Introduction
It is generally believed that fine grinding enables digestive secretions better access to substrates, improving digestion and absorption and subsequently increasing the growth efficiency (Behnke, Reference BEHNKE2001). However, it is increasingly recognised that the broilers may have a requirement for a certain degree of physical structure in their feed to meet their innate feeding behaviour (Ferket and Gernat, Reference FERKET and GERNAT2006). In recent years, the use of dietary structural components, such as coarse particles and whole grains in poultry diets, have attracted considerable attention due to their effects on the development and functionality of the gizzard (Svihus et al., Reference SVIHUS, HETLAND, CHOCT and SUNDBY2002). The beneficial effects of such practices may extend to a favourable influence on intestinal morphology and functionality (Nir et al., Reference NIR, HILLEL, SHEFET and NITSAN1994a; Amerah et al., Reference AMERAH, RAVINDRAN and LENTLE2007a; Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b) and microbiota profile (Engberg et al., Reference ENGBERG, HEDEMANN, STEENFELDT and JENSEN2004). However, published data on the effects of particle size on the gastrointestinal tract (GIT) parameters of broilers have been contradictory due largely to the confounding effects of feed physical form (mash vs. pelleted diets), with the impact of particle size being more pronounced in mash feeds than in pelleted or crumbled feeds (Svihus et al., Reference SVIHUS, JUVIK, HETLAND and KROGDAHL2004a; Péron et al., Reference PÉRON, BASTIANELLI, OURY, GOMEZ and CARRÉ2005; Amerah et al., Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b). The particle size effect is exacerbated during pelleting, which further reduces the feed particle size and may equalise the differences in particle size distribution (Svihus et al., Reference SVIHUS, KLØVSTAD, PEREZ, ZIMONJA, SAHLSTROM, SCHULLER, JEKSRUD and PRESTLØKKEN2004b; Amerah et al., Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b; Abdollahi et al., Reference ABDOLLAHI, RAVINDRAN, WESTER, RAVINDRAN and THOMAS2011). It has been reported that in birds fed pelleted diets, consisting of finely ground dietary ingredients, the upper regions of the gut may no longer be functional in the terms of mixing, grinding and may cause reverse peristalsis contractions (Cumming, Reference CUMMING1994; Duke, Reference DUKE1994).
Several extensive reviews are available on the influence of particle size and feed form on the nutrient utilisation, performance and development and health of GIT of broilers (Amerah et al., Reference AMERAH, RAVINDRAN and LENTLE2007a; Svihus, Reference SVIHUS2011; Abdollahi et al., Reference ABDOLLAHI, RAVINDRAN and SVIHUS2013b). The current review focuses on the physiological consequences of particle size and pelleting on GIT parameters, intestinal morphology and microbiota profile.
Effect of feed particle size on GIT development
Particle size has been reported to influence nutrient utilisation, growth performance, gizzard development and feed passage rate in broilers (Gabriel et al., Reference GABRIEL, MALLET and LECONTE2003a; Reference GABRIEL, MALLET, LECONTE, FORT and NACIRI2003b; Amerah et al., Reference AMERAH, RAVINDRAN and LENTLE2007a, Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2008a; Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2008b). The effect of particle size has been studied in wheat (Gabriel et al., Reference GABRIEL, MALLET, LECONTE, FORT and NACIRI2006; Amerah et al., Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2008a; Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2008b), maize (Zang et al., Reference ZANG, PIAO, HUANG, WANG, MA and MA2009; Jacobs et al., Reference JACOBS, UTTERBACK and PARSONS2010; Pacheco et al., Reference PACHECO, STARK, FERKET and BRAKE2013; Xu et al., Reference XU, STARK, FERKET, WILLIAMS, AUTTAWONG and BRAKE2015a; Reference XU, STARK, FERKET, WILLIAMS, NUSAIRAT and BRAKE2015b; Reference XU, STARK, FERKET, WILLIAMS and BRAKE2015c) and soybean meal (Pacheco et al., Reference PACHECO, STARK, FERKET and BRAKE2013).
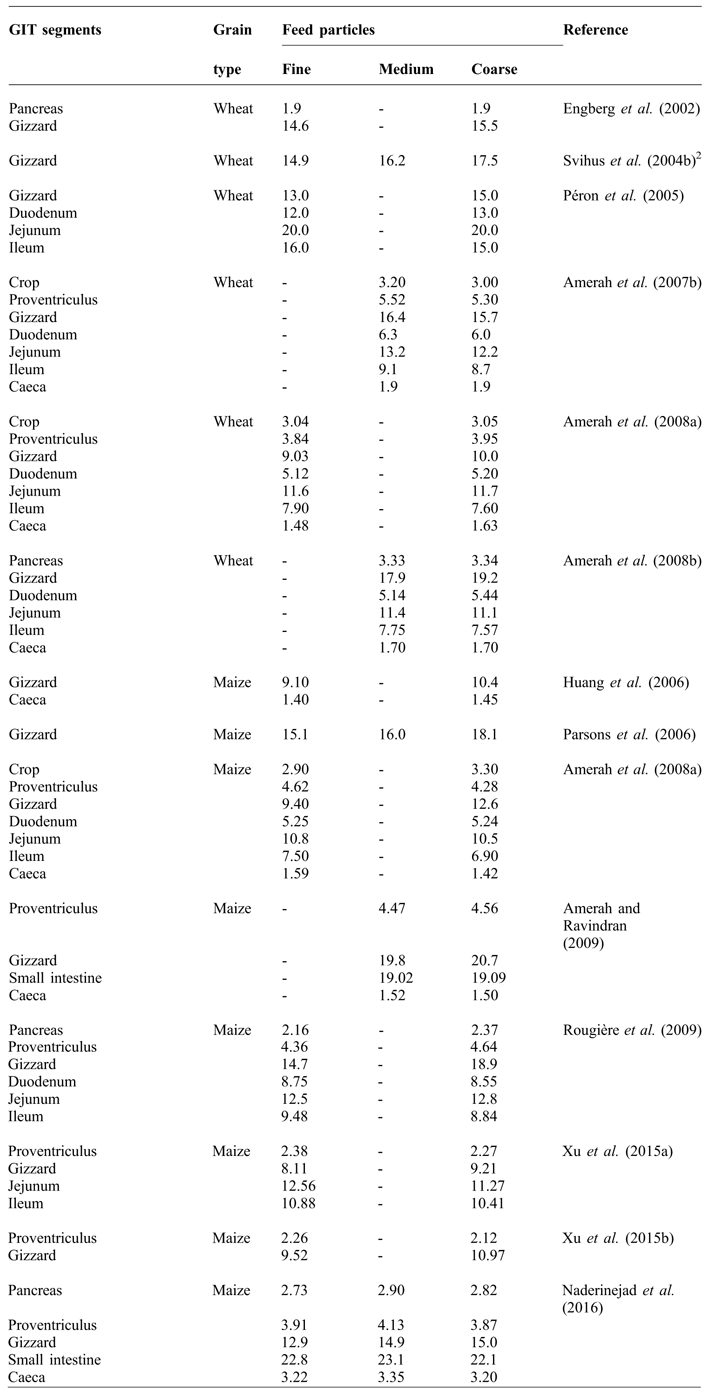
The influence of feed particle size on gizzard development is well documented (Table 1). The gizzard has a remarkable ability to grind the feed to a consistent particle size (Hetland et al., Reference HETLAND, CHOCT and SVIHUS2004). Early work of O'Dell et al. (Reference O'DELL, NEWBERNE and SAVAGE1959) showed that birds fed purified diets containing fine particles had an enlarged proventriculus and smaller gizzards compared to those containing large particles. It was found that the diets with fine particles moved through the GIT more rapidly (135 vs. 165 minutes) and the proventriculus of birds fed fine diets was filled with fluid rather than with feed. The birds were observed to drink more water, probably to aid in the swallowing and there was a tendency toward a pendulous crop.
Table 1 Influence of feed particle size on the relative weight (g/kg body weight) of GIT1 segments in broilers.

1 GIT, Gastrointestinal tract
2 Roller mill grinding
Moore (Reference MOORE1999) reported that, in geese, the folds of pylorus and its small opening play a sieving role which does not allow very large particle to pass through during contractions. Hetland et al. (Reference HETLAND, SVIHUS and OLAISEN2002) similarly found in broilers that, regardless of the original feed structure, the majority of particles were smaller than 0.04 mm in size when the digesta passed beyond the gizzard. According to Nir and Ptichi (Reference NIR and PTICHI2001), the relative gizzard weight was positively correlated to feed particle size when the diets were in mash form. In birds fed mash diets with coarsely ground particles, digesta were retained for longer in the gizzard along with greater gizzard development (Nir et al., Reference NIR, HILLEL, SHEFET and NITSAN1994a; Hetland and Svihus, Reference HETLAND and SVIHUS2001; Engberg et al., Reference ENGBERG, HEDEMANN and JENSEN2002). Nir et al. (Reference NIR, HILLEL, SHEFET and NITSAN1994a) reported that when day-old chicks were fed medium and coarse maize particles, gizzard weight increased by 26 and 41%, respectively, compared to those fed fine particles. Kilburn and Edwards (Reference KILBURN and EDWARDS2004) stated that larger particles were retained longer, allowing more time for nutrient digestion and absorption. These benefits were reduced when the diet was fed in pelleted or crumbled form, presumably as a result of concomitant degradation of larger particles during the pelleting process. Charbeneau and Roberson (Reference CHARBENEAU and ROBERSON2004) and Jacobs et al. (Reference JACOBS, UTTERBACK and PARSONS2010) similarly reported an increase in gizzard weight as particle size of dietary maize increased. Pacheco et al. (Reference PACHECO, STARK, FERKET and BRAKE2013) evaluated the effect of soybean meal and maize particle sizes on gizzard weight and found that the maize particle size had a greater influence on gizzard size than did soybean meal particle size. Xu et al. (Reference XU, STARK, FERKET, WILLIAMS, NUSAIRAT and BRAKE2015b) found that relative proventriculus weight was decreased and the relative gizzard weight was increased at 49 days of age by the inclusion of 500 g/kg coarse maize. It was suggested that this relative relationship between gizzard and proventriculus weight may be due to the fact that broilers may have adjusted their mechanical and enzymatic digestive functions according to the physical structure of feed. Increased gizzard weight due to increased maize particle size is a logical consequence of enhanced mechanical grinding activity (Dahlke et al., Reference DAHLKE, RIBEIRO, KESSLER, LIMA and MAIORKA2003; Parsons et al., Reference PARSONS, BUCHANAN, BLEMINGS, WILSON and MORITZ2006). Naderinejad et al. (Reference NADERINEJAD, ZAEFARIAN, ABDOLLAHI, HASSANABADI, KERMANSHAHI and RAVINDRAN2015) reported that, regardless of feed form, medium and coarse grinding of maize increased the gizzard weight compared to fine grinding. In contrast, Xu et al. (Reference XU, STARK, FERKET, WILLIAMS and BRAKE2015c) reported that relative gizzard weight was not affected by percentage of coarse maize (up to 500 g/kg) in crumble diet, while it increased in a linear manner with increasing of coarse maize inclusion in the mash diet.
Nir et al. (Reference NIR, HILLEL, PTICHI and SHEFET1995) suggested that coarser particles are better suited for poultry because of their stimulating effect on the gizzard size and gut motility. The stimulation of gut motility is an important effect of coarse particles (Sacranie, Reference SACRANIE2006) and has been hypothesised to improve intestinal strength due to greater muscular activity related to reverse peristalsis (Xu et al., Reference XU, STARK, FERKET, WILLIAMS, AUTTAWONG and BRAKE2015a).
Higher grinding activity in the gizzard increases the size of the gizzard muscles, which are myelinated and have a koilin layer (Preston et al., Reference PRESTON, MCCRACKEN and MCALLISTER2000; Svihus, Reference SVIHUS2011; Reference SVIHUS2014). Generally, a large and well-developed gizzard is associated with its musculature and increased grinding activity (Nir et al., Reference NIR, HILLEL, SHEFET and NITSAN1994a; Reference NIR, TWINA, GROSSMAN and NITSAN1994b; Amerah et al., Reference AMERAH, RAVINDRAN and LENTLE2007a; Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b; Svihus, Reference SVIHUS2011), increased pancreatic enzyme secretion through increased release of cholecystokinin (Svihus, Reference SVIHUS2011) and improved GIT motility (Ferket, Reference FERKET2000; González-Alvarado et al., Reference GONZÁLEZ-ALVARADO, JIMENEZ-MORENO, VALENCIA, LAZARO and MATEOS2008), with the overall result of improved nutrient utilisation. Garcia et al. (Reference GARCIA, GOMEZ, MIGNON-GRASTEAU, SELLIER and CARRÉ2007) reported that the increase in gizzard/intestine weight ratio was accompanied by an increase in apparent metabolisable energy (AME) values. They suggested that this ratio could be an efficient mean for predicting variations in nitrogen-corrected AME responses observed with xylanase.
Three distinct sites of reverse peristalsis can be observed in the GIT of birds (Duke, Reference DUKE1982): i) gastric reflux transferring the digesta from gizzard to proventriculus via gastro-duodenal contractions (two to four cycles per min), ii) the small intestinal reflux, that transfers digesta from the duodenum and jejunum into the gastric area (about four times per hour) and increases the digesta retention time, iii) cloaca-caecal reflux, which transfers urinary nitrogen to the caeca via the colon particularly when fed low-protein diets (Karasawa, Reference KARASAWA1999). A well-developed gizzard generates stronger reverse peristalsis contractions and increases proteolysis by pepsin, trypsin and other endogenous proteases in the small intestine (Ferket, Reference FERKET2000). Gabriel et al. (Reference GABRIEL, MALLET and LECONTE2003a) reported that large feed particles enhanced pepsin activity in the proventriculus.
When broilers were fed coarse structured feeds, in comparison to finely ground feeds, lower pH was recorded in the gizzard (Nir et al., Reference NIR, HILLEL, SHEFET and NITSAN1994a; Engberg et al., Reference ENGBERG, HEDEMANN and JENSEN2002; Jiménez-Moreno et al., Reference JIMÉNEZ-MORENO, FRIKHA, DE COCA-SINOVA, LÁZARO and and MATEOS2013) and proventriculus (Nir et al., Reference NIR, HILLEL, PTICHI and SHEFET1995). Jiménez-Moreno et al. (Reference JIMÉNEZ-MORENO, GONZÁLEZ-ALVARADO, DE COCA-SINOVA, LÁZARO and MATEOS2009; Reference JIMÉNEZ-MORENO, FRIKHA, DE COCA-SINOVA, LÁZARO and and MATEOS2013) and González-Alvarado et al. (Reference GONZÁLEZ-ALVARADO, JIMENEZ-MORENO, VALENCIA, LAZARO and MATEOS2008) found that inclusion of structural components, such as oat hulls and sugar beet pulp, in mash diets reduced the pH of the gizzard. The same result was reported by Sacranie et al. (Reference SACRANIE, SVIHUS, DENSTADLI, MOEN, IJI and CHOCT2012) for a mixture of oats and barley hulls. Higher HCl production as a result of longer retention time of digesta in the gizzard might be the reason for the reduced gizzard pH. However, Naderinejad et al. (Reference NADERINEJAD, ZAEFARIAN, ABDOLLAHI, HASSANABADI, KERMANSHAHI and RAVINDRAN2015) reported that gizzard pH was responsive to particle size only in pelleted maize diets. The lack of gizzard pH response to particle size in mash diets is in agreement with the findings by Charbeneau and Roberson (Reference CHARBENEAU and ROBERSON2004), Jacobs et al. (Reference JACOBS, UTTERBACK and PARSONS2010) and Singh et al. (Reference SINGH, RAVINDRAN, WESTER, MOLAN and RAVINDRAN2014). Naderinejad et al. (Reference NADERINEJAD, ZAEFARIAN, ABDOLLAHI, HASSANABADI, KERMANSHAHI and RAVINDRAN2016) found that, although pelleting reduced the proportion of coarse particles, it seemed that a minimum of 40 to 60 g/kg coarse particles of >2 mm was sufficient to stimulate secretion of hydrochloric acid and reduce the gizzard pH to the same level as mash-fed birds.
Feed particle size has also been found to influence GIT segments other than the gizzard, but the results are contradictory. Nir et al. (Reference NIR, HILLEL, SHEFET and NITSAN1994a) reported that, in addition to the gizzard, coarse maize diets increased the relative weights of jejunum, ileum and whole small intestine. Nir et al. (Reference NIR, TWINA, GROSSMAN and NITSAN1994b) reported that birds fed sorghum-soybean meal mash diets showed hypertrophy of the small intestine compared to pellet diets. In subsequent study, Nir et al. (Reference NIR, HILLEL, PTICHI and SHEFET1995) found no differences in the weight of intestinal segments with maize particle sizes increasing from 0.6 to 2.17 mm. However, Amerah et al. (Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b) reported a reduction in the relative length of all GIT components as wheat particle size increased. A decreased intestinal weight or length may result in improved feed efficiency due to reduced maintenance costs (Xu et al., Reference XU, STARK, FERKET, WILLIAMS, AUTTAWONG and BRAKE2015a).
Pelleting has been shown in a number of studies to even out the differences in particle size (Svihus et al., Reference SVIHUS, KLØVSTAD, PEREZ, ZIMONJA, SAHLSTROM, SCHULLER, JEKSRUD and PRESTLØKKEN2004b; Péron et al., Reference PÉRON, BASTIANELLI, OURY, GOMEZ and CARRÉ2005; Amerah et al., Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b; Abdollahi et al., Reference ABDOLLAHI, RAVINDRAN, WESTER, RAVINDRAN and THOMAS2011), but the beneficial influence of coarse particles might still exist after pelleting.
The study by Nir et al. (Reference NIR, HILLEL, PTICHI and SHEFET1995) showed that the effect of grain particle size was preserved even after pelleting. Naderinejad et al. (Reference NADERINEJAD, ZAEFARIAN, ABDOLLAHI, HASSANABADI, KERMANSHAHI and RAVINDRAN2016) reported that coarse grinding of maize, through enhanced gizzard development and functionality, was beneficial to nutrient and energy utilisation and growth performance in broilers fed pelleted diets. Amerah et al. (Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b) concluded that wheat particle size was more critical in mash than in pelleted or crumble diets. Péron et al. (Reference PÉRON, BASTIANELLI, OURY, GOMEZ and CARRÉ2005) found that pelleting coarse particles with very hard wheat-based diets increased gizzard weights compared to those with fine wheat, which was attributed to the resistance of hard particles to size reduction during the pelleting process. It would appear that the effect of grain particle size in pelleted diets on gizzard development depends on the cereal base used and grain hardness. Moreover, technical parameters of pelleting process, such as pellet diameter and the gap between the rollers and pellet die, may potentially influence the final particle size. A large pellet diameter and an increased gap between the rollers and the die will reduce the grinding of particles in the pellet press.
Effect of feed particle size on gut morphology and microbiota profile
There have been limited reports on the influence of feed particle size on intestinal morphology and the results are inconsistent. Nir et al. (Reference NIR, TWINA, GROSSMAN and NITSAN1994b; Reference NIR, HILLEL, PTICHI and SHEFET1995) reported that the duodenal villus height increased linearly as the dietary particle size increased. However, Amerah et al. (Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b) reported that villus height, crypt depth, and epithelial thickness in the duodenum were unaffected by maize particle size. Their results were confirmed by Zang et al. (Reference ZANG, PIAO, HUANG, WANG, MA and MA2009). However, Liu et al. (Reference LIU, PIAO, OU, CAO, HUANG and LI2006) reported that coarse maize inclusion reduced the number of mast cells in the duodenum, jejunum, and ileum as compared with finely ground maize. Xu et al. (Reference XU, STARK, FERKET, WILLIAMS, AUTTAWONG and BRAKE2015a) reported that 500 g/kg dietary inclusion of coarse maize increased the jejunal tip width and villi surface area, but decreased the thickness of muscularis layer. This finding was considered as a general response of the digestive and absorptive capacity of the proximal small intestine to greater digesta retention time to facilitate greater contact between the nutrients and villi.
A developed gizzard can be regarded as a barrier in preventing pathogenic bacteria from entering the distal GIT (Engberg et al., Reference ENGBERG, HEDEMANN, STEENFELDT and JENSEN2004). However, data on the effects of feed particle size on the intestinal microbiota profile are scanty. Harmful bacteria entering the GIT via the feed have a greater chance of being suppressed in a highly acidic environment. Engberg et al. (Reference ENGBERG, HEDEMANN and JENSEN2002) stated that an increase in Lactobacilli spp. populations is usually considered to be beneficial to the host because they can prevent colonisation of pathogens such as E. coli. These researchers compared coarse or finely ground mash or pelleted feed and reported that there was an increase in Lactobacilli spp. populations in the caeca when birds were given coarse mash diets, with the lowest counts of lactic acid bacteria being recorded in those given finely ground pelleted diets. Jacobs et al. (Reference JACOBS, UTTERBACK and PARSONS2010) observed an increase in Lactobacilli spp. and a decrease in Bifidobacteria spp. when birds were fed large maize particles (1.4 mm) compared to those fed fine maize particles (0.6 mm).
Singh et al. (Reference SINGH, RAVINDRAN, WESTER, MOLAN and RAVINDRAN2014) fed broilers diets with graded levels of coarse maize (0, 150, 300, 450, 600 g/kg) in mash diets and found that the counts of Lactobacillus spp. and Bifidobacteria spp. increased and those of Clostridium spp., Campylobacter spp., and Bacteroides spp. decreased with increasing inclusion levels of coarse maize. In birds fed fine particles, the feed is less exposed to low pH and proteases in the gizzard, and ingested feed appears more quickly in the duodenum (Hill, Reference HILL1971). The presence of such undigested material in the upper small intestine may result in aberrant bacterial populations such as Clostridium perfringens, the pathogenic agent of necrotic enteritis, or E. coli, as suggested by Cumming (Reference CUMMING1994).
The observed particle size effects on gut microbiota profiles may be explained by one or both of the following mechanisms: First, stimulation of gizzard development and increased secretion of hydrochloric acid, reduces the pH and subsequently has an antimicrobial effect on pathogenic bacteria entering the distal part of GIT (Engberg et al., Reference ENGBERG, HEDEMANN and JENSEN2002). Second, competitive exclusion may be promoted by encouraging colonisation of commensal bacteria and thus discouraging colonisation of harmful bacteria (Bjerrum et al., Reference BJERRUM, PEDERSEN and ENGBERG2005; Santos et al., Reference SANTOS, SHELDON, SANTOS and FERKET2008).
Effect of feed form on GIT development
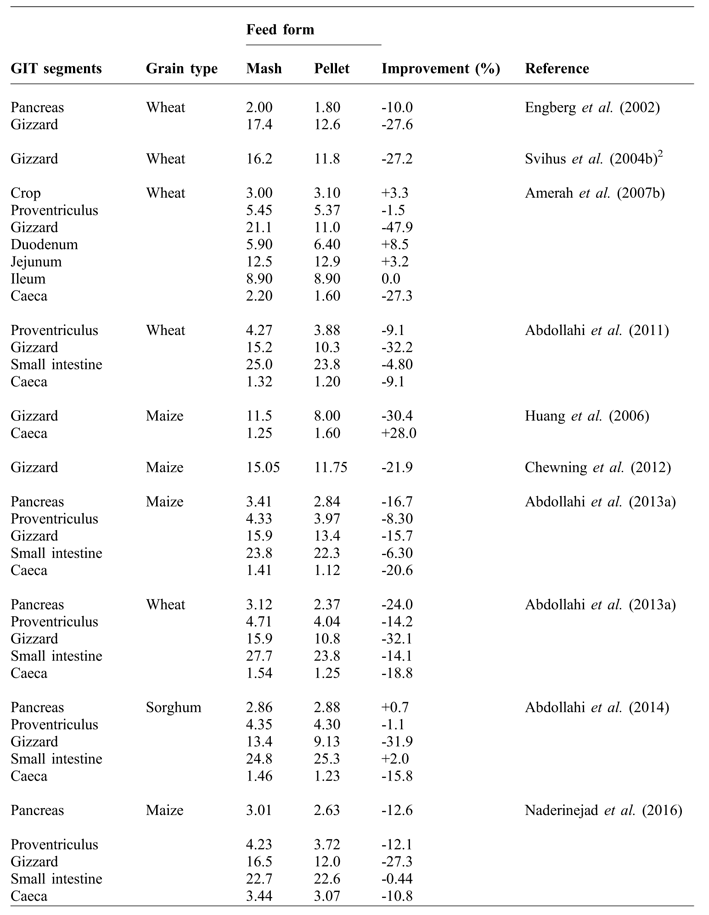
Although improved broiler performance is an advantage for pellet vs. mash feeding, there are negative physiological consequences on the development of GIT (Nir et al., Reference NIR, HILLEL, SHEFET and NITSAN1994a; Engberg et al., Reference ENGBERG, HEDEMANN and JENSEN2002; Amerah et al., Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b; Abdollahi et al., Reference ABDOLLAHI, RAVINDRAN, WESTER, RAVINDRAN and THOMAS2011). The birds do not fully develop their upper GIT when highly processed pelleted feeds are used. Nir et al. (Reference NIR, HILLEL, PTICHI and SHEFET1995) reported that pelleting reduced the proventriculus weight and its contents at 21 d of age, but not during the finisher period (40 d of age). Abdollahi et al. (Reference ABDOLLAHI, RAVINDRAN, WESTER, RAVINDRAN and THOMAS2011) observed lower relative weights of the proventriculus in pellet-fed birds than those fed mash diets. However, Mirghelenj and Golian (Reference MIRGHELENJ and GOLIAN2009) found that the relative weights of crop and proventriculus were unaffected by feed form.
The relative weight of the gizzard has been shown to decrease when birds are fed pelleted rather than mash diets (Table 2). The reduction in the size of gizzard, with pellet feeding, is a logical response to reduced grinding activity as a result of finer particle size caused by the pelleting process (Engberg et al., Reference ENGBERG, HEDEMANN and JENSEN2002; Svihus et al., Reference SVIHUS, JUVIK, HETLAND and KROGDAHL2004a; Péron et al., Reference PÉRON, BASTIANELLI, OURY, GOMEZ and CARRÉ2005; Abdollahi et al., Reference ABDOLLAHI, RAVINDRAN, WESTER, RAVINDRAN and THOMAS2011). Pelleting-induced particle size reduction has been suggested as a result of large particles being prone to grinding due to the narrow gap between pellet rollers and the pellet die (Svihus et al., Reference SVIHUS, KLØVSTAD, PEREZ, ZIMONJA, SAHLSTROM, SCHULLER, JEKSRUD and PRESTLØKKEN2004b), and frictional force inside the die itself (Abdollahi et al., Reference ABDOLLAHI, RAVINDRAN, WESTER, RAVINDRAN and THOMAS2011). Engberg et al. (Reference ENGBERG, HEDEMANN and JENSEN2002) reported that pelleting considerably reduced feed particle size and equalised the differences between coarsely and finely ground meals in wheat-based diets. In their study, due to pelleting, the fraction of feed particles with a size of over 1.0 mm reduced from 262 to 149 g/kg in the coarsely ground diet, and from 209 to 135 g/kg in the finely ground diet. In agreement, Abdollahi et al. (Reference ABDOLLAHI, RAVINDRAN, WESTER, RAVINDRAN and THOMAS2011) showed that the passage of diets through the pellet die reduced the proportion of coarse particles over 2.0 mm and increased the proportion of fine particles less than 0.075 mm.
Table 2 Influence of feed form on the relative weight (g/kg body weight) of GIT1 segments in broilers.

1 GIT, Gastrointestinal tract
2 Roller mill grinding
There appears to be an association between gizzard and pancreatic weights. Liu et al. (Reference LIU, TRUONG and SELLE2015) reported a positive correlation between relative weight of gizzard and pancreas. Engberg et al. (Reference ENGBERG, HEDEMANN and JENSEN2002) showed that birds fed pelleted diets had lower pancreas weights and pancreatic enzyme (amylase, lipase, and chymotrypsin) activities than those fed mash diets. This finding is consistent with the results of Agah and Norollahi (Reference AGAH and NOROLLAHI2008) showing a lower relative pancreas weight in broilers fed pelleted diets compared to those fed mash diets.
Published data on the effects of pelleting on the relative weight and length of small intestine are summarised in Table 2. Nir et al. (Reference NIR, TWINA, GROSSMAN and NITSAN1994b) stated that with increasing pelleting percent, relative weight of gizzard, as well as length of jejunum and ileum decreased. Pelleting levels in their study were: 0 (mash), 0.5 (mixture of mash and soft pellets (pelleted once)), 1 (soft pellets), 1.5 (mixture of soft pellets and hard pellets (pelleted twice)) and 2 (hard pellets). Amerah et al. (Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b) and Abdollahi et al. (Reference ABDOLLAHI, RAVINDRAN, WESTER, RAVINDRAN and THOMAS2011; Reference ABDOLLAHI, RAVINDRAN and SVIHUS2013a) found that relative empty weight of intestinal segments were greater in birds fed mash diets than those fed pelleted diets. Mirghelenj and Golian (Reference MIRGHELENJ and GOLIAN2009) showed that pelleting reduced the relative length of caeca. The same result was reported by Abdollahi et al. (Reference ABDOLLAHI, RAVINDRAN, WESTER, RAVINDRAN and THOMAS2011), who found a heavier caeca weight in mash-fed compared to pellet-fed birds. A lower weight of caeca may cause a higher amount of water being excreted relative to feed intake (Maisonnier et al., Reference MAISONNIER, GOMEZ and CARRÉ2001). Svihus et al. (Reference SVIHUS, CHOCT and CLASSEN2013) stated that feeding pelleted wheat-based diets increased excreta moisture. As recycling and reabsorption of water occurs largely in the caeca and colon, the poor caecal development in birds fed pelleted diets has implications for water loss and conservation.
The pH along the GIT of birds varies widely. The crop is moderately acidic (5.5), the proventriculus and gizzard are acidic (2.5-3.5) and the intestine is moderately neutral to moderately alkaline (5.0-7.5). It has been shown that gizzard pH is relatively higher with pelleted diets when compared to mash diets (Engberg et al., Reference ENGBERG, HEDEMANN and JENSEN2002; Huang et al., Reference HUANG, LI, XING, MA, LI and LV2006; Frikha et al., Reference FRIKHA, SAFAA, SERRANO, ARBE and MATEOS2009: Naderinejad et al., Reference NADERINEJAD, ZAEFARIAN, ABDOLLAHI, HASSANABADI, KERMANSHAHI and RAVINDRAN2016). Recently, Liu et al. (Reference LIU, TRUONG and SELLE2015) reported a negative correlation (r=-0.45) between the relative gizzard weight and gizzard pH and suggested that the consequence of heavier gizzard weight goes beyond grinding activity.
The upper GIT (crop, proventriculus and gizzard) are main sites of action of exogenous enzymes (Selle and Ravindran, Reference SELLE and RAVINDRAN2007). The average digesta retention time in the GIT of birds is short (135 to 280 minutes). As a result, digesta possibly spends only 60 to 90 min in the upper GIT, which gives only limited opportunity for enzyme action. As pelleting reduces gizzard development, the time feed spends in the upper GIT decreases even more and this can be limiting factor for exogenous enzyme efficiency in pelleted diets. According to Abdollahi et al. (Reference ABDOLLAHI, RAVINDRAN and SVIHUS2013a), considering the increased feed intake (up to 27%) and decreased gizzard size (up to 32%) in pellet-fed birds, the retention time per unit of feed becomes even shorter compared to mash-fed birds. The shorter digesta retention time and an elevated gizzard pH, due to an under-developed gizzard, are possible physiological limits to optimal digestion in pellet-fed birds (Ravindran, Reference RAVINDRAN2013).
Amerah et al. (Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b) observed that the pelleting process reduced the size of coarse particles and minimised the differences between the distribution of medium and coarse particles. However, in their study, pelleted diets with coarse particles showed heavier proventriculus and gizzard weights (by 4.9 and 5.6%, respectively) compared to pelleted diets with medium particle. Increased gizzard weight with medium and coarse grinding of maize in pelleted diets has also been reported by Dahlke et al. (Reference DAHLKE, RIBEIRO, KESSLER, LIMA and MAIORKA2003) and Naderinejad et al. (Reference NADERINEJAD, ZAEFARIAN, ABDOLLAHI, HASSANABADI, KERMANSHAHI and RAVINDRAN2016). As broiler diets are usually pelleted, it would appear that coarse grinding could be beneficial in terms of upper GIT development, depending on the cereal type and grain hardness.
Overall, it is evident that the poor gizzard development in pellet-fed birds is due mainly to the lack of mechanical stimulation by the feed. Pelleting decreases the grinding requirement of the gizzard so that its function is reduced to that of a transit organ (Amerah et al., Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b). Pellets disintegrate in the crop and pass directly through the proventriculus and gizzard to enter the duodenum. This explains the higher digesta dry matter content observed in the upper small intestine (duodenum) of pellet-fed birds (Engberg et al., Reference ENGBERG, HEDEMANN and JENSEN2002). A coarse mash diet stays longer in the gizzard, thus increasing the mechanical stimulation of this organ (Hetland and Svihus, Reference HETLAND and SVIHUS2001) and is extensively ground. Increased retention time in the gizzard may increase nutrient digestibility through the provision of more time for the secretion of hydrochloric acid (González-Alvarado et al., Reference GONZÁLEZ-ALVARADO, JIMENEZ-MORENO, VALENCIA, LAZARO and MATEOS2008; Svihus, Reference SVIHUS2011) and pepsin (Svihus, Reference SVIHUS2011), and by increasing gastric refluxes that serve to re-expose the digesta to pepsin (González-Alvarado et al., Reference GONZÁLEZ-ALVARADO, JIMENEZ-MORENO, VALENCIA, LAZARO and MATEOS2008). Longer retention time may potentially improve the efficacy of exogenous enzymes (Svihus, Reference SVIHUS2010) through facilitating the mixing of the feed with added enzymes (González-Alvarado et al., Reference GONZÁLEZ-ALVARADO, JIMENEZ-MORENO, VALENCIA, LAZARO and MATEOS2008).
Effect of feed form on gut morphology and microbiota profile
Relatively few studies have been reported on the effect of feed form on the intestinal morphology in broiler chickens. Dahlke et al. (Reference DAHLKE, RIBEIRO, KESSLER, LIMA and MAIORKA2003) found that pelleted diets increased the number of duodenal villi as compared with mash diets. Greater villus height and crypt depth in both the duodenum and jejunum of pellet-fed birds compared to mash-fed birds were also observed by Amerah et al. (Reference AMERAH, RAVINDRAN, LENTLE and THOMAS2007b). Zang et al. (Reference ZANG, PIAO, HUANG, WANG, MA and MA2009) reported an increase in villus height and villus height to crypt depth ratio in the small intestinal mucosa of broiler chickens fed pelleted diets compared with those given mash diets, with no effect on crypt depth. These findings might be considered as a general response of the digestive and absorptive capacity to the greater load of nutrients in pellet-fed birds. Higher villus height may increase the absorptive area and subsequently the transport of the nutrients at the villus surface (Cera et al., Reference CERA, MAHAN and CROSS1988). Higher crypt depth is an indication of increased turnover rate of intestinal mucosa, which in turn increases the maintenance requirement (Zang et al., Reference ZANG, PIAO, HUANG, WANG, MA and MA2009).
Since the intestinal microbiota profile competes for nutrients with the host and nutrient absorption mainly takes place in the small intestine, the species, numbers and activity of bacteria in this segment are of critical importance. In the small intestine of poultry, the major bacterial species are lactic acid-producing bacteria, especially Lactobacilli spp. (Barnes, Reference BARNES1972; Engberg et al., Reference ENGBERG, HEDEMANN and JENSEN2002). These bacteria are usually considered to offer health benefits for the host, as they prevent colonisation of pathogens bacteria such as E. coli and Salmonella spp. However, Lactobacilli spp. that reside in the small intestine seem to be responsible for deconjugation of bile salts (Feighner and Dashkevicz, Reference FEIGHNER and DASHKEVICZ1987), which may reduce the digestion of lipids (Smits et al., Reference SMITS, VELDMAN, VERKADE and BEYNEN1998). In addition to Lactobacilli spp., other commensal bacteria, including Enterococci, Bifidobacteria, Clostridium and Bacteroides spp., also catalyse bile acid deconjugation (Masuda, Reference MASUDA1981; Klaver and van der Meer, Reference KLAVER and VAN DER MEER1993; Smits et al., Reference SMITS, VELDMAN, VERKADE and BEYNEN1998). The microbiota profile and activity in the broiler GIT has shown to be influenced by the physical form of feed. Engberg et al. (Reference ENGBERG, HEDEMANN and JENSEN2002) reported that pellet-fed birds had lower counts of Lactobacilli spp. and C. perfringens and higher counts of Coliform and Enterococci spp. in the ileum compared to mash-fed birds. Undigested fine particles in pelleted diets can enter the caeca and become available for microbial fermentation. Microbial fermentation in terms of volatile fatty acid concentrations was found to be lower in the caeca of mash fed birds. Unfortunately data on the effect of feed form and cereal type on gut microbiota profile are scanty and more research is warranted in this area.
Conclusions
Optimum functionality of GIT in broilers is essential to enhance performance. Broilers appear to have the ability to adjust development of their GIT and digestive functions according to diet structure. In particular, particle size and feed form have major influence on gizzard development. Feeding birds with coarsely ground particles may have an advantage, since this strategy stimulates gastric functions, including secretion of hydrochloric acid, while increases the retention time of feed in the upper GIT.
It can be concluded that, although pelleting reduces particle size and can shorten the retention time in the gizzard as a consequence of reduced gizzard size, it seems that beneficial influence of coarse particles may still exist after pelleting, which would be preferable because of energy savings. However, it should be noted that the grain hardness and type of cereal used in the diet might have important effect. The current published data clearly highlights the need for well-planned studies to evaluate the effect of cereal type, feed form and particle size and their interactions on gut morphology and microbiota profile.