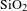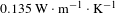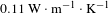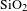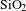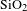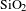1 Introduction
Antireflective (AR) coating is an important type of optical coating applied to the surface of transparent optical elements to reduce reflection. In high-power laser systems, laser-induced damage of the optical coating is the most important factor that limits the further development of high-power laser[Reference Bloembergen1]. Low laser-induced damage threshold (LIDT) not only reduces the laser beam quality, but leads to explosion of optical elements in vacuum. More and more attention is being paid to enhance LIDT of AR coating with the increase of laser power density. For this end, it is necessary to improve the surface quality of substrate and the interface energy diffusion between coating and substrate. The most frequently used methods for polishing substrate include ultrasonic cleaning, fine abrasive liquid polishing and super-polishing[Reference Bertussi, Natoli and Commandre2–Reference Shi, Shu, Dai, Peng and Li4]. On the other hand, the current experimental results indicate that microdefect and absorption of the coating are the major factors that influence the quality of coating. To reduce microdefect density and absorption, and improve LIDT of coating, researchers have developed many methods, such as laser conditioning, ion post-treatment[Reference Zhang, Fan, Cai, Shao and Fan5], effective cleaning process[Reference Field, Bellum and Kletecka6], modifying sols[Reference Shen, Li, Zhao and Tang7] and maintaining pressures[Reference Zhan, Tan, Zhang, He, Shao and Fan8] and temperature during deposition[Reference Field, Bellum and Kletecka9]. Silica AR coatings prepared by the sol–gel method have been widely used in high-power laser systems owing to its excellent optical performance, low cost, thermal mechanical properties and relatively high LIDT[Reference Xu, Wu, Sun, Huang, Jiang, Wei, Li, Dong and Wu10–Reference Li, Zou, Wu and Shen13]. The common method of improving the quality of AR coating is to modify silica sol with polyvinyl butyral (PVB)[Reference Chi, Yan, Lv, Wang and Yuan14] or polyvinylpyrrolidone (PVP)[Reference Zhang, Xu, Wu, Sun, Jiang and Wei15]. However, the addition of PVB and PVP into the silica sol decreases the porosity of coating, therefore, the refractive index of coating increases and the transmittance of coating decreases to 98%. Moreover, both of the methods are complex, and the thermal treatment of coating is needed. Hexagonal boron nitride (h-BN), a graphite-like inorganic compound composed of boron and nitrogen atoms in a hexagonal arrangement, has got recent attention[Reference Wang, Shi and Yin16, Reference Liu, Feng and Shen17]. Combining the high thermal conductivity (
![]() ${\sim}280~\text{W}\cdot \text{m}^{-1}\cdot \text{K}^{-1}$
in plane) and excellent electrical insulation, BN is a promising filler to enhance the thermal conductivity and retain the insulation of materials[Reference Gu, Zhang, Dang and Xie18]. In addition, it is also noted that BN nanosheets exhibit high optical transmittance in ultraviolet, visible and near-infrared bands[Reference Abdellaoui, Bouchikhi and Baehr19]. In recent years, many efforts have been made to fabricate low cost and high activity materials by incorporating silicon with BN[Reference Ferguson, Weimer and George20, Reference Lin, Ye and Huang21]. If BN nanosheets can be combined with silica coating, they may create a high LIDT AR coating.
${\sim}280~\text{W}\cdot \text{m}^{-1}\cdot \text{K}^{-1}$
in plane) and excellent electrical insulation, BN is a promising filler to enhance the thermal conductivity and retain the insulation of materials[Reference Gu, Zhang, Dang and Xie18]. In addition, it is also noted that BN nanosheets exhibit high optical transmittance in ultraviolet, visible and near-infrared bands[Reference Abdellaoui, Bouchikhi and Baehr19]. In recent years, many efforts have been made to fabricate low cost and high activity materials by incorporating silicon with BN[Reference Ferguson, Weimer and George20, Reference Lin, Ye and Huang21]. If BN nanosheets can be combined with silica coating, they may create a high LIDT AR coating.
2 Working principle
In this paper, BN/
![]() $\text{SiO}_{2}$
AR coating was successfully prepared by BN-incorporated
$\text{SiO}_{2}$
AR coating was successfully prepared by BN-incorporated
![]() $\text{SiO}_{2}$
sol. First, the BN nanosheets suspension was prepared by urea assisted solid exfoliation. Hexagonal boron nitride (Sinopharm Chemical, 98%) and urea (Sinopharm Chemical, 99.0%) were mixed in an agate milling container with the mass ratio of 1:60 and milled at a rotation speed of
$\text{SiO}_{2}$
sol. First, the BN nanosheets suspension was prepared by urea assisted solid exfoliation. Hexagonal boron nitride (Sinopharm Chemical, 98%) and urea (Sinopharm Chemical, 99.0%) were mixed in an agate milling container with the mass ratio of 1:60 and milled at a rotation speed of
![]() $600~\text{r}\cdot \text{min}^{-1}$
for 48 h at room temperature. After ball milling, urea was removed through dialysis with deionized water for several times. In this process, few-layer BN nanosheets can readily be dispersed in water with sonication. After the water was evaporated, the solid product was redissolved in ethanol (Sinopharm Chemical, 99.7%), forming homogeneous BN nanosheets ethanol suspension. The concentration of BN nanosheets ethanol suspension was
$600~\text{r}\cdot \text{min}^{-1}$
for 48 h at room temperature. After ball milling, urea was removed through dialysis with deionized water for several times. In this process, few-layer BN nanosheets can readily be dispersed in water with sonication. After the water was evaporated, the solid product was redissolved in ethanol (Sinopharm Chemical, 99.7%), forming homogeneous BN nanosheets ethanol suspension. The concentration of BN nanosheets ethanol suspension was
![]() $0.2~\text{mg}\cdot \text{mL}^{-1}$
. The
$0.2~\text{mg}\cdot \text{mL}^{-1}$
. The
![]() $\text{SiO}_{2}$
sol was prepared using tetraethyl orthosilicate (TEOS, ACROS, 98%) as precursor, ammonia (Sinopharm Chemical, 25%–28%) as catalyst and ethanol as solvent. The final molar ratio of
$\text{SiO}_{2}$
sol was prepared using tetraethyl orthosilicate (TEOS, ACROS, 98%) as precursor, ammonia (Sinopharm Chemical, 25%–28%) as catalyst and ethanol as solvent. The final molar ratio of
![]() $\text{SiO}_{2}$
sol was TEOS/ammonia/
$\text{SiO}_{2}$
sol was TEOS/ammonia/
![]() $\text{H}_{2}$
O/ethanol
$\text{H}_{2}$
O/ethanol
![]() $=$
1:0.57:2.45:38. The
$=$
1:0.57:2.45:38. The
![]() $\text{SiO}_{2}$
sol was stirred for 24 h and then aged for more than 7 days at room temperature. The BN/
$\text{SiO}_{2}$
sol was stirred for 24 h and then aged for more than 7 days at room temperature. The BN/
![]() $\text{SiO}_{2}$
sols were prepared by adding BN nanosheets ethanol suspension into
$\text{SiO}_{2}$
sols were prepared by adding BN nanosheets ethanol suspension into
![]() $\text{SiO}_{2}$
sol to form a mixture with 10wt% or 20wt% of BN nanosheets ethanol suspension. Then the BN/
$\text{SiO}_{2}$
sol to form a mixture with 10wt% or 20wt% of BN nanosheets ethanol suspension. Then the BN/
![]() $\text{SiO}_{2}$
sols were sonicated for 20 min at room temperature. The final sols were accordingly marked as 10wt% BN/
$\text{SiO}_{2}$
sols were sonicated for 20 min at room temperature. The final sols were accordingly marked as 10wt% BN/
![]() $\text{SiO}_{2}$
sol and 20wt% BN/
$\text{SiO}_{2}$
sol and 20wt% BN/
![]() $\text{SiO}_{2}$
sol, the concentration of BN nanosheets was
$\text{SiO}_{2}$
sol, the concentration of BN nanosheets was
![]() $0.02~\text{mg}\cdot \text{mL}^{-1}$
in 10wt% BN/
$0.02~\text{mg}\cdot \text{mL}^{-1}$
in 10wt% BN/
![]() $\text{SiO}_{2}$
sol and
$\text{SiO}_{2}$
sol and
![]() $0.04~\text{mg}\cdot \text{mL}^{-1}$
in 20wt% BN/
$0.04~\text{mg}\cdot \text{mL}^{-1}$
in 20wt% BN/
![]() $\text{SiO}_{2}$
sol. The BN/
$\text{SiO}_{2}$
sol. The BN/
![]() $\text{SiO}_{2}$
AR coatings were deposited via dip-coating on well-polished and well-cleaned fused silica (Heraeus 312) substrates which were named
$\text{SiO}_{2}$
AR coatings were deposited via dip-coating on well-polished and well-cleaned fused silica (Heraeus 312) substrates which were named
![]() $\text{SiO}_{2}$
coating, 10wt% BN/
$\text{SiO}_{2}$
coating, 10wt% BN/
![]() $\text{SiO}_{2}$
coating and 20wt% BN/
$\text{SiO}_{2}$
coating and 20wt% BN/
![]() $\text{SiO}_{2}$
coating.
$\text{SiO}_{2}$
coating.

Figure 1. (a) Optical absorption of BN nanosheets dispersion in ethanol. Inset: photographs of BN nanosheets dispersion in water (left) and ethanol (right). (b) XRD patterns of BN nanosheets we used here and h-BN bulky material. Inset: the curves in the region of
![]() $37^{\circ }$
–
$37^{\circ }$
–
![]() $57^{\circ }$
. (c) TEM and (d) HRTEM images of BN nanosheets.
$57^{\circ }$
. (c) TEM and (d) HRTEM images of BN nanosheets.
The exfoliation process assisted by urea generated functional amino groups at defective sites and edges of BN (002) planes that made the BN nanosheets suspension more stable[Reference Lei, Mochalin, Liu, Qin, Gogotsi and Chen22]. The left inset of Figure 1(a) shows a milky appearance of BN nanosheets water suspension, the concentration up to
![]() $1.0~\text{mg}\cdot \text{mL}^{-1}$
. After replacing the water with ethanol, the BN nanosheets ethanol suspension was very stable over several months under ambient conditions in a sealed bottle. In the right inset of Figure 1(a), the BN nanosheets ethanol suspension displays an obvious Tyndall effect due to the light scattering by BN nanosheets. Figure 1(a) also shows the distinct absorption peak at 204 nm (6.08 eV) which is attributed to the intrinsic exciton absorption band of BN nanosheets. The structure of BN nanosheets was also investigated by X-ray diffraction (XRD, Bruker D2). As shown in Figure 1(b), five peaks can be observed at
$1.0~\text{mg}\cdot \text{mL}^{-1}$
. After replacing the water with ethanol, the BN nanosheets ethanol suspension was very stable over several months under ambient conditions in a sealed bottle. In the right inset of Figure 1(a), the BN nanosheets ethanol suspension displays an obvious Tyndall effect due to the light scattering by BN nanosheets. Figure 1(a) also shows the distinct absorption peak at 204 nm (6.08 eV) which is attributed to the intrinsic exciton absorption band of BN nanosheets. The structure of BN nanosheets was also investigated by X-ray diffraction (XRD, Bruker D2). As shown in Figure 1(b), five peaks can be observed at
![]() $26.4^{\circ }$
,
$26.4^{\circ }$
,
![]() $41.4^{\circ }$
,
$41.4^{\circ }$
,
![]() $43.5^{\circ }$
,
$43.5^{\circ }$
,
![]() $49.0^{\circ }$
and
$49.0^{\circ }$
and
![]() $54.6^{\circ }$
, corresponding to the (002), (100), (101), (102) and (004) planes of h-BN. Clearly, the (002) and (004) diffraction peaks of BN nanosheets became much stronger compared with the (100), (101), (102) peaks, which might be attributed to the enhanced exposure of (002) and (004) planes upon exfoliation, indicating BN nanosheets were peeled off along the (002) and (004) planes without considerable destroy of crystalline structure. The morphology of BN nanosheets was tested by a transmission electron microscope (HRTEM, JEOL-2100). TEM image of BN nanosheets shows that the BN nanosheets were flat and quite thin and the lateral sizes were 500–1000 nm, suggesting that the BN nanosheets remain intact and are not broken down by ball exfoliation. From Figure 1(d), HRTEM image at the edges of BN nanosheets clearly shows the thicknesses of BN nanosheets were significantly reduced. The layer numbers of obtained BN nanosheets were 2–10, corresponding to the thickness of 1–4 nm. Incorporation of such thinner BN nanosheets into
$54.6^{\circ }$
, corresponding to the (002), (100), (101), (102) and (004) planes of h-BN. Clearly, the (002) and (004) diffraction peaks of BN nanosheets became much stronger compared with the (100), (101), (102) peaks, which might be attributed to the enhanced exposure of (002) and (004) planes upon exfoliation, indicating BN nanosheets were peeled off along the (002) and (004) planes without considerable destroy of crystalline structure. The morphology of BN nanosheets was tested by a transmission electron microscope (HRTEM, JEOL-2100). TEM image of BN nanosheets shows that the BN nanosheets were flat and quite thin and the lateral sizes were 500–1000 nm, suggesting that the BN nanosheets remain intact and are not broken down by ball exfoliation. From Figure 1(d), HRTEM image at the edges of BN nanosheets clearly shows the thicknesses of BN nanosheets were significantly reduced. The layer numbers of obtained BN nanosheets were 2–10, corresponding to the thickness of 1–4 nm. Incorporation of such thinner BN nanosheets into
![]() $\text{SiO}_{2}$
sols may have little influence on transmittance of
$\text{SiO}_{2}$
sols may have little influence on transmittance of
![]() $\text{SiO}_{2}$
AR coating.
$\text{SiO}_{2}$
AR coating.

Figure 2. (a) Raman-mapping image of 10wt% BN/
![]() $\text{SiO}_{2}$
coating: green dots are BN nanosheets. (b) The Raman spectrum of area (1) in the mapping. (c) TEM image of 10wt% BN/
$\text{SiO}_{2}$
coating: green dots are BN nanosheets. (b) The Raman spectrum of area (1) in the mapping. (c) TEM image of 10wt% BN/
![]() $\text{SiO}_{2}$
sol. Inset: TEM image of
$\text{SiO}_{2}$
sol. Inset: TEM image of
![]() $\text{SiO}_{2}$
particles. (d) HRTEM image of BN nanosheets in the 10wt% BN/
$\text{SiO}_{2}$
particles. (d) HRTEM image of BN nanosheets in the 10wt% BN/
![]() $\text{SiO}_{2}$
sol.
$\text{SiO}_{2}$
sol.
The detailed dispersion of BN nanosheets in the BN/
![]() $\text{SiO}_{2}$
coating was characterized by Raman spectroscopy mapping technology (RAMANforce, Nanophoton Corporation) at a laser wavelength of 532 nm. Figure 2(a) shows the Raman mapping of 10wt% BN/
$\text{SiO}_{2}$
coating was characterized by Raman spectroscopy mapping technology (RAMANforce, Nanophoton Corporation) at a laser wavelength of 532 nm. Figure 2(a) shows the Raman mapping of 10wt% BN/
![]() $\text{SiO}_{2}$
coating and the green dots display the locations of BN nanosheets and indicates the BN nanosheets are uniformly distributed in the BN/
$\text{SiO}_{2}$
coating and the green dots display the locations of BN nanosheets and indicates the BN nanosheets are uniformly distributed in the BN/
![]() $\text{SiO}_{2}$
coating. Figure 2(b) shows the Raman spectrum of area (1) in the mapping image. Only the typical peak of
$\text{SiO}_{2}$
coating. Figure 2(b) shows the Raman spectrum of area (1) in the mapping image. Only the typical peak of
![]() $\text{E}_{\text{2g}}$
mode can be found at
$\text{E}_{\text{2g}}$
mode can be found at
![]() ${\sim}1366~\text{cm}^{-1}$
, indicating few-layer BN nanosheets were present in the BN/
${\sim}1366~\text{cm}^{-1}$
, indicating few-layer BN nanosheets were present in the BN/
![]() $\text{SiO}_{2}$
coating. Figure 2(c) shows the TEM image of 10wt% BN/
$\text{SiO}_{2}$
coating. Figure 2(c) shows the TEM image of 10wt% BN/
![]() $\text{SiO}_{2}$
sol, in which the
$\text{SiO}_{2}$
sol, in which the
![]() $\text{SiO}_{2}$
particles were uniformly distributed on the BN nanosheets. That is consistent with the Raman-mapping result. The inset in Figure 2(c) shows the uniform particle size distribution of
$\text{SiO}_{2}$
particles were uniformly distributed on the BN nanosheets. That is consistent with the Raman-mapping result. The inset in Figure 2(c) shows the uniform particle size distribution of
![]() $\text{SiO}_{2}$
particles with diameter of about 10 nm, which determined the antireflection at 351 nm wavelength of BN/
$\text{SiO}_{2}$
particles with diameter of about 10 nm, which determined the antireflection at 351 nm wavelength of BN/
![]() $\text{SiO}_{2}$
coating[Reference Bazin, Andrew and Mcinnes23]. Figure 2(d) shows the HRTEM image of the 10wt% BN/
$\text{SiO}_{2}$
coating[Reference Bazin, Andrew and Mcinnes23]. Figure 2(d) shows the HRTEM image of the 10wt% BN/
![]() $\text{SiO}_{2}$
sol, in which the BN nanosheets can be clearly seen between several
$\text{SiO}_{2}$
sol, in which the BN nanosheets can be clearly seen between several
![]() $\text{SiO}_{2}$
particles.
$\text{SiO}_{2}$
particles.

Figure 3. AFM images of (a) bare fused silica substrate, (b)
![]() $\text{SiO}_{2}$
coating, (c) 10wt% BN/
$\text{SiO}_{2}$
coating, (c) 10wt% BN/
![]() $\text{SiO}_{2}$
coating and (d) 20wt% BN/
$\text{SiO}_{2}$
coating and (d) 20wt% BN/
![]() $\text{SiO}_{2}$
coating.
$\text{SiO}_{2}$
coating.
The surface morphology of BN/
![]() $\text{SiO}_{2}$
coating was confirmed by atomic-force microscopy (AFM) (Innova, Bruker). In order to obtain the more precise roughness values, each coating was measured at ten points of random selection and finally the average was taken. Figure 3 shows the AFM images of bare fused silica substrate,
$\text{SiO}_{2}$
coating was confirmed by atomic-force microscopy (AFM) (Innova, Bruker). In order to obtain the more precise roughness values, each coating was measured at ten points of random selection and finally the average was taken. Figure 3 shows the AFM images of bare fused silica substrate,
![]() $\text{SiO}_{2}$
coating, 10wt% BN/
$\text{SiO}_{2}$
coating, 10wt% BN/
![]() $\text{SiO}_{2}$
coating and 20wt% BN/
$\text{SiO}_{2}$
coating and 20wt% BN/
![]() $\text{SiO}_{2}$
coating which were randomly selected from obtained images. The root-mean-square roughness (
$\text{SiO}_{2}$
coating which were randomly selected from obtained images. The root-mean-square roughness (
![]() $R_{q}$
) values of coatings were calculated using the AFM images. As BN nanosheets were incorporated into
$R_{q}$
) values of coatings were calculated using the AFM images. As BN nanosheets were incorporated into
![]() $\text{SiO}_{2}$
sols, the
$\text{SiO}_{2}$
sols, the
![]() $R_{q}$
values show a small increase for 10wt% BN/
$R_{q}$
values show a small increase for 10wt% BN/
![]() $\text{SiO}_{2}$
coating and 20wt% BN/
$\text{SiO}_{2}$
coating and 20wt% BN/
![]() $\text{SiO}_{2}$
coating. Because the
$\text{SiO}_{2}$
coating. Because the
![]() $R_{q}$
values of all the BN/
$R_{q}$
values of all the BN/
![]() $\text{SiO}_{2}$
coatings were less than 2 nm, the surface scattering of coating was small enough to avoid additional decrease in transmittance. Meanwhile, there is little difference in
$\text{SiO}_{2}$
coatings were less than 2 nm, the surface scattering of coating was small enough to avoid additional decrease in transmittance. Meanwhile, there is little difference in
![]() $R_{q}$
between 10wt% BN/
$R_{q}$
between 10wt% BN/
![]() $\text{SiO}_{2}$
coating and 20wt% BN/
$\text{SiO}_{2}$
coating and 20wt% BN/
![]() $\text{SiO}_{2}$
coating, which indicates the lateral sizes and thicknesses of BN nanosheets were suitable for preparing BN/
$\text{SiO}_{2}$
coating, which indicates the lateral sizes and thicknesses of BN nanosheets were suitable for preparing BN/
![]() $\text{SiO}_{2}$
AR coating. Both AFM and TEM results were mutually complementary and offered a very detailed analysis of BN/
$\text{SiO}_{2}$
AR coating. Both AFM and TEM results were mutually complementary and offered a very detailed analysis of BN/
![]() $\text{SiO}_{2}$
coatings.
$\text{SiO}_{2}$
coatings.

Figure 4. Transmittance of
![]() $\text{SiO}_{2}$
coating, 10wt% BN/
$\text{SiO}_{2}$
coating, 10wt% BN/
![]() $\text{SiO}_{2}$
coating and 20wt% BN/
$\text{SiO}_{2}$
coating and 20wt% BN/
![]() $\text{SiO}_{2}$
coating. The inset is the photograph of 10wt% BN/
$\text{SiO}_{2}$
coating. The inset is the photograph of 10wt% BN/
![]() $\text{SiO}_{2}$
coating on fused silica substrate to show the high transmittance.
$\text{SiO}_{2}$
coating on fused silica substrate to show the high transmittance.
In order to investigate the optical property of BN/
![]() $\text{SiO}_{2}$
AR coating, fused silica substrates were coated by dipping into the
$\text{SiO}_{2}$
AR coating, fused silica substrates were coated by dipping into the
![]() $\text{SiO}_{2}$
sol, 10wt% BN/
$\text{SiO}_{2}$
sol, 10wt% BN/
![]() $\text{SiO}_{2}$
sol and 20wt% BN/
$\text{SiO}_{2}$
sol and 20wt% BN/
![]() $\text{SiO}_{2}$
sol. The refractive index and thickness of AR coatings were calculated using a spectroscopic ellipsometer (SC620, Sanco) at
$\text{SiO}_{2}$
sol. The refractive index and thickness of AR coatings were calculated using a spectroscopic ellipsometer (SC620, Sanco) at
![]() $60^{\circ }$
incidence, and listed in Table 1. The optical thickness of coating was controlled to match the desired antireflective wavelength 351 nm by adjusting the dipping speed. The optical transmittance results were measured by a UV–Vis–NIR spectrometer (U-4100, Hitachi) in the wavelength range from 200 to 800 nm. Figure 4 shows the transmittance spectra of
$60^{\circ }$
incidence, and listed in Table 1. The optical thickness of coating was controlled to match the desired antireflective wavelength 351 nm by adjusting the dipping speed. The optical transmittance results were measured by a UV–Vis–NIR spectrometer (U-4100, Hitachi) in the wavelength range from 200 to 800 nm. Figure 4 shows the transmittance spectra of
![]() $\text{SiO}_{2}$
coating, 10wt% BN/
$\text{SiO}_{2}$
coating, 10wt% BN/
![]() $\text{SiO}_{2}$
coating and 20wt% BN/
$\text{SiO}_{2}$
coating and 20wt% BN/
![]() $\text{SiO}_{2}$
coating. As shown in Table 1, the maximum transmittances of
$\text{SiO}_{2}$
coating. As shown in Table 1, the maximum transmittances of
![]() $\text{SiO}_{2}$
coating, 10wt% BN/
$\text{SiO}_{2}$
coating, 10wt% BN/
![]() $\text{SiO}_{2}$
coating and 20wt% BN/
$\text{SiO}_{2}$
coating and 20wt% BN/
![]() $\text{SiO}_{2}$
coating are all over 99.8% at 351 nm, which is an excellent antireflective performance for applying in high-power laser systems. Almost the same transmittance of coatings is mainly due to the high optical transmittance of BN nanosheets in ultraviolet, visible and near-infrared bands and the small concentration of BN nanosheets in sol which was too small to affect the transmittance of coating. The digital image of 10wt% BN/
$\text{SiO}_{2}$
coating are all over 99.8% at 351 nm, which is an excellent antireflective performance for applying in high-power laser systems. Almost the same transmittance of coatings is mainly due to the high optical transmittance of BN nanosheets in ultraviolet, visible and near-infrared bands and the small concentration of BN nanosheets in sol which was too small to affect the transmittance of coating. The digital image of 10wt% BN/
![]() $\text{SiO}_{2}$
coating on fused silica substrate is shown in Figure 4, which indicates a significantly improved transmittance of BN/
$\text{SiO}_{2}$
coating on fused silica substrate is shown in Figure 4, which indicates a significantly improved transmittance of BN/
![]() $\text{SiO}_{2}$
coating.
$\text{SiO}_{2}$
coating.

Figure 5. Laser-induced damage morphologies of AR coatings (a)
![]() $\text{SiO}_{2}$
coating, (b) 10wt% BN/
$\text{SiO}_{2}$
coating, (b) 10wt% BN/
![]() $\text{SiO}_{2}$
coating and (c) 20wt% BN/
$\text{SiO}_{2}$
coating and (c) 20wt% BN/
![]() $\text{SiO}_{2}$
coating.
$\text{SiO}_{2}$
coating.
Table 1. Optical parameters of BN/
![]() $\text{SiO}_{2}$
coatings with various BN nanosheets mass ratios.
$\text{SiO}_{2}$
coatings with various BN nanosheets mass ratios.

![]() $n_{\!f}$
is the refractive index of coating;
$n_{\!f}$
is the refractive index of coating;
![]() $T_{\text{max}}$
is the maximum transmittance at 351 nm;
$T_{\text{max}}$
is the maximum transmittance at 351 nm;
![]() $R_{q}$
is the root-mean-square roughness value of coating; LIDT means laser-induced damage threshold with 6.60 ns pulse of 351 nm laser.
$R_{q}$
is the root-mean-square roughness value of coating; LIDT means laser-induced damage threshold with 6.60 ns pulse of 351 nm laser.
Laser-induced damage threshold test was performed via the ‘R-on-1’ mode according to ISO standard 11254-2: 2000, using a 351 nm pulse laser with a pulse width of 6.6 ns and the laser spot area of
![]() $1.02~\text{mm}^{2}$
. The LIDT data of coatings are shown in Table 1. There was a small increase in the threshold from 8.69 to
$1.02~\text{mm}^{2}$
. The LIDT data of coatings are shown in Table 1. There was a small increase in the threshold from 8.69 to
![]() $8.92~\text{J}\cdot \text{cm}^{-2}$
as the traditional
$8.92~\text{J}\cdot \text{cm}^{-2}$
as the traditional
![]() $\text{SiO}_{2}$
coating was deposited on the fused silica substrate. Under laser irradiation, the coating was destroyed when the surface temperature increased to the melting point after the laser energy was absorbed by coating. The LIDT result of 10wt% BN/
$\text{SiO}_{2}$
coating was deposited on the fused silica substrate. Under laser irradiation, the coating was destroyed when the surface temperature increased to the melting point after the laser energy was absorbed by coating. The LIDT result of 10wt% BN/
![]() $\text{SiO}_{2}$
coating is significantly higher than that of bare
$\text{SiO}_{2}$
coating is significantly higher than that of bare
![]() $\text{SiO}_{2}$
coating on fused silica substrate, especially the 10wt% BN/
$\text{SiO}_{2}$
coating on fused silica substrate, especially the 10wt% BN/
![]() $\text{SiO}_{2}$
coating shows 23.1% increase of LIDT without any other treatment. The enhanced LIDT of 20wt% BN/
$\text{SiO}_{2}$
coating shows 23.1% increase of LIDT without any other treatment. The enhanced LIDT of 20wt% BN/
![]() $\text{SiO}_{2}$
coating is only
$\text{SiO}_{2}$
coating is only
![]() $8.96~\text{J}\cdot \text{cm}^{-2}$
, which is much smaller than that of 10wt% BN/
$8.96~\text{J}\cdot \text{cm}^{-2}$
, which is much smaller than that of 10wt% BN/
![]() $\text{SiO}_{2}$
coating.
$\text{SiO}_{2}$
coating.
In addition to the LIDT, damage morphology of coating was tested to analyze the laser damage mechanism of coating. Figure 5 shows the laser-induced damage morphology of
![]() $\text{SiO}_{2}$
coating, 10wt% BN/
$\text{SiO}_{2}$
coating, 10wt% BN/
![]() $\text{SiO}_{2}$
coating and 20wt% BN/
$\text{SiO}_{2}$
coating and 20wt% BN/
![]() $\text{SiO}_{2}$
coating. As can be seen from Figures 5(a) and 5(b), the damage area of 10wt% BN/
$\text{SiO}_{2}$
coating. As can be seen from Figures 5(a) and 5(b), the damage area of 10wt% BN/
![]() $\text{SiO}_{2}$
coating is smaller than that of the bare
$\text{SiO}_{2}$
coating is smaller than that of the bare
![]() $\text{SiO}_{2}$
coating, which indicates the 10wt% BN/
$\text{SiO}_{2}$
coating, which indicates the 10wt% BN/
![]() $\text{SiO}_{2}$
coating suffered the milder damage[Reference Chi, Yan, Lv, Wang and Yuan14]. Seen from Figure 5(c), there are some tiny damage dots on the surface of 20wt% BN/
$\text{SiO}_{2}$
coating suffered the milder damage[Reference Chi, Yan, Lv, Wang and Yuan14]. Seen from Figure 5(c), there are some tiny damage dots on the surface of 20wt% BN/
![]() $\text{SiO}_{2}$
coating. This may be attributed to plenty of BN nanosheets in coating, the more BN nanosheets in coating, the worse adhesion of coating would be.
$\text{SiO}_{2}$
coating. This may be attributed to plenty of BN nanosheets in coating, the more BN nanosheets in coating, the worse adhesion of coating would be.
As an excellent thermally conductive material, BN has been used to prepare electron-insulative and thermally conductive composites. Sato et al.
[Reference Sato, Horibe, Shirai, Hotta, Nakano, Nagai, Mitsuishi and Watari24] prepared an h-BN/polyimide composite film with high thermal conductivity up to
![]() $7~\text{W}\cdot \text{m}^{-1}\cdot \text{K}^{-1}$
, in which the active edge planes of h-BN filler most probably interacted chemically with the surrounding polyimide matrix. The enhanced interactions resulted in fewer flaws, coherent interfaces and minimal interfacial phonon scattering. Zhou and co-workers[Reference Zhou, Qi, Zhao and Liu25] reported a thermally conductive silicone rubber used BN powder as conductive fillers. The inclusion of BN increases both thermal conductivity and thermal stability, while decreases the coefficient of thermal expansion. Cho et al.
[Reference Cho, Tokoi, Tanaka, Suematsu, Suzuki, Jiang, Niihara and Nakayama26] reported a facile technique that modified BN nanosheets with iron oxides in order to fabricate highly oriented polysiloxane/BN nanosheet composite films. The thermal property of polysiloxane/BN nanosheet composite film was determined by the orientation of BN, and the thermal conductivity of composite film was enhanced by 20 times after addition of BN nanosheets.
$7~\text{W}\cdot \text{m}^{-1}\cdot \text{K}^{-1}$
, in which the active edge planes of h-BN filler most probably interacted chemically with the surrounding polyimide matrix. The enhanced interactions resulted in fewer flaws, coherent interfaces and minimal interfacial phonon scattering. Zhou and co-workers[Reference Zhou, Qi, Zhao and Liu25] reported a thermally conductive silicone rubber used BN powder as conductive fillers. The inclusion of BN increases both thermal conductivity and thermal stability, while decreases the coefficient of thermal expansion. Cho et al.
[Reference Cho, Tokoi, Tanaka, Suematsu, Suzuki, Jiang, Niihara and Nakayama26] reported a facile technique that modified BN nanosheets with iron oxides in order to fabricate highly oriented polysiloxane/BN nanosheet composite films. The thermal property of polysiloxane/BN nanosheet composite film was determined by the orientation of BN, and the thermal conductivity of composite film was enhanced by 20 times after addition of BN nanosheets.
Here, BN nanosheets played an important role in enhancing the diffusion of heat because of its extremely high in-plane thermal conductivity and insulation, but low coefficient of thermal expansion and superior thermal stability[Reference Zhou, Qi, An, Zhao and Liu27, Reference Williams28]. Because traditional basic silica coating was porous, the absorbed thermal energy cannot effectively transfer through the separated silica particles and the surrounding voids. BN nanosheets have good thermal conductivity, so the thermal conductivity of BN/
![]() $\text{SiO}_{2}$
coating must be higher than that of the traditional basic silica coating. With the laser radiation, the BN/
$\text{SiO}_{2}$
coating must be higher than that of the traditional basic silica coating. With the laser radiation, the BN/
![]() $\text{SiO}_{2}$
coating has good slow-release ability to the local strain caused by the absorbed laser energy. A laser thermal conductivity testing instrument (NanoTR, NETZSCH) was used to evaluate the thermal conductivity of
$\text{SiO}_{2}$
coating has good slow-release ability to the local strain caused by the absorbed laser energy. A laser thermal conductivity testing instrument (NanoTR, NETZSCH) was used to evaluate the thermal conductivity of
![]() $\text{SiO}_{2}$
coating and 10wt% BN/
$\text{SiO}_{2}$
coating and 10wt% BN/
![]() $\text{SiO}_{2}$
coating. The thermal conductivity coefficient of 10wt% BN/
$\text{SiO}_{2}$
coating. The thermal conductivity coefficient of 10wt% BN/
![]() $\text{SiO}_{2}$
coating is
$\text{SiO}_{2}$
coating is
![]() $0.135~\text{W}\cdot \text{m}^{-1\!}\cdot \text{K}^{-1}$
, 23% higher than
$0.135~\text{W}\cdot \text{m}^{-1\!}\cdot \text{K}^{-1}$
, 23% higher than
![]() $0.110~\text{W}\cdot \text{m}^{-1\!}\cdot \text{K}^{-1}$
of bare
$0.110~\text{W}\cdot \text{m}^{-1\!}\cdot \text{K}^{-1}$
of bare
![]() $\text{SiO}_{2}$
coating, which was consistent with the increased LIDT.
$\text{SiO}_{2}$
coating, which was consistent with the increased LIDT.
In summary, the antireflective BN/
![]() $\text{SiO}_{2}$
coatings have been successfully prepared through incorporating BN nanosheets into traditional basic silica sol without any additional treatment. The BN/
$\text{SiO}_{2}$
coatings have been successfully prepared through incorporating BN nanosheets into traditional basic silica sol without any additional treatment. The BN/
![]() $\text{SiO}_{2}$
coatings revealed very promising antireflective properties at 351 nm and also enhanced LIDT performance. This is a good candidate for the future application of AR coating in optical components for a high-power laser system. In contrast with other modifying silica sol methods, this work has very low cost and simple process. By optimizing the content of BN nanosheets, coating material, and other design parameters, the antilaser-damage ability may be further enhanced.
$\text{SiO}_{2}$
coatings revealed very promising antireflective properties at 351 nm and also enhanced LIDT performance. This is a good candidate for the future application of AR coating in optical components for a high-power laser system. In contrast with other modifying silica sol methods, this work has very low cost and simple process. By optimizing the content of BN nanosheets, coating material, and other design parameters, the antilaser-damage ability may be further enhanced.
Acknowledgement
This work was supported by National Natural Science Foundation of China (Nos. U1530148 and 61605188).