At present there is an increasing interest in the capacity of food and food components to reduce the risk of non-infectious diseases related to diet. Dietary fibres have gained a considerable interest due to their favourable health effects. According to recent studies it has been proven that dietary fibre intake is inversely correlated with several CVD factors, which supports its protective role against cardiovascular diseases and recommendations for its increased consumption (Lairon et al. Reference Lairon, Arnault, Bertrais, Planells, Clero, Hercberg and Boutron-Ruault2005; Erkkila & Lichtenstein, Reference Erkkila and Lichtenstein2006).
From past studies it has been shown that some plant fibres have a hypocholesterolaemic effect, which may be due to fibre-induced alterations of intestinal absorption, intestinal or pancreatic hormone secretion, lipoprotein metabolism, bile acid metabolism, or fermentation by-products and their effects on hepatic cholesterol synthesis (Kay, Reference Kay1982). When animal dietary fibres are concerned, relatively few studies have been carried out to investigate the effect on lipid metabolism. Normally, it is believed that those who are at risk of CHD should consume less red meat due to adverse health impacts.
Bovine Achilles' tendons and arteries are waste products of the beef industry that could be used as a potential source of functional protein for possible food applications due to their favourable amino acid composition and indigestible behaviour. This hypothesis may be supported by previous findings showing that dietary proteins with favourable amino acid composition reduced serum cholesterol level in rats (Morita et al. Reference Morita, Oh-hashi, Takei, Ikai, Kasaoka and Kiriyama1997; Gudbrandsen et al. Reference Gudbrandsen, Wergedahl, Liaset, Espe and Berge2005). The indigestible behaviour of animal protein may be due to the abundant collagen concentration, as Achilles' tendons and artery protein contain high glycine and proline concentrations (Schubert et al. Reference Schubert, Harris, Devine and Heinemann1974). In the present study, we investigated the effect of some bovine dietary proteins on serum lipids, liver lipids, faecal lipids, bacterial population in the colon, and hepatic mRNA in rats.
Experimental methods
Animals and diets
Male Crj/Wistar rats (5 weeks old) were purchased from Charles River Japan Inc. (Yokohama, Japan). They were housed individually in cages with free access to food and water. The animal facility was maintained on a 12 h light–dark cycle at a temperature of 23 ± 1°C and relative humidity of 60 ± 5 %. The rats were randomly assigned to three groups (n 5). There were no significant differences in body weight and serum total cholesterol concentration among groups at the beginning of the experiment. The composition of each diet is shown in Table 1. The experimental rats were fed for 4 weeks, with a 20 % casein diet (CON), in comparison with two diets containing 15 % casein and 5 % of either bovine Achilles' tendons (AC) or artery (AR) protein preparations. The AC and AR proteins were prepared as follows; first, raw bovine Achilles’ tendons and arteries were defatted by washing and then boiling for 2 h. The boiled Achilles' tendons and arteries were freeze-dried and milled at 300 mesh. The compositions of AC and AR were as follows (g/100g dry weight): moisture, 2·1 and 4·1; protein, 73·9 and 81·0; lipid, 23·1 and 8·4; carbohydrate, 0·5 and 5·9; ash, 0·4 and 0·6, respectively. Total moisture, protein, lipid and carbohydrate content were determined by the procedure of the Association of Official Analytical Chemists (1990). Dietary cholesterol level in the AC and AR was determined (g/kg diet), and it was 0·1 (0·01 %) and 0·06 (0·006 %), respectively. The control group consisted of rats fed 200 g casein/kg. The amino acid compositions of the AC, AR and CON are shown in Table 2. The amino acid compositions were determined as follows; first 4 mg of AC and AR samples were hydrolysed in 2 ml of 6 m-HCl at 110°C for 24 h, vacuum dried and then reconstituted with 1 ml of 0·2 m-HCl, filtered with 0·45 μm diameter filter (W-13-5; Tosoh, Tokyo, Japan) and analysed with a Hitachi-8700 amino acid analyzer (Hitachi Ltd, Tokyo, Japan). The rats were allowed free access to food and water for 4 weeks. Body weight and food consumption were recorded weekly and daily, respectively. This experimental design was approved by the Animal Experiment Committee of Obihiro University of Agriculture and Veterinary Medicine. All animal procedures conformed to standard principles described in Guide for the Care and Use of Laboratory Animals (National Research Council, 1985).
Table 1 Composition of the experimental diets (g/kg diet)
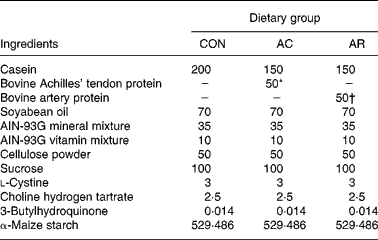
CON, casein control diet; AC, bovine Achilles’ tendon diet; AR, bovine artery diet.
* Cholesterol 0·1 g/kg diet.
† Cholesterol 0·06 g/kg diet.
Table 2 Amino acid composition of bovine proteins (mmol/g nitrogen)

CON, casein; AC, bovine Achilles’ tendon powder; AR, bovine artery powder.
Analytical procedures
Blood samples (1 ml) were collected between 08.00 and 10.00 hours from the jugular veins of fasting rats anaesthetised by sodium pentobarbital. The samples were taken into tubes without an anticoagulant. After the samples were allowed to stand at room temperature for 2 h, the sera were separated by centrifugation at 1500 g for 20 min. All faecal excretions were collected during the last 3 d of the experimental period (4 weeks). Faecal dry weight did not differ among groups. The rats were killed by diethyl ether inhalation, and the livers and caecum quickly removed, washed with cold saline (9 g NaCl/l), blotted dry on filter paper, and weighed before freezing for storage.
Chemical analysis
Total cholesterol, HDL-cholesterol, and TAG concentrations in the serum were determined enzymically using commercially available reagent kits (assay kits for the TDX system; Abbott Laboratory Co., Irving, TX, USA). The non-HDL-cholesterol concentration was calculated as follows: non-HDL-cholesterol = total cholesterol − HDL-cholesterol.
Total lipids were extracted from liver and faeces by a mixture of chloroform–methanol (2:1, v/v) (Folch et al. Reference Folch, Lees and Sloane Stanley1957). The neutral steroids in each lipid sample obtained by saponification were acetylated (Matsubara et al. Reference Matsubara, Sawabe and Iizuka1990) and analysed by GLC using a Shimadzu 14A chromatograph (Kyoto, Japan) with a DB17 capillary column (0·25 mm × 30 m; J&W Scientific, Folsom, CA, USA) with N2 as the carrier gas. Acidic sterols in faeces were measured by GLC following the method of Grundy et al. (Reference Grundy, Ahrens and Miettinen1965). Sterol balance was calculated as sterol balance = (faecal cholesterol + faecal coprostanol+faecal bile acids) – dietary cholesterol intake. A part of the caecal content was taken out into desalting water in a vial without exposure to air, and suspended. The suspension of caecum was deproteinised with perchloric acid and to form Na salts of the SCFA. Individual SCFA were measured by GLC with a glass column (2000 × 3 mm) packed with 80–100 mesh chromosorb W-AW DMCS with H3PO4 (100 ml/l) as the liquid phase after adding H3PO4 according to the procedure of Hara et al. (Reference Hara, Saito, Nakashima and Kiriyama1994). Faecal N content was determined by Kjeldahl's method. Apparent digestibility of protein was calculated as apparent protein digestibility = (protein intake – faecal protein)/protein intake × 100.
Ribonucleic acid isolation, reverse transcription-polymerase chain reaction and Southern blot analysis
Total RNA was isolated from the liver by the acid guanidium–phenol–choloroform method using Isogen (Nippon Gene, Tokyo, Japan; Chomczynski & Sacchi, Reference Chomczynski and Sacchi1987). mRNA encoding the LDL receptor (LDL-R), cholesterol 7α-hydroxylase (CYP7A1), fatty acid synthase (FAS), hydroxyl methyl glutaryl (HMG)-CoA reductase, scavenger receptor type B1 (SR-B1), sterol regulatory elementary binding protein (SREBP-1c), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; used as an invariant control) were analysed by semi-quantitative RT-PCR and subsequent Southern hybridisation of the PCR products with each inner oligonucleotide probe. Total RNA samples were treated with DNase RQ1 (Promega, Madison, WI, USA) to remove genomic DNA and subjected to RT-PCR by using Moloney murine leukaemia virus RT (Gibco-BRL, Gaithersburg, MD, USA) and EX-Taq polymerase (Takara, Tokyo, Japan) with LDL-R primers of oligonucleotides (upstream primer, 5′-ATTTTGGAGGATGAGAAGCAG-3′; downstream primer, 5′-CAGGGCGGGGAGTGTGAGAA-3′), CYP7A1 primers of oligonucleotides (upstream primer, 5′-GCCGTCCAAGAAATCAAGCAGT-3′; downstream primer, 5′-TGTGGGCAGCGAGAACAAAGT-3′), FAS primers of oligonucleotides (upstream primer, 5′-GCTGGAGCCCCTTTTTGTCTT-3′; downstream primer, 5′-ACCCCAGCACTGCAGTTTTCT-3′), HMG-CoA reductase primers of oligonucleotides (upstream primer, 5′-GCGTGCAAAGACAATCCTGGAG-3′; downstream primer, 5′-GTTAGACCTTGAGAACCCAATG-3′), SR-B1 primers of oligonucleotides (upstream primer, 5′-GTAGGGCCCAGAAGACACCAC-3′; downstream primer, 5′-CGCCTGCTTCACCACCTTCTT-3′), SREBP-1c primers of oligonucleotides (upstream primer, 5′-GAGCCACAATGAAGACCGCA-3′; downstream primer, 5′-CAAGGACAAGGGGCTACTCT-3′) and GAPDH primers of oligonucleotides (upstream primer, 5′-GCCATCAACGACCCCTTCATT-3′; downstream primer, 5′-CGCCTGCTTCACCACCTTCTT-3′). The reaction mixtures for the PCR contained 25 pmol of each primer, 1·25 U of EX-Taq polymerase, 1x PCR buffer (Takara), and 200 μm-dNTP in a 50 μl reaction volume. Temperature cycling was as follows: first cycle, denaturation at 94°C for 3 min, annealing at 60°C for 1 min, and extension at 72°C for 2 min. Subsequent cycles were denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 2 min. The thermal cycling was completed by terminal extension at 72°C for 10 min. In total, twenty-five cycles were performed for LDL-R and CYP7A1, thirty cycles for HMG-CoA reductase and SR-B1, and twenty cycles for FAS, GAPDH and SREBP-1c. Amplification products were electrophoresed on 2 % agarose gel and transferred to a nylon membrane (Biodyne B; Pall Bio-Support, East Hills, NY, USA). Blots were hybridised with an LDL-R probe of a fifty-four-base oligonucleotide (5′-CCAGGGTTGGTCGCTTTGCCCTGGAGCTGATCTGTCACCTCCAGCTCTCCCTC-3′), CYP7A1 probe of a fifty-four-base oligonucleotide (5′-CCCGAAGGCCTGTTTAAGTGATGACTCTCAGCCGCCAAGTGACATCATCCAGTG-3′), FAS receptor probe of a fifty-four-base oligonucleotide (5′-CTGCTCTCTGTGGATAGGACTGAATGCTGTGGCCTTCTGATAGACTCTTCTGGA-3′), HMG-CoA reductase probe of a fifty-four-base oligonucleotide (5′-GATCTGTTGTGAACCATGTGACTTCTGACAAGATGTCCTGCTGCCAATGCTGCC-3′), SR-B1 probe of a fifty-four-base oligonucleotide (5′-TGCCGTGTGGACAGTGTGACATCTTGGGGCTCAGGACGTGGCACTGGCGGGTTG-3′), SREBP-1c probe of a fifty-four-base oligonucleotide (5′-GCCGGCGTCTGAGGGTGGAGGGGTCAGCGTTTCTACCACTTCAGGTTTCATGCC-3′) and GAPDH probe of a fifty-four-base oligonucleotide (5′-TGATGACCAGCTTCCCATTCTCAGCCTTGACTGTGCCGTTGAACTTGCCGTGGG-3′). The probe was 3′-tailing labelled with digoxigenin, using a DIG oligonucleotide tailing kit (Boehringer Mannheim, Mannheim, Germany). Prehybridisation, hybridisation, and detection were carried out with a DIG luminescent detection kit (Boehringer Mannheim) as recommended by the manufacturer. The relative quantity of mRNA was estimated by densitometry scanning with X-ray film.
Growth of bacteria in the caecum
Coliforms in the caecum were inoculated and grown for 2 d on DHL agar (Eiken Chemical Co. Ltd, Tokyo, Japan) plates at 37°C. Anaerobes, Lactobacillus and Bifidobacterium in the caecum were incubated for 5 d on GAM agar (Nissui Pharmaceutical Co. Ltd, Tokyo, Japan), Rogosa agar (Merck KGaA, Darmstadt, Germany) and BL agar (Eiken Chemical Co. Ltd, Tokyo, Japan) at 37°C by the gaspak method according to the procedure of Mitsuoka et al. (Reference Mitsuoka, Sega and Yamamoto1964, Reference Mitsuoka, Sega and Yamamoto1965, Reference Mitsuoka, Ohno, Benno, Suzuki and Namba1976).
Statistical analysis
Data are presented as means and standard deviations for serum total cholesterol, HDL-cholesterol and non-HDL-cholesterol at prescribed times. The significance of differences among treatment groups was determined by ANOVA with Duncan's multiple range test and Student's t test (SAS Institute, Cary, NC, USA). Differences were considered significant at P < 0·05.
Results
There was no significant difference in the food intake, body-weight gain, liver weight, caecum weight, faecal dry weight and caecal pH among groups at the end of the experimental period (data not shown). The apparent digestibility of AC and AR was about 92–93 %, which was lower than CON (96 %).
Table 3 shows the serum total cholesterol, non-HDL-cholesterol, HDL-cholesterol and TAG concentrations of rats. The serum total cholesterol and non-HDL-cholesterol concentration in the AR-fed group were significantly lower than the CON group at the end of the 4-week feeding period. There was no significant difference in the HDL-cholesterol concentration among the groups. The serum TAG concentration was significantly lower in the AC- and AR-fed groups than that in the CON group through out the feeding period. The liver cholesterol concentrations in rats at the end of the experimental period are shown in Fig. 1. The liver cholesterol concentration in rats fed AC was significantly higher than in the CON group at the end of the 4-week feeding period.
Table 3 Serum total cholesterol, high-density-lipoprotein-cholesterol, very-low-density-lipoprotein-cholesterol + intermediate-density-lipoprotein-cholesterol + low-density-lipoprotein-cholesterol, and triacylglycerol concentrations in rats fed bovine proteins for 4 weeks (mmol/l) (Mean values and standard deviations for five rats)
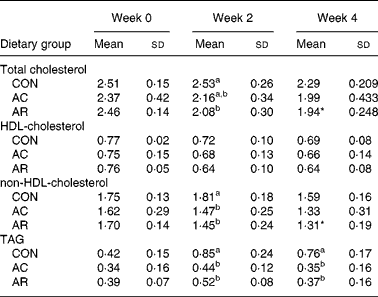
CON, casein control diet; AC, bovine Achilles’ tendon diet; AR, bovine artery diet.
a,b Mean values within a column with unlike superscript letters were significantly different (P < 0·05) (Duncan's multiple range test).*P < 0·05 v. CON by Student's t test.

Fig. 1 Liver cholesterol concentration in rats fed animal proteins for 4 weeks. CON, casein diet; AC, bovine Achilles' tendon protein diet; AR, bovine artery protein diet. Values are means for five rats, with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P < 0·05).
Table 4 shows the dietary cholesterol intake, faecal neutral steroid and acidic steroid concentrations of rats. The bovine dietary cholesterol intake in rats fed the AC and AR diets were 7·20 (sd 1·03) and 6·91 (sd 0·61) μmol/rat per d, respectively. The faecal neutral sterol excretion was significantly higher in the AC- and AR-fed groups than those in the CON group, and those in the AC-fed group were the highest among all the groups. However, the data on faecal coprostanone level are lacking in Table 4 due to unavailability. Further, there were no significant differences in total bile acid, cholic acid, chenoceoxycholic acid, deoxycholic acid and lithocholic acid concentrations among the groups. Sterol balance in rats fed CON, AC and AR were 14·64, 26·96 and 14·16 μmol/rat per d, respectively. There were no significant differences in total SCFA, acetic acid, propionic acid and n-butyric acid excretions in the caecum among the three groups (data not shown).
Table 4 Cholesterol intake, and faecal neutral steroid and acidic steroid concentrations in rats fed bovine proteins for 4 weeks (μmol/rat per d) (Mean values and standard deviations for five rats)

CON, casein control diet; AC, bovine Achilles’ tendon diet; AR, bovine artery diet; LCA, lithocholic acid; DCA, deoxycholic acid; CDCA, chenodeoxycholic acid; CA, cholic acid; TBA, total bile acids.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
The relative quantities of mRNA were determined by the Southern hybridisation of PCR-amplified LDL-R cDNA, CYP7A1 cDNA, HMG-CoA reductase cDNA, SR-B1 cDNA, SREBP-1c cDNA and FAS cDNA in the rat liver. The values of LDL-R, CYP7A1, HMG-CoA reductase, SR-B1, SREBP-1c and FAS mRNA were normalised to 1·00. There was no significant difference in relative quantity of LDL-R mRNA among the three groups and the quantities for CON, AC and AR were 0·856, 0·809 and 0·796 respectively. No significant difference was observed for the relative quantity of CYP7A1 mRNA among the three groups (Fig. 2). The relative quantity of hepatic HMG-CoA reductase mRNA in the AR-fed group was significantly lower than in the CON group (P < 0·05), and that in the AC-fed group tended to be lower compared with the CON group (Fig. 2). The relative quantity of FAS mRNA level in the AC- and AR-fed groups was significantly lower than that in the CON group (Fig. 3). No significant difference was observed for the relative quantity of SR-B1 mRNA level, and the quantities for CON, AC and AR were 0·68, 0·67 and 0·66, respectively. The SREBP-1c mRNA level in the AR-fed group was significantly lower than that in the CON group, and that in the AC-fed group tended to be lower compared with the CON group (Fig. 3). The FAS mRNA level was positively correlated with SREBP-1c mRNA level (r 0·660; P < 0·01).

Fig. 2 Hepatic hydroxyl methyl glutaryl (HMG)-CoA reductase mRNA (A) and cholesterol 7α-hydroxylase mRNA (B) expressions in rats fed animal proteins for 4 weeks. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CON, casein diet; AC, bovine Achilles' tendon protein diet; AR, bovine artery protein diet. Values are means for five rats, with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P < 0·05).

Fig. 3 Hepatic sterol regulatory element binding protein-1c (SREBP-1c) (A) and fatty acid synthase mRNA (B) expressions in rats fed animal proteins for 4 weeks. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CON, casein diet; AC, bovine Achilles' tendon protein diet; AR, bovine artery protein diet. Values are means for five rats, with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P < 0·05).
Table 5 shows the caecal bacterial population in rats. Anaerobe bacterial population was significantly higher in the AC- and AR-fed groups compared with the CON group. Bifidobacterium population in the AR-fed group was significantly higher compared with the CON group, and that in the AC-fed group tended to be elevated compared with the CON group. Coliform bacterial population was significantly higher in the AC-fed group compared with the CON group, and that in the AR-fed group tended to be elevated compared with the CON group.
Table 5 Caecal bacterial populations in rats fed bovine proteins for 4 weeks (log10 cfu/wet g) (Mean values and standard deviations for five rats)
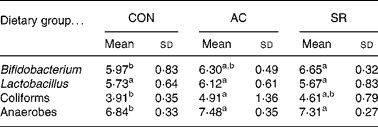
CON, casein control diet; AC, bovine Achilles’ tendon diet; AR, bovine artery diet.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Discussion
In the present study, we examined the effects of some bovine proteins on serum lipids, liver lipids, faecal lipids, caecal lipids and bacterial population in the colon, and hepatic mRNA in rats. The serum total cholesterol and non-HDL-cholesterol concentration in the AR-fed group were significantly lower than those in the CON group at the end of the 4-week feeding period. Several mechanisms may have caused these findings. One possible reason may be that bovine proteins act as dietary fibres. This could be supported by the lower digestibility of AC and AR (92–93 %) compared with CON (96 %). Jorgensen et al. (Reference Jorgensen, Zhao, Theil, Gabert and Bach Knudsen2003) have reported that increased intake of dietary fibre was associated with a concomitant loss of protein and energy to faeces. Previous findings have showed that dietary fibres lower plasma cholesterol levels in animals and human subjects compared with casein-based diets (Khosla et al. Reference Khosla, Samman and Carrol1991; Anderson et al. Reference Anderson, Johnstone and Cook-Newell1995). The factor lowering the cholesterol concentration in the AR-fed group was the lowering of the non-HDL-cholesterol level, which was in agreement with previous findings (Fukushima et al. Reference Fukushima, Nakano, Morii, Ohashi, Fujiwara and Sonoyama2000; Li et al. Reference Li, Wang, Kaneko, Qin and Sato2004). However, there was no significant difference in hepatic LDL-R mRNA level in rats fed bovine proteins compared with the CON group. Thus, the lower non-HDL-cholesterol concentration in the AR-fed group may be due to any other reason. This result was in agreement with previous findings showing that there was no correlation between plasma LDL-cholesterol concentration and hepatic LDL-R mRNA level (Sorci-Thomas et al. Reference Sorci-Thomas, Wilson, Johnson, Williams and Rudel1989). The CYP7A1 mRNA level and faecal bile acid concentration were not significantly different among the groups. The rate-limiting enzyme in endogenous sterol biosynthesis is HMG-CoA reductase, which catalyses the synthesis of mevalonate, and the activity of HMG-CoA reductase is also regulated by changes in the exogenous cholesterol concentration (Brown & Goldstein, Reference Brown and Goldstein1997). In the present experiment, HMG-CoA reductase mRNA expression was significantly lower in the AR-fed group, and tended to be lower in the AC-fed group compared with the CON group. This may be another reason for the low total serum cholesterol level which was positively correlated with HMG-CoA reductase mRNA level in the AR-fed group (r 0·742; P < 0·01). The decrease in HMG-CoA reductase mRNA is surprising and conflicts with previous reports related to dietary fibre. Lund et al. (Reference Lund, Salf and Johnson1993) and Moundras et al. (Reference Moundras, Behr, Remesy and Demigne1997) have reported that dietary fibre increases hepatic reductase activity. The mechanism of reduction of HMG-CoA reductase activity might be due to the increased liver cholesterol concentration which may inhibit the hepatic HMG-CoA reductase mRNA level. In rats, most of the serum cholesterol (60–80 %) is transported in HDL and only 5–10 % in LDL, since they lack lipid transfer protein (Day et al. Reference Day, Phillips and Schurr1979). In contrast, the present study showed only 30 % of serum cholesterol was from HDL-cholesterol even in rats fed the 0·01 and 0·006 % bovine dietary cholesterol diets. The dietary cholesterol level and 7 % soyabean oil content with 0·4 % plant sterols in the AIN 93G diet may be a reason for lower HDL-cholesterol concentration in the AC- and AR-fed groups. However, it has been shown that feeding normal Wistar–Furth or Sprague–Dawley rats with diets containing 1 % cholesterol had negligible effects on serum cholesterol level (Ness & Gertz, Reference Ness and Gertz2004).
The amino acid composition of animal protein might be an important factor affecting the behaviour of animal protein. The indigestible behaviour of animal protein may be due to the abundant collagen concentration, as Achilles' tendons and arteries contain high glycine and proline concentrations (Schubert et al. Reference Schubert, Harris, Devine and Heinemann1974). In addition, it has been suggested in previous studies that favourable amino acid composition is at least partially responsible for the suppressive effect on plasma cholesterol (Kayashita et al. Reference Kayashita, Shimaoka and Nakajoh1995, Reference Kayashita, Shimaoka, Nakajoh, Yamazaki and Kato1997; Morita et al. Reference Morita, Oh-hashi, Takei, Ikai, Kasaoka and Kiriyama1997; Tomotake et al. Reference Tomotake, Shimaoka, Kayashita, Yokoyama, Nakajoh and Kato2000; Gudbrandsen et al. Reference Gudbrandsen, Wergedahl, Liaset, Espe and Berge2005). Lower methionine level or lower methionine:glycine (Table 2) ratio (80–85 %) in the AC and AR diets might have hindered the transfer of cholesterol from the liver into the bloodstream (Morita et al. Reference Morita, Oh-hashi, Takei, Ikai, Kasaoka and Kiriyama1997). In fact, the lower methionine level may reduce phosphatidyl choline synthesis via phosphatidyl ethanolamine, leading to the depression of apolipoprotein release into the circulation (Morita et al. Reference Morita, Oh-hashi, Takei, Ikai, Kasaoka and Kiriyama1997). Moreover, dietary arginine concentration was comparatively higher in AC and AR diets, which was negatively correlated with serum non-HDL-cholesterol level (r 0·863; P < 0·01) in a previous study (Eklund & Sjoblom, Reference Eklund and Sjoblom1980). These facts may suggest another mechanism of serum cholesterol lowering in the AR-fed group. On the other hand, the effect of methionine would give a reason for higher liver cholesterol levels in both the AC- and AR-fed groups. The liver cholesterol concentration was significantly higher in the AC-fed group, and tended to be higher in the AR-fed group, than that in the CON group. Han et al. (Reference Han, Fukushima, Kato, Kojima, Ohba, Shimada, Sekikawa and Nakano2003) also have reported that liver cholesterol concentration in groups fed the enzyme-resistant fractions of beans was significantly higher than in a cellulose powder-fed group, although the reason for this remained unclear; however, it may be speculated that the increase in liver cholesterol concentration was due to an enhanced hepatic SR-B1 mRNA level. However, in the present study no significant difference was observed in SR-B1 mRNA level among groups. This finding does not agree with previous findings in mice showing that dietary fibres lower liver lipid concentration (Van Bennekum et al. Reference Van Bennekum, Nguyen, Schulthess, Hauser and Phillips2005). However, the significantly higher liver cholesterol level in the AC-fed group may be a compensatory response to the higher sterol excretion compared with control.
The faecal cholesterol and coprostanol concentrations in the AC and AR groups were significantly higher than those in the CON group. Neutral sterols occupy a major part of steroid excretion, and it is noteworthy that the excretions of cholesterol and coprostanol were brought about by feeding AC and AR. This may be another reason for the lower serum cholesterol concentration in the AR-fed group. This was in agreement with findings for some dietary plant fibres (Kritchevsky et al. 1982; Eastwood, Reference Eastwood1992; Moundras et al. Reference Moundras, Behr, Remesy and Demigne1997). Although the excretion of neutral sterols was affected by bovine protein, acid sterols were unaffected compared with casein. This was in contrast with previous findings showing that some plant fibres lower serum cholesterol by increasing cholesterol elimination as bile acids and neutral sterols (Fukushima et al. Reference Fukushima, Nakano, Morii, Ohashi, Fujiwara and Sonoyama2000; Buhman et al. Reference Buhman, Furumoto, Domkin and Story1998). Furthermore, Hara et al. (Reference Hara, Haga, Kasai and Kiriyama1998) reported that sugar-beet fibre reduces serum cholesterol level by reducing cholesterol synthesis in rats.
According to the results of dietary cholesterol intake, faecal sterol excretion and HMG-CoA reductase mRNA level, the sterol balance was 1·86-fold higher in the AC-fed group, and that in the AR-fed group was not different, compared with the control group. However, HMG-CoA reductase mRNA level in the AC-fed group was not significantly different compared with the control group and that in the AR-fed group was significantly lower relative to the control. In the present study, AC and AR diets may maintain the body sterol balance by two different mechanisms.
Serum TAG concentration was significantly lower in the AC- and AR-fed groups compared with the CON group. This was in agreement with previous findings showing that dietary fibres reduce serum TAG levels in rats (Li et al. Reference Li, Wang, Kaneko, Qin and Sato2004). Low TAG level in the AC- and AR-fed groups was accompanied by the reduction in FAS mRNA level in the animal protein-fed groups. In fact, the mRNA expression of FAS was significantly lower in the AR-fed group, and tended to be lower in the AC-fed group compared with the CON group. The FAS is a mutant enzyme complex that catalyses the synthesis of long-chain fatty acids from acetyl CoA and malonyl CoA (Wakil et al. Reference Wakil, Stoops and Joshi1983). Moreover, SREBP-1c mRNA level was significantly lower in the AR-fed group, and it tended to be lower in the AC-fed group compared with the CON group. The correlation between SREBP-1c and FAS was r 0·660 (P < 0·01). It is suggested that the bovine protein-dependent decrease in FAS gene expression is due to the suppression of the gene expression of SREBP-1c, which is the transcriptional factor to generate FAS mRNA. SREBP are membrane-bound transcriptional factors which regulate the gene expression of enzymes in fatty acid and cholesterol biosynthesis (Brown & Goldstein, Reference Brown and Goldstein1997; Horton & Shimomura, Reference Horton and Shimomura1999). SREBP-1c preferentially enhances the transcription of genes required for fatty acid synthesis (Horton et al. Reference Horton, Goldstein and Brown2002).
According to the results of the present study, the anaerobe bacteria population of the caecum in the AC- and AR-fed groups was significantly higher than that of the CON group. These results were accompanied by a higher faecal coprostanol concentration, which may be an index of the activity of intestinal flora (Arjmandi et al. Reference Arjmandi, Ahn, Nathani and Reeves1992). It was suggested that protein was one of the major substrates for caecal fermentation in rats when an animal protein-based diet was fed (Tsukahara & Ushida, Reference Tsukahara and Ushida2000). Fatty acids of anaerobic bacteria are a major source of energy for the colonic mucosa in man (Roediger, Reference Roediger1980). The Bifidobacterium population was significantly higher in the AR-fed group, and tended to be higher in the AC-fed group compared with the CON group. In addition, the coliform bacteria population was significantly higher in the AC-fed group and tended to be higher in the AR-fed group than that in the CON group. However, there was no significant difference in caecal pool size of SCFA among the groups. The reason for higher bifidobacteria and coliform populations in the bovine protein-fed groups remains unclear. However, the undigested AC and AR proteins might be used as N sources by caecal bacteria.
In conclusion, the effects of some bovine proteins were most clearly seen when compared with rats fed casein. Bovine artery protein (AR) reduced the total serum cholesterol by reducing non-HDL-cholesterol, accompanied by lower HMG-CoA reductase mRNA level, and by increasing faecal neutral sterol excretion. Animal proteins, such as bovine Achilles' tendon protein and artery protein, reduced TAG concentration by reducing fatty acid synthesis in the liver accompanied by lower FAS mRNA level compared with the casein-fed group. In view of some of these facts, we conclude that some bovine animal proteins show dietary fibre-like properties by virtue of their low digestibility.
Acknowledgements
The present research was partially supported by a grant from the 21st Century COE Program (A-1), Ministry of Education, Culture, Sports, Science and Technology, Japan, and by Special Coordination Funds for Promoting Science and Technology (Leading Research Utilizing Potential of Regional Science and Technology) of the Japanese Ministry of Education, Culture, Sports, Science and Technology.










