Introduction
Protected areas have been recognized as a primary and effective tool for preserving both marine and terrestrial environments. To achieve worldwide conservation objectives, the countries that are signatories to the Convention on Biological Diversity (CBD) have agreed to substantially increase the extent of protected areas across the globe in the coming years (O'Leary et al., Reference O'Leary, Winther-Janson, Bainbridge, Aitken, Hawkins and Roberts2016; Claudet et al., Reference Claudet, Loiseau, Sostres and Zupan2020). Targeting top predator species has been identified as an important conservation strategy. It ensures their own protection and benefits other species involved in the food chain, maintaining a healthier environment (Zacharias and Roff, Reference Zacharias and Roff2001; Sergio et al., Reference Sergio, Caro, Brown, Clucas, Hunter, Ketchum, McHugh and Hirald2008). However, common species tend to be overlooked when priority measures and conservation strategies are set. Neglecting once-common species can lead to their decline and even local disappearance when faced with threats (Vermeulen and Bräger, Reference Vermeulen and Bräger2015).
To establish effective conservation action plans, information on demographic parameters and population dynamics in both common and less abundant species is essential. Likewise, identifying central areas where biologically and socially relevant behaviours are associated (e.g. feeding, reproduction, rest) is critical (Smith et al., Reference Smith, Frère, Kobryn and Bejder2016; Tardin et al., Reference Tardin, Maciel, Espécie, Melo-Santos, Simão and Alves2020). Combining data on density, abundance, distribution and habitat use is key to identifying priority conservation areas. Therefore, efforts to obtain robust estimates of demographic and ecological parameters are necessary to evaluate and monitor populations (Azevedo et al., Reference Azevedo, Lailson-Brito, Cunha and Van Sluys2004; Cantor et al., Reference Cantor, Wedekin, Daura-Jorge, Rossi-Santos and Simões-Lopes2012). However, obtaining such information is challenging, particularly for marine mammals like cetaceans. These animals spend long periods underwater, occupy large areas, can distance themselves from the coast and present complex social organization and patterns of spatial use (Whitehead et al., Reference Whitehead, Reeves, Tyack, Mann, Connor, Tyack and Whitehead2000; Rollo, Reference Rollo2002; Sandercock, Reference Sandercock2006; Azevedo et al., Reference Azevedo, Oliveira, Viana and Van Sluys2007; Dawson et al., Reference Dawson, Wade, Slooten and Barlow2008).
Sotalia guianensis (van Bénéden, 1864) is one of the most observed resident species along the Atlantic coast of South and Central America. It is a small cetacean found primarily in bays and estuaries, with its occurrence generally associated with mangroves, shallow waters, beaches with slopes and rocky coasts (Borobia et al., Reference Borobia, Siciliano, Lodi and Hoek1991; Da Silva et al., Reference Da Silva, Fettuccia, Rodrigues, Edwards, Moreno, Moura, Wedekin, Bazzalo, Emin-Lima, Carmo, Siciliano and Utreras2010; Cantor et al., Reference Cantor, Wedekin, Daura-Jorge, Rossi-Santos and Simões-Lopes2012). Due to its coastal habits, near-shore distribution and high site fidelity, the species is highly susceptible to cumulative and constant anthropogenic pressure and urbanization (Borobia et al., Reference Borobia, Siciliano, Lodi and Hoek1991; Cantor et al., Reference Cantor, Wedekin, Daura-Jorge, Rossi-Santos and Simões-Lopes2012).
Over the last decades, studies addressing ecological and reproductive parameters, abundance and population density of S. guianensis throughout its distribution have intensified (Borobia et al., Reference Borobia, Siciliano, Lodi and Hoek1991; Lodi and Hetzel, Reference Lodi and Hetzel1998; Geise et al., Reference Geise, Gomes and Cerqueira1999; Ramos et al., Reference Ramos, Di Beneditto and Lima2000; Rollo, Reference Rollo2002; Rosas and Monteiro-Filho, Reference Rosas and Monteiro-Filho2002; Rosas et al., Reference Rosas, Barreto and Monteiro-Filho2003; Di Beneditto and Ramos, Reference Di Beneditto and Ramos2004; Araújo et al., Reference Araújo, Araújo, Souto, Parente and Geise2007; Crespo et al., Reference Crespo, Alarcon, Alonso, Bazzalo, Borobia, Cremer, Filla, Lodi, Magalhães, Marigo, Queiróz, Reynolds JE, Schaeffer, Dorneles, Lailson-Brito and Wetzel2010; Da Silva et al., Reference Da Silva, Fettuccia, Rodrigues, Edwards, Moreno, Moura, Wedekin, Bazzalo, Emin-Lima, Carmo, Siciliano and Utreras2010; Hardt et al., Reference Hardt, Cremer, Tonello and Simões-Lopes2010; Santos et al., Reference Santos, Cremer, Secchi, Flach, Filla, Hubner and Dussán-Duque2010a; Havukainen et al., Reference Havukainen, Monteiro-Filho and Filla2011; Lima et al., Reference Lima, Carvalho, Azevedo, Barbosa and Silveira2017; Monteiro-Filho et al., Reference Monteiro-Filho, Deconto, Louzada, Wanderley, Godoy, Medeiros, Rossi-Santos and Finkl2018; Santos et al., Reference Santos, Laílson-Brito, Flach, Oshima, Figueiredo, Carvalho, Ventura, Molina and Azevedo2019; Tardin et al., Reference Tardin, Maciel, Espécie, Melo-Santos, Simão and Alves2020). The distance sampling method has been widely used and recommended for estimating the density and abundance of cetacean species and all sorts of biological populations (Thomas et al., Reference Thomas, Buckland, Burnham, Anderson, Laake, Borchers, Strindberg, El-Shaarawi and Piegorsch2002).
Different populations of the species have been accessed for these data in Brazil, at the Baía do Emboraí (Pará state), Baía de Guanabara and Baía de Sepetiba (Rio de Janeiro state), Cananéia estuary (São Paulo state), Paranaguá and Guaratuba estuaries (Paraná state) and Baía de Babitonga (Santa Catarina state) (Santos et al., Reference Santos, Cremer, Secchi, Flach, Filla, Hubner and Dussán-Duque2010a; Azevedo et al., Reference Azevedo, Carvalho, Kajin, Van Sluys, Bisi, Cunha and Lailson-Brito2017; Monteiro-Filho et al., Reference Monteiro-Filho, Deconto, Louzada, Wanderley, Godoy, Medeiros, Rossi-Santos and Finkl2018). Population estimates are also available for Venezuela (Gulf of Venezuela), Colombia (Gulf of Morrosquillo) and Nicaragua (Miskito Cayos Reserve) (Santos et al., Reference Santos, Cremer, Secchi, Flach, Filla, Hubner and Dussán-Duque2010a; Espinoza-Rodríguez et al., Reference Espinoza-Rodríguez, De Turris-Morales, Takahiro and Barrios-Garrido2019).
Despite increasing efforts, there is still a significant gap in knowledge regarding the abundance, density, survival rates and population trends of S. guianensis, which is classified as near threatened in the IUCN Red List of Threatened Species and as vulnerable in the Red Book of Brazilian Endangered Fauna (ICMBio, 2018; Secchi et al., Reference Secchi, Santos and Reeves2018; IUCN, 2019). The Cananéia estuarine-lagoon complex, an estuarine region located in southern São Paulo state, has previously been studied in terms of population estimates of S. guianensis, as observed in Geise et al. (Reference Geise, Gomes and Cerqueira1999), Bisi (Reference Bisi2001), Rollo (Reference Rollo2002) and Havukainen et al. (Reference Havukainen, Monteiro-Filho and Filla2011). However, considering the conservation status of this species in Brazil and the threats faced by this region in recent years (including fishing, tourism, harbour activities and changes in environmental protection laws), it is necessary to regularly update these estimates. Therefore, the present study aims to provide additional data regarding abundance and density estimates and to consolidate distribution information for the S. guianensis population in the Cananéia estuary, located at the natural confluence of several protected areas, such as Ilha do Cardoso State Park, Lagamar de Cananéia State Park and Mandira Extractive Reserve.
Materials and methods
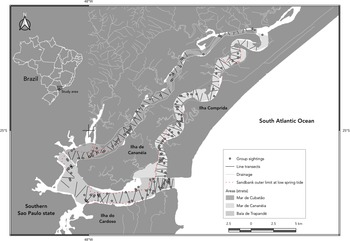
The Cananéia estuarine-lagoon complex (Lat. 25°1′28″S; Lon. 47°55′56″W) has a land area of approximately 3400 km2 and a marine area of 2450 km2 (Tessler and Mahiques, Reference Tessler and Mahiques1998) (Figure 1) and is part of a vital continuum of rainforest preservation in the São Paulo state of Brazil, known for its elevated levels of ecological diversity (Secretaria do Meio Ambiente, São Paulo, 1990). This UNESCO World Heritage site is also included in the Federal Environmental Protection Area of Cananéia-Iguape-Peruíbe and in the Wilderness Conservation Zone, forming part of the Lagamar Mosaic of Protected Areas (Federal Decree No. 90.347 dated 23 October 1984, and complemented by Decree No. 91.892 of 06/11/1985). The complex is home to rare and common species of cetaceans, such as Pontoporia blainvillei and S. guianensis, respectively (Secchi et al., Reference Secchi, Ott, Crespo, Kinas, Pedraza and Bordino2001; Desvaux, Reference Desvaux2013).

Figure 1. Study area showing the survey design with line transects and group sightings of Sotalia guianensis at the Cananéia estuarine-lagoon complex, São Paulo, Brazil.
The entire complex is highly productive, dominated by mangroves, with the predominant species being Rhizophora mangle, Laguncularia racemosa and Avicennia schaueriana, which are characteristics of the Atlantic Forest biome. The system is characterized by its year-round concentration of nutrients and fish (Domit, Reference Domit2006; Oliveira and Monteiro-Filho, Reference Oliveira and Monteiro-Filho2008). The rainfall in the area is highest between December and April, and the dry season is between May and November (Kumpera, Reference Kumpera2007). The mean annual temperature is 21.5 °C, while the sea temperature is 23.9 °C (Oliveira and Monteiro-Filho, Reference Oliveira and Monteiro-Filho2008).
The estuary surrounding Ilha de Cananéia is characterized by varying physical features in the Baía de Trapandé, Mar de Cananéia and Mar de Cubatão, which separate it from the continent and the neighbouring Ilha Comprida and Ilha do Cardoso. The Mar de Cananéia and Mar de Cubatão are narrow channels, ranging from 1 to 3 km in width and reaching depths of up to 20 m in some areas, with an average depth of around 6 m (Tessler and Souza, Reference Tessler and Souza1998). The Mar de Cananéia is larger than the Mar de Cubatão, covering areas of 24.25 and 13.53 km2, respectively, and is closer to the open sea. The Baía de Trapandé, which connects the two channels and opens to the Atlantic Ocean, has an area of 37.92 km2 and presents the highest values of width, depth and salinity in the estuary.
The tidal waves and temporal variation of freshwater discharge have a major influence on the channels and bay, affecting the salinity levels that vary according to the tides and freshwater inputs, with the highest levels recorded in winter and the lowest in summer (Domit, Reference Domit2006; Kumpera, Reference Kumpera2007). Although there are few beaches in the area, the estuary is a unique ecosystem shaped by the interplay between its physical features and environmental factors.
Estimates of abundance and density of S. guianensis in the Cananéia estuary were obtained using distance sampling methods, which involve scanning a series of transect lines for sightings of individual animals or groups (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001, Reference Buckland, Rexstad, Thomas, Borchers, Aston, Mulholland and Tant2016; Thomas et al., Reference Thomas, Buckland, Burnham, Anderson, Laake, Borchers, Strindberg, El-Shaarawi and Piegorsch2002). The distances from the sightings are measured to estimate the total number of individuals present in the area (Thomas et al., Reference Thomas, Buckland, Burnham, Anderson, Laake, Borchers, Strindberg, El-Shaarawi and Piegorsch2002; Cullen and Rudran, Reference Cullen, Rudran, Cullen, Rudran and Valladares-Padua2003; Buckland et al., Reference Buckland, Rexstad, Thomas, Borchers, Aston, Mulholland and Tant2016). For the method to be effective, it is important to adhere to several premises: (1) all objects located on the transect line will be sighted; (2) objects are detected in its initial place; (3) distances and angles are accurately measured; (4) the same animal, or group, cannot be counted more than once in the same sampling effort. Another recommendation is to achieve an appropriate number n of records, such as a minimum of 60–80 observations per line transect survey (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001; Thomas et al., Reference Thomas, Buckland, Burnham, Anderson, Laake, Borchers, Strindberg, El-Shaarawi and Piegorsch2002; Cullen and Rudran, Reference Cullen, Rudran, Cullen, Rudran and Valladares-Padua2003).
Each detected object is recorded with its radial distance and angle of detection from the transect line, and the perpendicular distance from the object to the line is then calculated. A decrease in detectability is expected with increasing perpendicular distance. Thus, observed distances are incorporated into a detection function to estimate the proportion of objects lost in the sampling. The function g (x) represents the probability of an object being detected at a distance x, where g (0) = 1 is assumed, that is, all objects in the path line are detected, respecting the premise of the method. Therefore, the density is estimated based on the number of individuals sighted, the length of the transects travelled and the area sampled, the distances recorded and the probabilities of detection of individuals using available software (Distance; Miller et al., Reference Miller, Rextad, Thomas, Marshall and Laake2019).
The study area was previously surveyed in a pilot effort to establish the sampling sectors and design the transects. The experimental design developed by Rollo (Reference Rollo2002) was used as a reference, in which transects were drawn within the contour of the study area previously digitized in AutoCAD (Autodesk Inc.). The angles between the lines were adjusted to maintain a constant ratio of length(l)/area(a). Zigzag transects were used to increase the distance between consecutive transects, reducing the likelihood of multiple sightings of the same individual and ensuring more uniform coverage in the area (Rollo, Reference Rollo2002; Chen et al., Reference Chen, Zheng, Zhai, Xu, Sun, Wang and Yang2008; Dawson et al., Reference Dawson, Wade, Slooten and Barlow2008). A total of 218 zigzag transects were distributed in the three sampled areas (Baía de Trapandé, Mar de Cananéia and Mar de Cubatão) and a stratified analysis was chosen due to the previous knowledge of the heterogeneous distribution of the species in the habitat and the different physical characteristics of the estuary (Rollo, Reference Rollo2002).
The data were collected during four seasonal campaigns, each comprising of four survey days, between July 2011 and July 2012. Each transect around Ilha de Cananéia was completed over 2 days, resulting in two full paths per campaign. The starting and direction point of the route, i.e. north or south, were randomly chosen on the first day of the campaign. Eight replicates were performed for each of the 218 transects established. The surveys were conducted between 8 am and 4 pm, with good sea and wind conditions (Beaufort 0–3), using an aluminium boat of 4–5 m in length, elevated 1.10 m from sea level, with an average speed of 16.08 km h−1 and a crew of three to four people (pilot and observers) respecting a visual field of 90° left and 90° right of the line of sight.
For each individual or group observed, the date and time, sampling area, geographical position, group size, radial distance and observation angles were recorded. Group was defined as a set of individuals with close spatial cohesion developing similar behavioural activities (Chen et al., Reference Chen, Qiu, Jia, Hung and Liu2011). The perpendicular distance was estimated from the angle and radial distances with the aid of binoculars with reticles and a laser rangefinder. Perpendicular distances over 500 m were truncated to avoid difficulties in fitting the functions to the data due to the discontinuous occurrence of records at greater distances (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001; Rollo, Reference Rollo2002). The lengths of the transects were measured using Google Earth, Garmin BaseCamp and GPS TrackMaker tools, and the areas of the sample sectors were calculated using the GE-PATH 1.4.6 software.
The analysis ran using the Distance software and tested the models half-normal with cosine adjustment, half-normal with simple polynomial adjustment, half-normal with hermite polynomial adjustment and uniform with cosine adjustment. The model and adjustment with the lowest Akaike information criterion (AIC) value was selected. The AIC is a quantitative method of estimator selection that identifies which model best fits the data with the smallest number of parameters used and less violation of assumptions (Akaike, Reference Akaike1981).
Results
During the field surveys, a total of 1339.91 km of transects were covered in 75.70 km2 with 83 h 05 min of effort. Throughout the year and along the estuary, 241 groups of S. guianensis were observed, composed by 1–20 individuals, and totalling 975 individuals sighted.
The model that best fits the data with support from the AIC in the Distance software was the half-normal with cosine adjustments. With the model and adjustment selected, the estimated total population abundance was 193 individuals (10.46% CV, 95% CI 158–237) and the average density was 2.55 ind km−2 (10.46% CV, 95% CI 2.08–3.13). The expected mean group size was 4.15 individuals (4.93% CV, 95% CI 3.76–4.57). The coefficient of variation (CV) is the ration of standard deviation to the mean, and the confidence interval (CI) is a range of values that lies between an upper and lower interval that is likely to include a population value with a certain degree of confidence.
The distribution of the species was heterogeneous in the study area, with 48.72% (n = 120, Δ = 475) of the sightings in Baía de Trapandé, followed by the Mar de Cananéia 32.72% (n = 78, Δ = 319), and Mar de Cubatão 18.56% (n = 43, Δ = 181) (Figure 1).
A stratified analysis confirmed the heterogeneous distribution of the species in the sampled sectors, with the Mar de Cananéia and Baía de Trapandé being more densely populated with 2.76 ind km−2 (18.35% CV, 95% CI 1.93–3.96) and 2.76 ind km−2 (14.64% CV, 95% CI 2.07–3.66), respectively, and the less dense Mar de Cubatão, with 1.59 ind km−2 (21.93% CV, 95% CI 1.04–2.44). Tables 1 and 2 present the results found by the Distance analyses.
Table 1. Parameters from half-normal model and cosine adjust with the lowest AIC value, for density and abundance estimates of Sotalia guianensis at the Cananéia estuarine-lagoon complex, São Paulo, Brazil

D, density; DS, group density; N, abundance.
Table 2. Parameters from half-normal model and cosine adjust with the lowest AIC value, for density and abundance estimates of Sotalia guianensis in each surveyed area at the Cananéia estuarine-lagoon complex, São Paulo, Brazil

D, density; DS, group density; N, abundance; n, number of records; L, transect length.
Discussion
The basis for the design and development of this survey in the Cananéia estuary was the experimental model applied by Rollo (Reference Rollo2002), which estimated 118 individuals. The effort and total area sampled in this survey were very similar to that model. In this study, the estimated abundance value was 193 individuals, which is comparable to Havukainen et al.'s (Reference Havukainen, Monteiro-Filho and Filla2011) estimate of 195 individuals in the estuary. However, Havukainen et al. (Reference Havukainen, Monteiro-Filho and Filla2011) surveyed only the Baía de Trapandé region, which is a smaller area than that sampled in this study. When comparing the abundance obtained only for the Baía de Trapandé region, Havukainen et al. (Reference Havukainen, Monteiro-Filho and Filla2011) estimate of 105 individuals (95% CI 79–139) is slightly higher than the value presented here, considering the CI. Geise et al. (Reference Geise, Gomes and Cerqueira1999) obtained the highest abundance estimate for the estuary, with 704.8 individuals (±367.7). However, their use of the negative exponential model, which tends to inflate values, may have contributed to this high estimate, as well as the experimental design applied and the low survey effort of about 82 km, which is below the recommended minimum for reliable results (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001).
The density of dolphins estimated in this study, 2.55 ind km−2, is relatively high compared to some physiographically similar regions, such as Baía de Guaratuba (0.15 ind km−2; Filla, Reference Filla2004) and Miskito Cayos Reserve in Nicaragua, with 0.486–0.647 ind km−2 (Edwards and Schnell, Reference Edwards and Schnell2001). Rollo (Reference Rollo2002) and Havukainen et al. (Reference Havukainen, Monteiro-Filho and Filla2011) found some of the highest densities recorded for the area, with 24.36 and 12.41 ind km−2, respectively. Rollo (Reference Rollo2002) suggests that remarkably high values of local density in some sampled sectors, in contrast with the small number found in other sectors, could have influenced the overall value. Havukainen et al. (Reference Havukainen, Monteiro-Filho and Filla2011) concentrated their surveys in Baía de Trapandé, which is known for its intense use by dolphins, and this could also explain the high value estimated by their analysis.
The average group size of S. guianensis, at 4.15 individuals, was higher than previously recorded in the Cananéia estuary by Geise et al. (Reference Geise, Gomes and Cerqueira1999), Rollo (Reference Rollo2002), Filla and Monteiro-Filho (Reference Filla and Monteiro-Filho2009) and Havukainen et al. (Reference Havukainen, Monteiro-Filho and Filla2011). Typically, S. guianensis forms small groups of 2–16 individuals (Lodi and Hetzel, Reference Lodi and Hetzel1998; Geise et al., Reference Geise, Gomes and Cerqueira1999; Da Silva et al., Reference Da Silva, Fettuccia, Rodrigues, Edwards, Moreno, Moura, Wedekin, Bazzalo, Emin-Lima, Carmo, Siciliano and Utreras2010), although larger groups have been observed in the bays of Rio de Janeiro state (Lodi, Reference Lodi2003; Azevedo et al., Reference Azevedo, Viana, Oliveira and Van Sluys2005; Flach et al., Reference Flach, Flach and Chiarello2008), Baía de Paranaguá (PR) and Baía do Norte (SC) (Daura-Jorge et al., Reference Daura-Jorge, Wedekin, Piacentini and Simões-Lopes2005; Santos et al., Reference Santos, Cremer, Secchi, Flach, Filla, Hubner and Dussán-Duque2010a), and in the Gulf of Venezuela (Espinoza-Rodríguez et al., Reference Espinoza-Rodríguez, De Turris-Morales, Takahiro and Barrios-Garrido2019). The formation of larger groups is driven by social purposes such as reproduction, protection against injury and predation and foraging when prey abundance is higher, and it varies based on the activity performed (Edwards and Schnell, Reference Edwards and Schnell2001; Araújo et al., Reference Araújo, Araújo, Souto, Parente and Geise2007; Santos et al., Reference Santos, Oshima, Pacífico and Silva2010b). The distribution of trophic resources can influence the composition and size of groups, as well as social organization and vagility (Chen et al., Reference Chen, Qiu, Jia, Hung and Liu2011). In areas with few predators, such as the study area, the presence of the species may be related to the physical characteristics of the habitat that affect the availability of resources, which may vary seasonally (Lodi, Reference Lodi2003; Godoy et al., Reference Godoy, Andriolo and Filla2015).
A stratified analysis confirmed that dolphins in the Cananéia estuary use different areas heterogeneously. The most densely populated areas, Mar de Cananéia and Baía de Trapandé, are closer to the ocean and have less variation in salinity and reduced freshwater intake. This pattern has been found for S. guianensis in many studies (Geise et al., Reference Geise, Gomes and Cerqueira1999; Rollo, Reference Rollo2002; Oliveira and Monteiro-Filho, Reference Oliveira and Monteiro-Filho2008; Godoy et al., Reference Godoy, Andriolo and Filla2015; Oshima and Santos, Reference Oshima and Santos2016; Mello et al., Reference Mello, Molina, Kajin and Santos2019). Areas close to the open sea, such as Baía de Trapandé, may offer greater diversity and availability of resources, with deeper depth gradients providing more feeding strata. Conversely, shallower areas such as Mar de Cananéia provide protection for mothers and calves against predators (Garaffo et al., Reference Garaffo, Dans, Pedraza, Crespo and Degrati2007). The preference for shallow or deep water varies among populations and their habitats, and the fact that the species is found at different depths demonstrates its behavioural flexibility under different environmental conditions (Araújo et al., Reference Araújo, Araújo, Souto, Parente and Geise2007; Azevedo et al., Reference Azevedo, Oliveira, Viana and Van Sluys2007; Da Silva et al., Reference Da Silva, Fettuccia, Rodrigues, Edwards, Moreno, Moura, Wedekin, Bazzalo, Emin-Lima, Carmo, Siciliano and Utreras2010). The distribution pattern of the species in each area demonstrates areas with greater intensity of use and behaviours strongly associated with specific sites (Wedekin et al., Reference Wedekin, Daura-Jorge, Piacentini and Simões-Lopes2007; Cremer et al., Reference Cremer, Hardt, Tonello and Simões-Lopes2011; Godoy et al., Reference Godoy, Andriolo and Filla2015; Monteiro-Filho et al., Reference Monteiro-Filho, Deconto, Louzada, Wanderley, Godoy, Medeiros, Rossi-Santos and Finkl2018).
The distribution and abundance of cetacean prey and other marine predators can be influenced by various environmental factors, both short and long term. Topography and associated currents are among these factors, which can lead to vertical mixing of water layers and nutrient distribution. Additionally, the presence of tides in channels can concentrate fish and impact the local distribution and foraging behaviour of cetaceans, as demonstrated in certain areas (Anderwald et al., Reference Anderwald, Evans, Dyer, Dale, Wright and Hoelzel2012). Food availability has been identified as the main factor influencing the spatial, behavioural and foraging strategies of delphinids (Bräger and Bräger, Reference Bräger and Bräger2019). Marine environments are complex, three-dimensional habitats with specific physical and chemical regimes (Bräger et al., Reference Bräger, Harraway and Manly2003). The distribution of cetaceans reflects their preference for specific habitats, which are associated with a wide range of biotic and abiotic factors that may directly affect species according to their physiological limits or by influencing prey availability (Bräger et al., Reference Bräger, Harraway and Manly2003; Garaffo et al., Reference Garaffo, Dans, Pedraza, Crespo and Degrati2007).
The persistence of the species throughout the year indicates that the environment offers sufficient resources for the population (Hardt et al., Reference Hardt, Cremer, Tonello and Simões-Lopes2010; Monteiro-Filho et al., Reference Monteiro-Filho, Deconto, Louzada, Wanderley, Godoy, Medeiros, Rossi-Santos and Finkl2018). Residency is common for this species throughout its distribution, as observed in Caravelas (Rossi-Santos et al., Reference Rossi-Santos, Wedekin and Monteiro-Filho2007), Baía de Sepetiba (Campos et al., Reference Campos, Fernandes, Marques and Simão2004), Baía de Babitonga (Hardt et al., Reference Hardt, Cremer, Tonello and Simões-Lopes2010; Cremer et al., Reference Cremer, Hardt, Tonello and Simões-Lopes2011), Costa Rica (Gamboa-Poveda and May-Collado, Reference Gamboa-Poveda and May-Collado2006) and the Gulf of Venezuela (Espinoza-Rodríguez et al., Reference Espinoza-Rodríguez, De Turris-Morales, Takahiro and Barrios-Garrido2019). Activities such as feeding, reproduction and parental care usually indicate a residential life area (Gamboa-Poveda and May-Collado, Reference Gamboa-Poveda and May-Collado2006). Therefore, residency patterns may reflect differences in individual responses to essential activities such as feeding and reproduction in a heterogeneous environment (Rossi-Santos et al., Reference Rossi-Santos, Wedekin and Monteiro-Filho2007).
The distribution of natural resources in small patches creates differences in densities among populations and within a habitat, which is a common tendency for most populations (Begon et al., Reference Begon, Harper and Townsend1996). The variations observed in the estimates for the Cananéia estuary probably reflect fluctuations in the population due to natural interannual changes in the balance between additions (births and immigration) and deletions (death and emigration). The movement of individuals in and out of the Cananéia estuary occurs daily, and some individuals may move between the two estuarine complexes that form the Lagamar estuarine complex, Cananéia and Paranaguá estuaries, which are connected by a long artificial channel constructed in the 1950s (Geise et al., Reference Geise, Gomes and Cerqueira1999; Oshima and Santos, Reference Oshima and Santos2016; Mello et al., Reference Mello, Molina, Kajin and Santos2019; Santos et al., Reference Santos, Laílson-Brito, Flach, Oshima, Figueiredo, Carvalho, Ventura, Molina and Azevedo2019).
Anthropogenic impacts can also lead to differences in densities in the same habitat (Chen et al., Reference Chen, Zheng, Zhai, Xu, Sun, Wang and Yang2008; Cremer et al., Reference Cremer, Hardt, Tonello and Simões-Lopes2011; Azevedo et al., Reference Azevedo, Carvalho, Kajin, Van Sluys, Bisi, Cunha and Lailson-Brito2017). Coastal species are particularly vulnerable to the cumulative impact of anthropogenic activities due to their proximity to areas of intense human presence and high site fidelity, which may exacerbate these impacts (Ramos et al., Reference Ramos, Di Beneditto and Lima2000; Chen et al., Reference Chen, Zheng, Zhai, Xu, Sun, Wang and Yang2008; Dawson et al., Reference Dawson, Wade, Slooten and Barlow2008; Smith et al., Reference Smith, Frère, Kobryn and Bejder2016; Azevedo et al., Reference Azevedo, Carvalho, Kajin, Van Sluys, Bisi, Cunha and Lailson-Brito2017). Habitat degradation, fisheries, urbanization and industrial development, chemical and noise pollution and dolphin and whale watching have all been identified as negatively affecting population decline (Chen et al., Reference Chen, Qiu, Jia, Hung and Liu2011; Smith et al., Reference Smith, Frère, Kobryn and Bejder2016; Karczmarski et al., Reference Karczmarski, Huang and Chan2017). Repeated exposure to these threats can lead to long-term effects, disrupting critical behaviours, changing acoustic communication patterns and causing displacement and area abandonment (Rollo et al., Reference Rollo, Jodas and Natal2016; Smith et al., Reference Smith, Frère, Kobryn and Bejder2016).
Sotalia dolphins face several direct threats such as bycatch in fishing nets, harvesting of natural resources, vessel traffic and tourism (Crespo et al., Reference Crespo, Alarcon, Alonso, Bazzalo, Borobia, Cremer, Filla, Lodi, Magalhães, Marigo, Queiróz, Reynolds JE, Schaeffer, Dorneles, Lailson-Brito and Wetzel2010; Flores et al., Reference Flores, Da Silva, Fettuccia, Würsig, Thewissen and Kovacs2018; Secchi et al., Reference Secchi, Santos and Reeves2018). The Scientific Committee of the International Whaling Commission (IWC) has identified incidental mortality of Sotalia as a significant threat (Crespo et al., Reference Crespo, Alarcon, Alonso, Bazzalo, Borobia, Cremer, Filla, Lodi, Magalhães, Marigo, Queiróz, Reynolds JE, Schaeffer, Dorneles, Lailson-Brito and Wetzel2010). Bycatch is considered one of the most significant threats to marine life globally, affecting not only individual species but also entire communities (Breen et al., Reference Breen, Brown, Reid and Rogan2017).
The world's oceans have suffered from centuries of human exploitation and overfishing, resulting in local extinctions, high pollution levels, habitat loss and degradation and altered ecosystems with compromised trophic relations (Costello and Ballantine, Reference Costello and Ballantine2015; Woodcock et al., Reference Woodcock, O'Leary, Kaiser and Pullin2017). Constant and increasing threats to marine diversity have raised concerns about ocean preservation and local population maintenance. As a result, several protected areas have been designated and established worldwide (Bossley et al., Reference Bossley, Steiner, Rankin and Bejder2017; Venter et al., Reference Venter, Magrach, Outram, Klein, Possingham, Di Marco and Watson2017). The implementation of protected areas and habitat management improvements has shown positive effects towards species presence and population increase, especially for coastal species (Bossley et al., Reference Bossley, Steiner, Rankin and Bejder2017). For example, Bossley et al. (Reference Bossley, Steiner, Rankin and Bejder2017) observed population growth in an industrial estuary where previously there had been a decline due to environmental degradation, particularly from tourism and port dredging.
However, over 90% of protected areas still allow some form of fishing, and more than half of coastal countries have not designated any protected areas (Costello and Ballantine, Reference Costello and Ballantine2015; Woodcock et al., Reference Woodcock, O'Leary, Kaiser and Pullin2017). In Brazilian waters, studies show that most protected areas do not include the entire home range of many marine mammals and still allow human activities that interfere with the species' distribution and habitat use, including S. guianensis, leading to negative effects on local biodiversity (Santos et al., Reference Santos, Figueiredo and Bressem2017; Tardin et al., Reference Tardin, Maciel, Espécie, Melo-Santos, Simão and Alves2020).
Identifying the habitats used by wildlife for essential natural behaviours, such as feeding, resting, parental care and breeding, is critical for determining priority areas for conservation and designing strategies to mitigate human impacts on animal populations (Smith et al., Reference Smith, Frère, Kobryn and Bejder2016; Venter et al., Reference Venter, Magrach, Outram, Klein, Possingham, Di Marco and Watson2017; Tardin et al., Reference Tardin, Maciel, Espécie, Melo-Santos, Simão and Alves2020). This is the first step in developing effective conservation strategies that can achieve long-term goals, using scientific evidence to evaluate the effectiveness of the actions implemented and identify the factors that influence it (Karczmarski et al., Reference Karczmarski, Huang and Chan2017; Woodcock et al., Reference Woodcock, O'Leary, Kaiser and Pullin2017). Long-term assessments and continuous monitoring of population demographic patterns are therefore necessary, as they allow for the identification of key habitats and the evaluation of potential anthropogenic threats and animal responses (Bailey and Thompson, Reference Bailey and Thompson2009; Campbell et al., Reference Campbell, Thomas, Whitaker, Douglas, Calambokidis and Hildebrand2015).
The Cananéia estuary has been identified as a vital area of residence for S. guianensis, providing resources for this and other marine populations. This species is highly valued by the local population of Cananéia, as well as by traditional fishermen, and is also a popular attraction for tourism, contributing significantly to the community's income. Reinforcement of existing protective measures for the habitat and greater involvement by the local community and stakeholders are crucial to achieving priority goals. The combination of different protected areas may bring balance between biodiversity, habitat protection and natural resource use and exploitation, leading to sustainable development and the continued presence of this species.
Data
The authors confirm that the data supporting the findings of the current study are available within the article. Interested readers to other materials and datasets could request them from the corresponding authors.
Acknowledgements
We would like to express our gratitude to several individuals and organizations for their support during our field research. The Instituto Oceanográfico, Universidade de São Paulo (IOUSP) provided valuable logistical assistance. Dr Felipe Fornazari, Júlia Dombroski (MSc.), Rodrigo Araújo de Souza (MSc.) and Ingrid Zwar (MSc.) generously assisted us during our field surveys. Dr Ricardo S. Bovendorp provided us with helpful information during our population estimates. We are also thankful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the Scholarship and Cetacean Society International (CSI) for the small grants.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Inaê Guion de Almeida and Mario Manoel Rollo Jr. The first draft of the manuscript was written by Inaê Guion de Almeida and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Financial support
The author Inaê Guion de Almeida received a doctoral scholarship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and three small grants from the Cetacean Society International (CSI) to carry out field surveys and participate in national and international scientific events.
Competing interest
None.
Ethical standards
Not applicable.