The ductus arteriosus provides a physiologic right-to-left shunt between the pulmonary artery and the aorta during intrauterine life. Post-natally, the shunt direction normally changes from left to right and the ductus arteriosus undergoes functional closure, which is followed by definite anatomic remodelling.Reference Hamrick and Hansmann1 Especially, in the most immature preterm infants the ductus arteriosus often fails to close (patent ductus arteriosus). A haemodynamically significant patent ductus arteriosus can be associated with several adverse outcomes such as pulmonary oedema, bronchopulmonary dysplasia, left ventricular dysfunction, and impaired renal and cerebral blood flow.Reference Sallmon, Koehne and Hansmann2 These observations provide the rationale to consider treatment in infants with haemodynamically significant patent ductus arteriosus. Pharmacological treatment is usually initiated with the cyclooxygenase inhibitors ibuprofen or indomethacin. However, cyclooxygenase inhibitor treatment is associated with several complications and the responses of individual infants differ and are difficult to predict.Reference Jain and Shah3
Therefore, the identification of patient-specific risk factors is of great interest to provide targeted (tailored) therapies. Hypoxia-regulated vascular endothelial growth factor is an essential mediator of ductal constriction and closure. Vascular endothelial growth factor and its receptors have been shown to be developmentally regulatedReference Weber, Rheinlaender and Sarioglu4 and functional in endothelial cells of the ductus. Specifically, vascular endothelial growth factor promotes vasa vasorum ingrowth and recruitment of mononuclear cells to the ductal intima which constitutes a pivotal step during ductus arteriosus anatomic remodelling.Reference Clyman, Seidner and Kajino5,Reference Waleh, Seidner and McCurnin6 We have recently shown that ibuprofen and indomethacin differentially regulate vascular endothelial growth factor and its receptors in primary endothelial cells of the rat ductus arteriosus.Reference Sallmon, Akanbi and Weber7 In addition, several studies discussed vascular endothelial growth factor polymorphisms as potential risk factors for several diseases in neonates, including retinopathy of prematurityReference Ali, Hussien, Samy and Husseiny8,Reference Kwinta, Bik-Multanowski and Mitkowska9 and bronchopulmonary dysplasia.Reference Kwinta, Bik-Multanowski and Mitkowska10–Reference Mahlman, Huusko and Karjalainen12
The aim of the current study was to investigate the incidence of the vascular endothelial growth factor polymorphism rs2010963 status in a large cohort of preterm infants with and without patent ductus arteriosus and its potential association with cyclooxygenase inhibitor treatment success rates.
Methods
Study population and treatment algorithm
This case–control study was conducted at the Department of Neonatology, Charité University Medical Center, Berlin, Germany (1995–2008). All very low birth weight infants born in the respective period were included if blood from dry-spot cards for the German national neonatal screening program could be obtained. Parental consent for data collection was obtained at the time the German national newborn screening program blood draw was performed. The study was approved by the local ethics committee at Charité Berlin.
Infants were examined for haemodynamically significant patent ductus arteriosus on days of life 4–5 and when clinically indicated. Echocardiography included evaluation of ductus arteriosus shunt direction (high upper parasternal short axis) as well as assessment of the minimal internal ductal diameter. The left atrium to aortic root ratio was measured by M-mode (parasternal long axis). Doppler measurement of the resistance index in the anterior cerebral artery was performed at the same time. Examinations were performed as previously described.Reference Sallmon, Weber and Huning13
Cyclooxygenase inhibitor treatment was initiated in all infants with a haemodynamically significant patent ductus arteriosus. A patent ductus arteriosus with left-to-right shunt was considered haemodynamically significant if (1) a respiratory set back with a fraction of inspired oxygen >0.3 and/or mechanical ventilation, (2) a left atrium to aortic root ratio ≥1.4, (3) a ductal diameter ≥2.5 mm, and/or (4) a decreased end diastolic flow in the anterior cerebral artery with a resistance index ≥0.85 were present. There has been no substantial change in the clinical and echocardiographic criteria used to determine the haemodynamic significance of a patent ductus arteriosus during the study period.
Infants received indomethacin or ibuprofen exactly as previously described in detail (including dosing, duration, etc.).Reference Sallmon, Weber and Huning13 Dosing had been constant throughout the study period and we did not use high-dose regimens as a rescue therapy. Successful response to cyclooxygenase inhibitor treatment was defined as absent or minimal ductal shunt flow 24–48 hours after therapy; all other cases were defined as cyclooxygenase inhibitor treatment failure. Ductus ligation was performed after failed pharmacologic therapy based upon individual decision (secondary ligation). Primary ligation was only performed in a minority of patients with severe haemodynamically significant patent ductus arteriosus during the first years of the study period and is not practiced anymore.
DNA isolation and polymerase chain reaction
DNA isolation from dry-spot cards was performed using standard phenol–chloroform extraction. Nano Drop (peqlab, Erlangen, Germany) measurements were used to assess the quality of the extracted DNA. Polymerase chain reaction-based DNA amplification was carried out using a modified previously published protocol.Reference Young, Summers and Bhushan14 In contrast to the reported experimental design, we changed the annealing temperature to 60°C and the DNA concentration to 25 ng. The following primer sequences were used for human vascular endothelial growth factor: 5’-GACGGCTTGGGGAGATTGCT-3’ (forward primer) and 5’-TCAGCTGCGGGATCCCAAGG-3’ (reverse primer), respectively (BioTEZ, Berlin, Germany).
Restriction fragment length polymorphism analysis and DNA sequencing
Restriction fragment length polymorphism analysis was performed following standard protocols. Briefly, digestion by the restriction enzyme Faq1 (BsmF1) was achieved by using a standard preparation (polymerase chain reaction product 10 µl, ddH2O 18 µl, 10 × Buffer Tango 2 µl, 50 × SAM 0.6 µl Faq1/BsmF1 1 µl – all Fermentas, York, United Kingdom), which was digested for 960 minutes (37°C) followed by enzyme inactivation for 20 minutes (65°C). After storage at 4°C the restriction fragments were separated by electrophoresis in an ethidium bromide-containing 3% agarose gel (3 g Agarose/100 ml 1 × TAE Puffer + 3 µl 1% ethidium bromide – all Fermentas, except for ethidium bromide, Merck, Darmstadt, Germany) and visualised by ultraviolet light. The polymerase chain reaction as described produces a 245 base pairs fragment. Depending on rs2010963 status either one band (245 base pairs, rs2010963 C/C), two bands (175 base pairs and 70 base pairs, rs2010963 G/G), or three bands (245 base pairs, 175 base pairs, and 70 base pairs, rs2010963 G/C) could be detected after restriction enzyme digestion.
DNA sequencing was performed using the Sanger methodReference Sanger, Nicklen and Coulson15 in representative samples of each group in order to verify accurate detection of the polymorphism (n = 3). DNA sequencing experiments were commercially carried out by AGOWA GmbH, Berlin, Germany.
Statistics
Comparisons between groups were made by Mann–Whitney U-test for continuously scaled data and by chi-square test for categorical data. p values <0.05 were considered statistically significant. Statistical analyses were carried out using IBM SPSS Statistics 22 (SPSS Inc., Chicago, Illionois, United States of America) und Microsoft® Excel 2010 software.
Results
Study population
We included a total of 1053 very low birth weight preterm infants, out of which 520 were diagnosed with a patent ductus arteriosus (49.4%). A total of 30 infants underwent primary ligation and 225 did not require therapy (no haemodynamically significant patent ductus arteriosus). In the remaining 265 infants with haemodynamically significant ductus arteriosus, pharmacological cyclooxygenase inhibitor treatment was initiated with either ibuprofen or indomethacin (Table 1, Fig 1). Out of 265 infants, 95 underwent secondary ligation after failed medical treatment due to a persistently patent significant ductus arteriosus. In total, 814 infants could be successfully screened for vascular endothelial growth factor polymorphism rs2010963 status for further analyses.
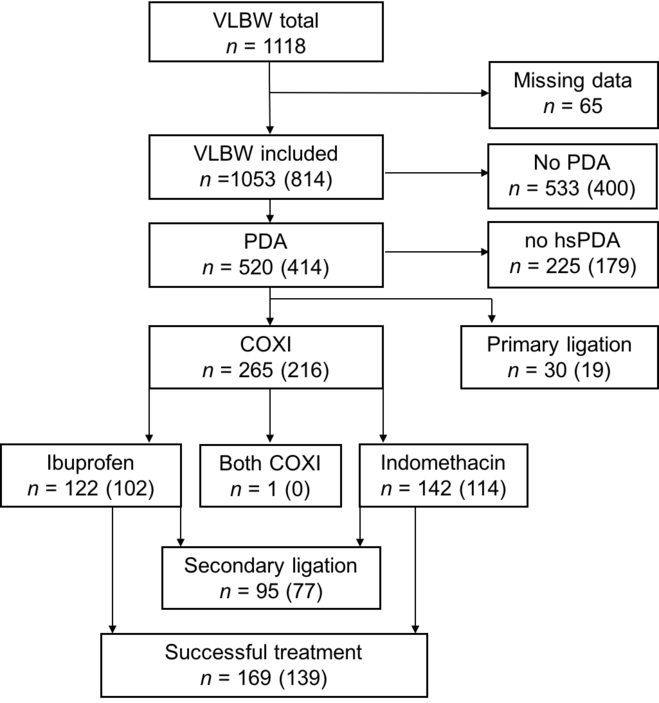
Figure 1. Study population. Displayed are the total numbers of patients in the different clinical outcome groups. In paracentesis, the number of infants screened for VEGF polymorphism rs2010963 status is given. COXI = cyclooxygenase inhibitor, hsPDA = haemodynamically significant PDA, PDA = patent ductus arteriosus, VLBW = very low birth weight infants.
Table 1. Demographic characteristics of the study population.

BPD = bronchopulmonary dysplasia; IVH = intraventricular haemorrhage; NEC = necrotising enterocolitis; PDA = patent ductus arteriosus; PVL = periventricular leukomalacia; RDS = respiratory distress syndrome; ROP = retinopathy of prematurity; VLBW = very low birth weight.
* p-value <0.05.
Incidence of patent ductus arteriosus and haemodynamically significant patent ductus arteriosus and vascular endothelial growth factor rs2010963 polymorphism status
A percentage of 42.3 (344/814) exhibited a homozygous G/G genotype, while 35.4% (288/814) were heterozygous for G/C and 22.4% (182/814) homozygous for C/C. We did not find any significant differences between infants with and without patent ductus arteriosus with regard to rs2010963 status (Table 2). Of note, rs2010963 polymorphism status did not differ between infants with haemodynamically significant and those with haemodynamically non-significant patent ductus arteriosus (not shown).
Table 2. Vascular endothelial growth factor polymorphism rs2010963 status in very low birth weight infants with and without patent ductus arteriosus.

PDA = patent ductus arteriosus; VEGF = vascular endothelial growth factor.
In the top row, data in parentheses represent percentages referring to the total number of 814 included very low birth weight preterm infants (horizontal comparison within the first row). For all other data shown, percentages in parentheses refer to the total number in the upper first row of the same column (vertical comparison within the same column).
Vascular endothelial growth factor rs2010963 polymorphism status and initial cyclooxygenase inhibitor treatment success
In a next step, we sought to investigate whether rs2010963 polymorphism status influenced cyclooxygenase inhibitor therapy success. We found no significant differences between infants who responded to cyclooxygenase inhibitor treatment and those who were non-responders with regard to rs2010963 polymorphism status (Fig 2). Of note, these observations were made irrespective of the administered agent (indomethacin or ibuprofen). In addition, vascular endothelial growth factor rs2010963 polymorphism status did not differ between infants that required 1 versus ≥ 2 COX inhibitor cycles and did not affect the overall time to achieve ductal closure in our cohort (not shown).
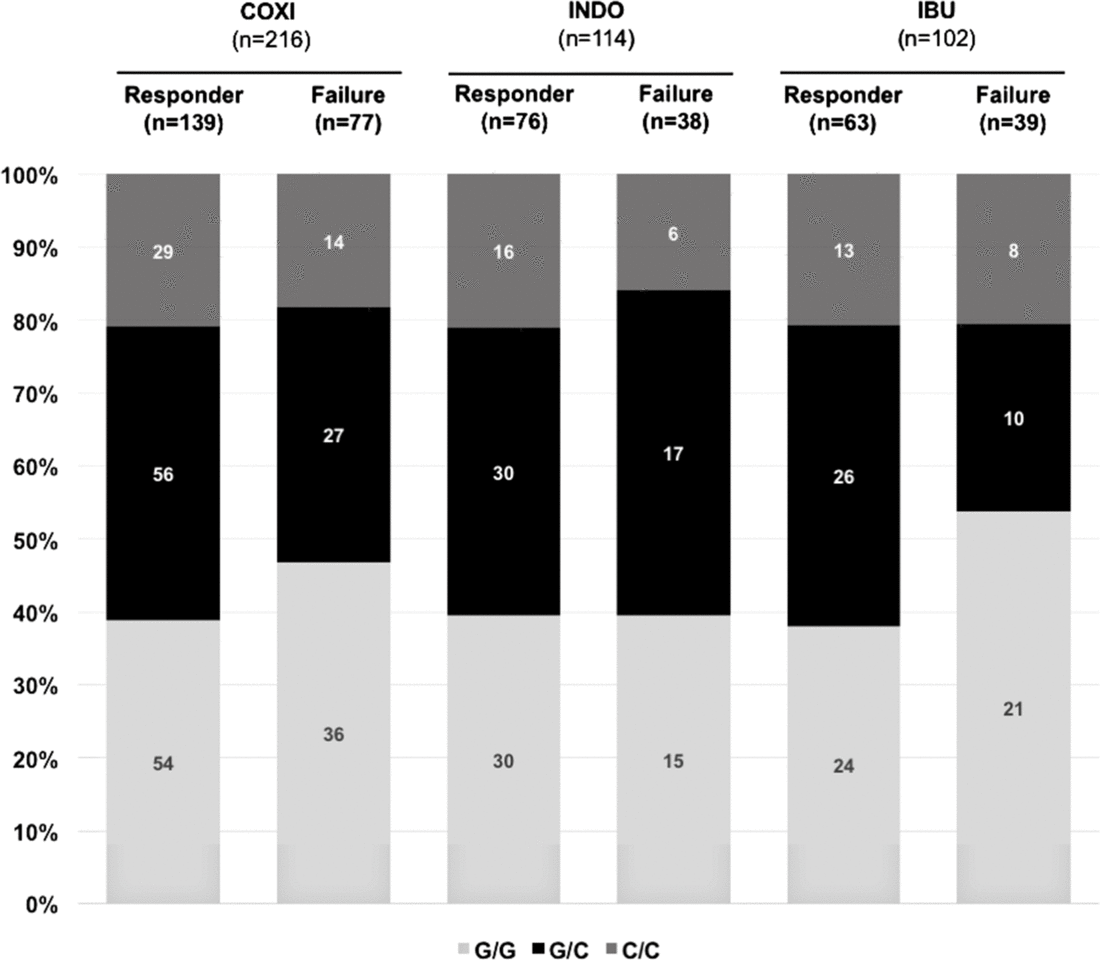
Figure 2. Vascular endothelial growth factor polymorphism rs2010963 status and response to cyclooxygenase inhibitor treatment for haemodynamically significant patent ductus arteriosus. Displayed are the total numbers and percentages for each genotype (C/C, G/C, G/G) in the different groups. There was no association between VEGF rs2010963 status and cyclooxygenase inhibitor treatment response, irrespective of the cyclooxygenase inhibitor used. COXI = cyclooxygenase inhibitor, IBU = ibuprofen, INDO = indomethacin, VEGF = vascular endothelial growth factor.
Discussion
Vascular endothelial growth factor plays an important role in ductal closure in neonates. Different responses of preterm infants to pharmacological patent ductus arteriosus treatment with cyclooxygenase inhibitors suggest a potential role of genetic modifiers, such as polymorphisms in genes involved in physiologic ductus arteriosus closure. We herein investigated the potential impact of vascular endothelial growth factor polymorphism rs2010963 on the incidence and success rates of patent ductus arteriosus treatment in a large cohort of preterm infants. We found no significant association between rs2010963 status and the incidence of patent ductus arteriosus or the response to cyclooxygenase inhibitor treatment in our cohort.
Vascular endothelial growth factor mediates ductal closure by promoting intimal proliferation, vasa vasorum ingrowth, smooth muscle cell migration, and recruitment of mononuclear cells, all of which are required for definite ductal closure.Reference Clyman, Seidner and Kajino5,Reference Waleh, Seidner and McCurnin6 The expression of vascular endothelial growth factor and its receptors changes throughout gestation. In a human study using ductal tissue specimens, vascular endothelial growth factor receptor 1 and 3 expression was marked in the endothelium during early maturity stages and vascular endothelial growth factor receptor 3 decreased during development, while vascular endothelial growth factor receptor 2 predominated in the media during later developmental stages.Reference Weber, Rheinlaender and Sarioglu4 We recently found a differential response of primary rat ductus arteriosus endothelial cells to ibuprofen and indomethacin. While ibuprofen induced vascular endothelial growth factor and vascular endothelial growth factor receptor 2 expression, indomethacin did not affect the expression levels of the vascular endothelial growth factor system in ductus arteriosus endothelial cells.Reference Sallmon, Akanbi and Weber7
Several authors investigated vascular endothelial growth factor polymorphisms and their potential association with neonatal morbidities such as bronchopulmonary dysplasia,Reference Kwinta, Bik-Multanowski and Mitkowska10–Reference Mahlman, Huusko and Karjalainen12 retinopathy of prematurity,Reference Ali, Hussien, Samy and Husseiny8, Reference Kwinta, Bik-Multanowski and Mitkowska9 and intraventricular haemorrhageReference Prasun, Madan and Puthuraya16 in preterm infants. However, the role of the vascular endothelial growth factor polymorphism rs2010963 on patent ductus arteriosus incidence and treatment success rates has not been previously investigated. Vascular endothelial growth factor polymorphism rs2010963 is located within a regulatory element of the vascular endothelial growth factor A gene (5´-untranslated region) and influences vascular endothelial growth factor gene expression levels in several human tissues (the C allele of rs2010963 is related to higher gene expression levels).Reference Ma, Xin and Chen17
Genetic factors likely influence ductus arteriosus closure. A recent review article addressed the known genetic factors which contribute to prolonged ductal patency and variability in response to drug therapy for patent ductus arteriosus.Reference Lewis, Shelton and Van Driest18 One striking finding is the different ethnic response to cyclooxygenase inhibitor treatment.Reference Waleh, Barrette and Dagle19 Our study has only been conducted in a single centre and is therefore unable to examine those relationships. Furthermore, only one polymorphism has been examined and it cannot be excluded that other vascular endothelial growth factor polymorphisms modify ductal closure or pharmacological treatment response.
In general, the indications and treatment strategies for preterm infants with patent ductus arteriosus are still unclear.Reference Sallmon, Koehne and Hansmann2 An expectant strategy, especially for small patent ductus arteriosus, is nowadays preferred by many clinicians in order to avoid potential harmful effects of pharmacological, interventional, and surgical therapies.Reference Hamrick and Hansmann1–Reference Jain and Shah3,Reference Weber, Weiss and Buhrer20,Reference Barikbin, Sallmon and Wilitzki21 However, a longer duration of exposure to a haemodynamically significant patent ductus arteriosus has been associated with several adverse events providing the rationale for treatment in selected cases.Reference Sallmon, Koehne and Hansmann2,Reference Weisz, Mirea and Rosenberg22 Further research on the individual genetic mechanisms underlying ductal closure might contribute to the development of individual (tailored) therapies to achieve an optimal balance between intended and adverse drug effects in individual patients. Future prospective trials on the optimal indications and strategies for patent ductus arteriosus treatment are therefore highly warranted and might benefit from incorporating genetic risk assessment.
Author ORCIDs
Hannes Sallmon, 0000-0002-7152-5379
Acknowledgement
We thank Boris Metze (Berlin) for his expert statistical assistance.
Financial Support
This study was supported by a Lydia-Rabinowitsch-Scholarship (Charité University Medical Center Berlin to P.K.).
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (German federal and local laws) and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees (Charité Berlin).






