be relevantly biased. A further limitation of our study is the elaborate design of the DONALD Study that results in a select population which is not representative with respect to socio-economic status(Reference Kroke, Manz and Kersting44). Nevertheless, measured UIE rates in 2004–6 were in the same range as the results of the nationwide representative KiGGS Study in 2003–6 (KiGGS median urinary iodine concentration (μg/l) of boys: 127·0 (7–10 years), 123·2 (11–13 years); girls: 115·6 (7–10 years), 104·1 (11–13 years)(Reference Thamm, Ellert and Thierfelder9, Reference Thamm, Karaolis-Dankert and Kroke47); DONALD (6–12 years) boys: 120·1, girls: 99·2) and therefore underline the nationwide relevance of our results. The distinct advantage of the DONALD Study is the simultaneous collection of repeated 24-h urine samples and weighed dietary records. The combination of 24-h urinary iodine measurements with dietary data provides a sensitive monitoring instrument for the characterisation of iodine status, its most important dietary determinants, and the underlying changes and time trends.
In conclusion, the iodine status of German schoolchildren, only recently classified as almost adequate, apparently began to deteriorate from 2007. A decreased use of iodised salt in industrially produced foods may be one possible reason for the declining trend. Since it is known that in children in particular, even a mild iodine deficiency leads to an increased risk of developing mental impairment(Reference Gordon, Rose and Skeaff10, Reference Santiago-Fernandez, Torres-Barahona and Muela-Martinez12), our data emphasise the need for promoting the use of iodised salt, especially in processed foods (currently, only about 26 % of industrially used salt is iodised in Germany). Iodised salt added to processed foods has been shown to be a suitable vehicle for iodine fortification as it has emerged to be highly stable, especially in cereal products where the observed retention of iodine was 100 %(Reference Thomson48). The examination of the stability of iodised salt added to meat products revealed iodine losses of 7–25 % after storage and cooking(Reference Wirth and Kühne49). Against the background of the recommendations for reduction in salt intake as a public health measure worldwide, an increment of the iodine concentrations in salt should be considered. The present investigation can be seen as an initial health warning for the probable future development of iodine status in populations consuming relevant amounts of processed foods.
Acknowledgements
The present examination was supported in part by the Federal Ministry of Food, Agriculture and Consumer Protection (BMELV) through the Federal Agency for Agriculture and Food (BLE), grant no. 07HS002. The DONALD Study is funded by the Ministry of Science and Research of North Rhine Westphalia, Germany. The authors are very grateful to the staff of the Research Institute of Child Nutrition for carrying out the anthropometric measurements, and for collecting and coding the dietary records. In particular, the authors thank Monika Friedrich and Brigitte Nestler for expert laboratory assistance. None of the authors has any conflict of interest in regard to the present study. The contributions of the authors to this study are as follows: T. R. designed the research; S. A. J. and A. L. B. G. performed the statistical analysis; S. A. J. wrote the paper; S. A. J. and T. R. had primary responsibility for the final content. All authors made significant contributions to and approved the final manuscript.
The paper is dedicated to Professor Friedrich Manz, the former director of the Research Institute of Child Nutrition, on the occasion of his 70th birthday.







