Introduction
The mosquito-borne chikungunya virus (CHIKV) affects human health worldwide and is transmitted mainly through the vectors Aedes aegypti and Ae. albopictus. From its discovery in 1953 in the Newala district of Tanganyika (present-day Tanzania), until the first years of the 21st century, CHIKV was recognised as an arbovirus that caused small outbreaks in Asia and Africa and had high attack rates but low case fatality rates. After 2005, there was a significant increase in the number of cases and an expansion in the transmission area in several regions of the world, which included continental Europe after 2007 [1, Reference Weaver and Forrester2]. During 2005–2006, a major epidemic hit the Réunion Island, a French overseas region located in the Indian Ocean, where severe cases and deaths were reported [Reference Renault3–Reference Gérardin6]. In 2006, the island had an estimated 266 000 symptomatic patients and 255 deaths, with a fatality rate of one death per 1000 cases. Since then, several fatal cases have been described in the literature [Reference Tandale7–Reference Jean-Baptiste12].
At the end of 2013, an autochthonous transmission of CHIKV was identified in Saint Martin, Guadeloupe and Martinique [Reference Cauchemez13]. The transmission quickly spread through almost all the islands of the Caribbean and continental America. By the end of the 2014 epidemic, 1 118 578 CHIKV cases were reported in the Americas with 194 deaths, which were reported to Pan American Health Organization (PAHO), which resulted in a lethality rate of 0.02% which is much lower than what was reported in the literature [14]. Ae. aegypti was the suspected vector of the arbovirus because of the absence of Ae. albopictus on these islands. In fact, a recent study has shown that for the first time, CHIKV infections occurred in natural populations of Ae. aegypti on Martinique [Reference Farraudière15].
By the end of the epidemics in Guadeloupe and Martinique, 153 400 CHIKV cases were reported out of estimated 306 800 symptomatic patients [16]. Considering these data, the fatality rates in Guadeloupe and Martinique would be 0.04% and 0.06%, respectively, which are both half of the fatality rate observed in Réunion Island in 2006. Because in 2006, elderly individuals accounted for 9.6% of the population in Réunion Island and in 2014, they accounted for 15.7% and 17.6% of the population in Guadeloupe and Martinique [17], respectively, and because CHIKV deaths occur at more advanced ages, higher mortality would have been expected in Martinique and Guadeloupe (2014) than in Réunion (2006).
In Réunion Island, excess deaths during the CHIKV epidemic were estimated at 267 [Reference Josseran18], which were very close to the 255 deaths that were recorded in the death certificates. However, these findings were different from those reported in Brazil, Ahmedabad, the Andaman and Nicobar Islands (belonging to India) and Mauritius, where a significant number of excess deaths did not coincide with those reported in the epidemiological surveillance system [Reference Manimunda19–Reference Freitas23]. Thus, there are some inconsistencies in the estimated mortality data being reported in CHIKV circulation regions.
The objective of this study was to assess excess deaths by sex and age in the Guadeloupe and Martinique Islands during the 2014 CHIKV epidemic using official data from the Institut National de La Statistique et des Études Économiques (INSEE). We hypothesise that there may have been excess deaths above the number of deaths identified by certificates.
Methods
A time series study of reported CHIKV incidence rates and mortality by sex and age group was conducted in Guadeloupe and Martinique in the period between 2011 and 2015.
Locality
Guadeloupe and Martinique are French overseas regions in the Caribbean and are islands of tropical climate (Classification Af according to the Köppen Climate System) located in the Lesser Antilles of the French West Indies. In 2014, Guadeloupe and Martinique had populations of 400 186 and 383 911 inhabitants, respectively [17]. We analysed the two French overseas regions together because they had similar climate, similar epidemiological and sociodemographic profiles and practically simultaneous CHIKV epidemics [16].
Data collection
Estimated by sex and age group, population and mortality data were accessed from the INSEE of the French government [24]. Epidemiological data on CHIKV, including number of cases, hospitalisations and deaths, were obtained in the official epidemiological reports of the Cellule de Institut de Veille Sanitaire (InVS) en Région (English Regional Office of the French Institute for Public Health Surveillance) [16]. As the influenza virus is known to be a cause of seasonal increases in overall mortality, we also extracted monthly influenza-like syndrome data reported to sentinel surveillance systems from the bulletins of InVS [25, 26]. Numerical data were extracted from the graphs of bulletins using the program Engauge Digitizer [Reference Mitchell27].
Statistical analysis
We calculated both monthly and annual age-specific mortality rate (ASMR) by sex from 2010 to 2013. The monthly number of expected deaths for 2014 and 2015 by age group and sex was calculated using the average mortality rates of each month of the reference period for the estimated population of the French regions of Guadeloupe and Martinique in 2014 and 2015. The reference period used was the 3 years prior to 2013, in which no transmission was reported in the study areas [Reference Hérmon and Jougla28, Reference Josseran29]. For each month in 2014 and 2015, excess deaths were calculated as the difference between the monthly observed deaths and monthly expected deaths for all ages. The expected deaths by age group were calculated based on an average of ASMR of the reference years (2011–2013) applied using the estimated population of 2014. We also calculated a conservative 99% confidence interval (CI) for the monthly deaths (2014–2015) and total deaths for each sex and age group (2014).
The standardised mortality ratio (SMR) was calculated by dividing the monthly observed deaths by the monthly expected deaths. This value was compared with the null hypothesis (SMR = 1.00) according to formula (1) of the z score [Reference Jougla30]:
where n = observed deaths and A = expected deaths.
Statistical analysis was performed using IBM® SPSS® 24.0 software.
Results
CHIKV epidemic curve and monthly deaths
The monthly reported cases of CHIKV diagnosed by sentinel doctors, number of hospitalisations and excess deaths in 2014 in Guadeloupe and Martinique are presented in Table 1. The 2014 estimates of clinical cases of CHIKV in Guadeloupe and Martinique reported by InVS are also given. There was a strong correlation between the monthly incidence rates of CHIKV and excess deaths (R = 0.81, p < 0.005), and the correlation was even higher with a 1-month lag between CHIKV cases and excess deaths (R = 0.87, p < 0.001) (Table 1). We observed a strong correlation between monthly hospitalisation rates for CHIKV and excess deaths with a delay of 1 month (R = 0.87, p < 0.0005), while a moderate correlation was found between monthly hospitalisation rates and excess deaths (R = 0.66, p < 0.05) (Table 1).
Table 1. Monthly cases of chikungunya, influenza-like illness, excess deaths and SMR (Guadaloupe and Martinique, 2014)

a Without biological plausibility.
Figure 1 shows the number of observed deaths, the number of expected deaths per month and the upper limit of the 99% CI in Guadeloupe and Martinique for 2014–2015, and it also presents the number of monthly cases of CHIKV estimated by InVS. The peak of the epidemic was in June and coincided with the peak of deaths. Excess deaths remained above the 99% CI for April–November, which is the period with the highest incidences of CHIKV. In the months with a small number of CHIKV cases in 2014 (January–March and December) and in 2015, the number of monthly deaths was below the upper limit of the 99% CI.
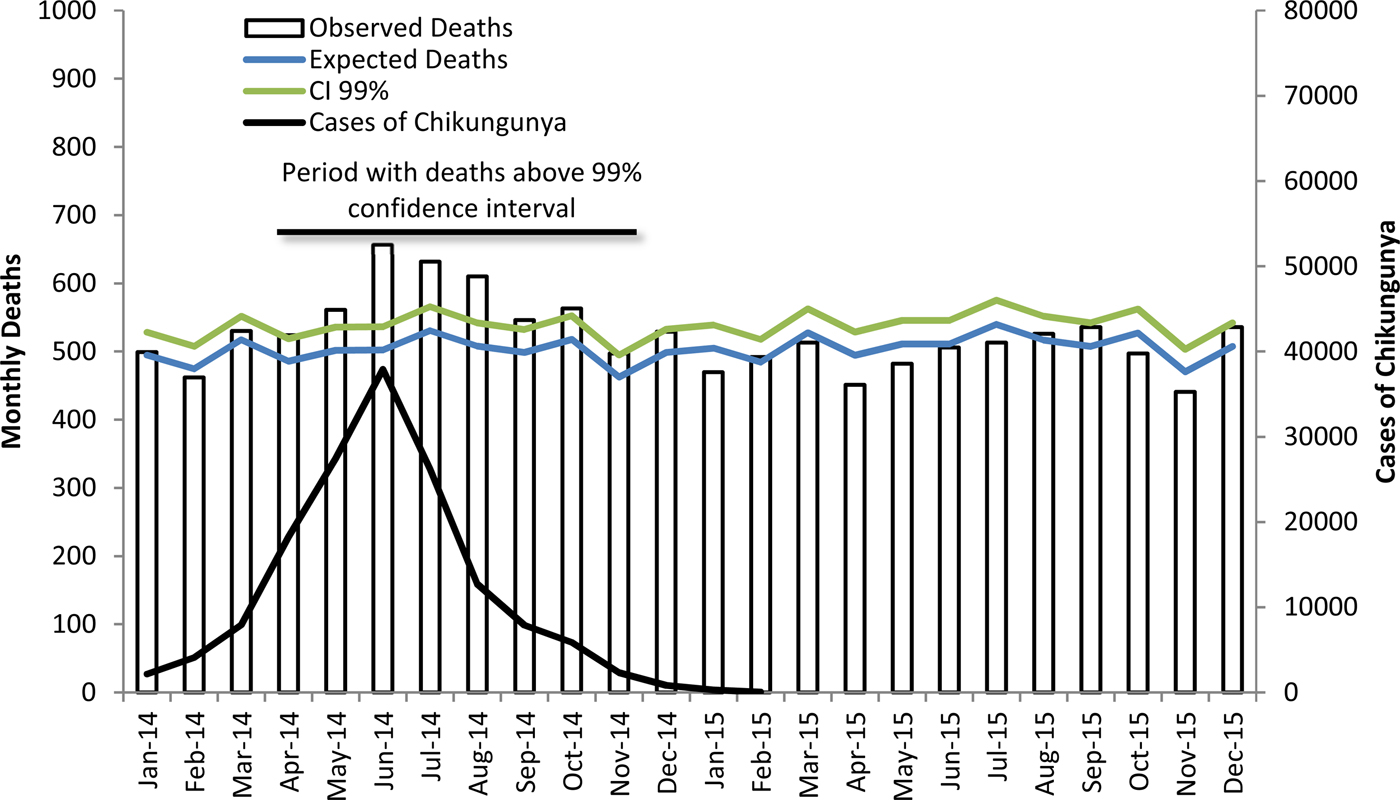
Fig. 1. Cases of chikungunya, expected and observed monthly deaths and 99% CI upper limit (Guadeloupe and Martinique 2014–2015).
Mortality by sex and age group
Except for the <19-year age group, all other age groups had a higher number of deaths than expected. ASMR was above the upper limit of the 99% CI in both sexes (Fig. 2) and in all age groups above 20 years of age, with the exception of the 40–59-year age group (Table 2). Excess deaths increased with age in both sexes. In 2015, ASMR was lower than that in 2014 and was below the upper limit of the CI in all age groups.
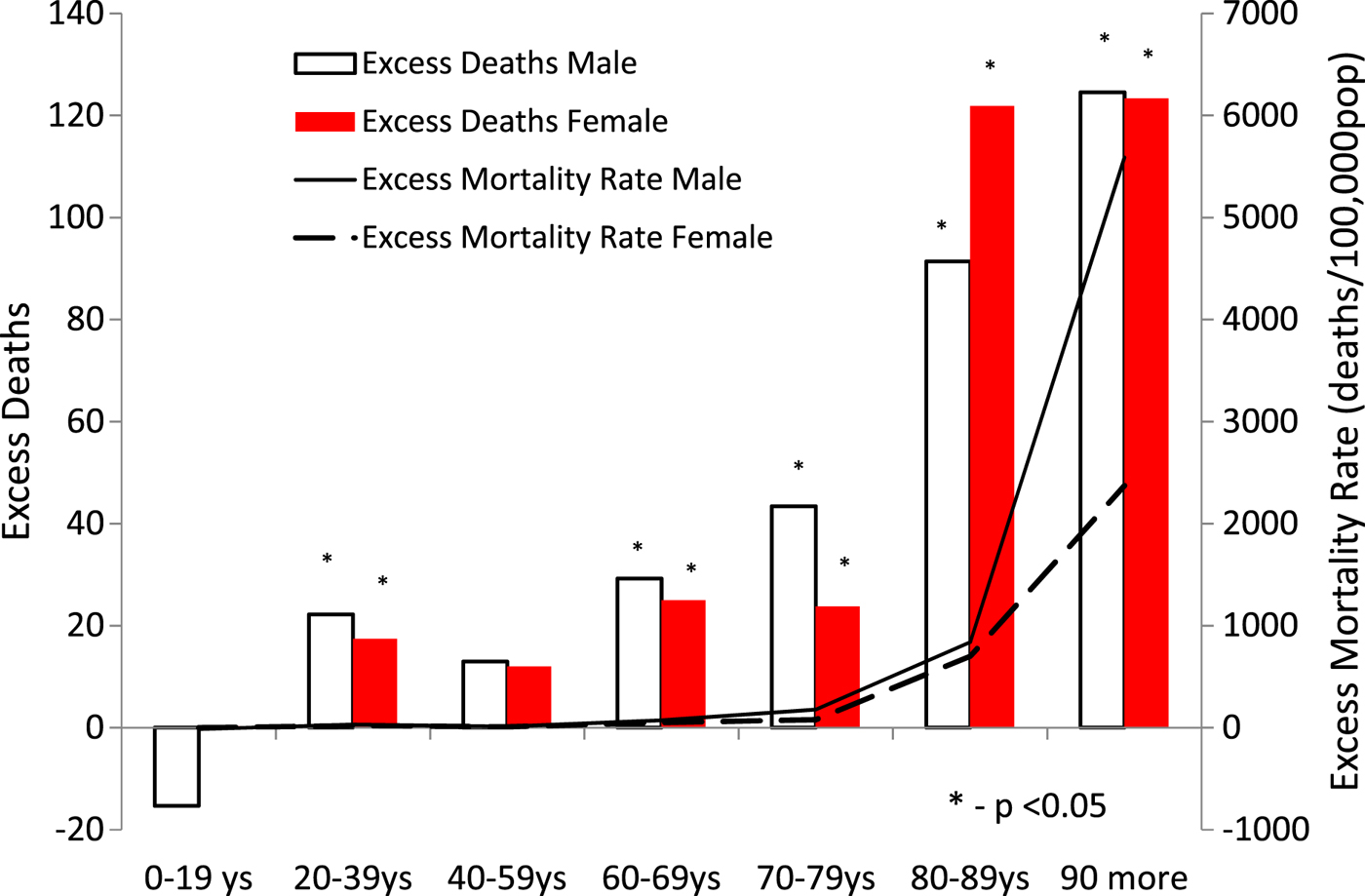
Fig. 2. Excess deaths and excess mortality rate by sex and age group (Guadeloupe and Martinique, 2014).
Table 2. Population, mortality data, observed and estimated by age group in Guadaloupe and Martinique (2014)

ASMR, age-specific mortality rate.
a According to the death certificate listed chikungunya as a cause or contributing condition to death.
The underlined values are those referring to the age groups whose mortality was above 99% CI.
Overall excess deaths and fatality rate
During 2014, in Guadeloupe and Martinique, there were 639 excess deaths, which is four times greater than the number of deaths reported to InVS through death certificates. The excess mortality rate was 81.5 deaths per 100 000 inhabitants, and the case fatality rate in 2014 was estimated at 2.08 deaths per 1000 cases of CHIKV (Table 2).
Discussion
Time series analysis of deaths in the Guadeloupe and Martinique regions shows that mortality increased above the 99% CI during the CHIKV epidemic of 2014 and returned to normal soon after the end of the epidemic. In June, the peak of deaths occurred simultaneously with the peak of CHIKV cases. There was a strong temporal correlation between excess deaths, cases of CHIKV and hospitalisations. This correlation was stronger when we used 1 month of delay between clinically identified cases and deaths. This delay can be explained by prolonged hospitalisations before death [Reference Crosby10]. The correlation between monthly hospitalisation rates and excess deaths reinforces this hypothesis. Excess mortality increased with age but did not occur only among older people; among people aged 20–39 years, there were excess deaths above the upper limit of the 99% CI. This same mortality pattern was observed in studies that reported CHIKV as a cause of death on death certificates [Reference Renault3] and in studies that used laboratory test results (RT-PCR, viral isolation or IgM) to confirm the disease [Reference Economopoulou4, Reference Tandale7]. The 2014 excess mortality rate by age group in Guadeloupe and Martinique (Table 2) was very close to the CHIKV-specific mortality rate observed in Réunion in 2006 based on the declarations of deaths [Reference Renault3, Reference Renault8]. These findings reinforce our hypothesis that increased mortality was associated with the CHIKV epidemic. The case fatality rate in the current study was 2.08 deaths per 1000 cases of CHIKV, which is practically twice the registered case fatality rate reported in Réunion (0.96 deaths per 1000 cases of CHIKV) in 2006 [Reference Renault3, Reference Renault8]. These differences may be due to the fact that the proportion of older people in the French Antilles in 2014 was higher than that in Réunion Island in 2006.
There was no positive correlation between the increase in deaths and circulation of influenza virus, another frequent cause of increased overall mortality. There were no other phenomena in these islands that could be related to an increase in mortality during this period. There was an epidemic of dengue that began in mid-2013 and ended on March 2014 and April 2014 in Guadeloupe and Martinique, respectively, and there were no reports of other arboviruses [Reference Cassadou31, Reference Daudens-Vaysse32].
A total of 639 excess deaths were associated with CHIKV epidemic in 2014, which is four times higher than the number of CHIKV-caused deaths reported through death certificates. In the 2006 Réunion epidemic, the estimated excess was very similar to the number of deaths in which there was mention of CHIKV in death certificates as the basic or contributing cause [Reference Renault8, Reference Josseran18]. Because it was the first major epidemic in French territory, the aetiologic investigation and the detailed preparation of the death certificates may have been carried out more carefully by the health professionals. Considering that the InVS estimated the number of cases of symptomatic patients at 307 400, the estimated fatality rate was two deaths per 1000 cases, 10 times higher than the estimated fatality rate reported to PAHO by overall countries of Americas in 2014 [14]. In 2006, the fatality rate attributed to CHIKV in the Réunion Island was one death for 1000 CHIKV cases, and twice this rate was observed in the Antilles in 2014. This divergence probably was due to the difference in the age profile of the islands since the proportion of elderly people in the Antilles in 2014 was higher than the proportion of elderly people in Réunion in 2006 [Reference Renault8]. A published study evaluating excess mortality in India in 2006 posed the hypothesis that deaths could be linked to increased virulence related to mutations in the East/Central/South African lineage of CHIKV [Reference Mavalankar20]. The current study found excess mortality to be associated with an epidemic of the Asian lineage of chikungunya suggesting that increased mortality during epidemics is a characteristic of the virus and not a consequence of mutations in a specific strain.
Although some researchers still consider that the deaths caused by CHIKV are rare [Reference Rajapakse, Rodrigo and Rajapakse33], data from Martinique and Guadeloupe regions and others studies [Reference Renault3, Reference Economopoulou4, Reference Manimunda19, Reference Mavalankar20, Reference Freitas23, Reference Josseran29, Reference Cavalcanti34, Reference Brito and Teixeira35] suggest the opposite. The lack of consensus on the possibility of CHIKV leading to serious manifestations may induce physicians not to attribute deaths to CHIKV in death certificates, leading to underreporting as noted in the current study. Recent studies carried out in the Antilles have shown that the elderly present an acute clinical picture that is different from the clinical picture of young people [Reference Economopoulou4, Reference Gérardin6, Reference Godaert36]. Another explanation for the difficulty in recognising CHIKV as the cause of death is the fact that many patients who die from chikungunya are carriers of chronic diseases such as cardiovascular diseases and diabetes [Reference Economopoulou4, Reference Jean-Baptiste12, Reference Cavalcanti34] which may induce medical assistants to erroneously indicate the previous diseases as the basic cause of death on the death certificate. Many studies conducted in the Americas and other continents have shown that CHIKV can lead to severe illness and death in patients with and without previous chronic diseases and in the various age groups [Reference Economopoulou4, Reference Tandale7–Reference de la Hoz11]. Therefore, we suggest that public health authorities should be clearer in risk communication, recognising that CHIKV can manifest as severe forms and lead to death. Unclear messages from public health officials about the risk of death by CHIKV may lead the population to a false sense of security lead health professionals to neglect the potential severity of the disease by delaying hospitalisations, and discourage research in this field.
The main limitation of this study is associated with the ecological study design that is based on a temporal series of deaths with no aetiological definition of each death individually. Moreover, it is not appropriate to infer directly for the individual as the results were obtained at the population level. Other phenomena may have been responsible for this increase in overall mortality in Guadeloupe and Martinique. However, the temporal pattern and age at death are very similar to those found in epidemics that occurred in other localities, in which laboratory criteria were used to confirm cases. Our results are compatible with the association of CHIKV transmission with excess mortality and higher impact in the elderly. The lack of aetiological diagnosis of the infection may not mean that the disease does not increase risk of death. A situation similar to this is observed for influenza in which only a small number of deaths are diagnosed as such. Most influenza-related deaths result from secondary bacterial pneumonia or from decompensation of chronic diseases caused by viral infection and death is not classified as influenza on the death certificate. Thus, the impact of influenza on mortality is estimated by assessing the excess of influenza mortality [Reference Simonsen37, Reference Nielsen38]. Using similar methodology to the current study, excess deaths due to influenza among those over 65 years of age are estimated at 73 deaths per 100 000 per year [Reference Simonsen37]. The excess deaths associated with a CHIKV epidemic are 324 per 100 000 inhabitants over 60 years or 4.4 times higher than the annual average of mortality associated with influenza [Reference Simonsen37].
Mortality rate and case lethality rate are very expressive indicators for assessing disease burden and are important for use in prioritising public health investments, including vaccine production. Official documents have stated that mortality associated with CHIKV is a rare, less important event that occurs only in the elderly population and that CHIKV participates only partially (not solely) in the cause of death [39–41]. Studies in Réunion Island, India and the Americas have shown that a significant proportion of deaths occurred in chikungunya patients who had relatively common and non-severe conditions such as high blood pressure and diabetes, and even occurred in patients who did not have underlying diseases [Reference Economopoulou4, Reference Tandale7, Reference Sá and de9, Reference Crosby10, Reference Rajapakse, Rodrigo and Rajapakse33].
Between 2004 and 2015 in Guadeloupe and Martinique, 49 deaths were attributed to dengue and 171 550 cases were estimated resulting in a fatality rate of 0.29 deaths per 1000 cases [42]. According to official data from the InVS that considers death certificates, the number of deaths per CHIKV in Guadeloupe and Martinique in a single year is 3.3 times higher (160 deaths) than the accumulated dengue in 10 years and fatality rate almost twice as high (0.52 deaths per 1000 cases). The current study suggests that excess deaths associated with the CHIKV epidemic in a single year were 13 times greater than that accumulated in 10 years of dengue epidemics in the same locality, and the case fatality rate of CHIKV was 7.7 times greater than that of dengue.
Although it is not possible to make an aetiological diagnosis of all deaths associated with CHIKV infection, well-known statistical tools can contribute to an evaluation of the impact of this virus on mortality in different age groups, and these tools are currently used to assess deaths attributed to extreme weather phenomena and seasonal and pandemic influenza [Reference Nielsen38, Reference Hoshiko43, Reference Freitas and Donalisio44].
Understanding CHIKV epidemic's potential for morbidity and mortality in the most vulnerable populations may help health professionals during epidemics.







