Introduction
The Hoary-throated Spinetail Synallaxis kollari (Furnariidae) and Rio Branco Antbird Cercomacra carbonaria (Thamnophilidae) are both restricted to riverine forests in the far northern state of Roraima, Brazil, and adjacent Guyana (Vale Reference Vale and Schulenberg2020; Zimmer et al. Reference Zimmer, Whittaker and Stotz1997, Reference Zimmer, Isler, Sharpe, del Hoyo, Elliot, Sargental, Christie and de Juana2020). Their ranges lie primarily within the Roraima–Rupununi savanna, a region of Amazonian grassland embedded within the extensive humid forests of the Guiana Shield (Robbins et al. Reference Robbins, Braun and Finch2004). Vale et al. (Reference Vale, Bell, Alves and Pimm2007) were the first to determine the extent of both species’ geographical ranges on tributaries of the Rio Branco (Figure 1), and presented the first estimates of local densities and global population sizes. Using a series of playback surveys and extrapolating local densities to each species’ observed distribution, they calculated a mean density of 60 individuals/km2 for the spinetail and 76 individuals/km2 for the antbird, yielding global population estimates of approximately 5,000 and 15,000 individuals, respectively. Hoary-throated Spinetail has the more limited distribution of the two species, being mostly restricted to tributaries of the Rio Branco, whereas the Rio Branco Antbird occurs both on tributaries and along the Rio Branco itself, including on islands, almost as far south as the river’s mouth (Naka et al. Reference Naka, Cohn-Haft, Mallet-Rodrigues, Santos and de Fátima Torres2006, Reference Naka, Laranjeiras, Lima, Plaskievicz, Mariz and Da Costa2019; Santos Reference Santos2003; Vale Reference Vale and Schulenberg2020; Vale et al. Reference Vale, Bell, Alves and Pimm2007) (Figure 1).

Figure 1. The known distributions of Rio Branco Antbird Cercomacra carbonaria (RBAN) and Hoary-throated Spinetail Synallaxis kollari (HTSP) in the upper Rio Branco drainage as determined by Vale et al. (Reference Vale, Bell, Alves and Pimm2007), in relation to the area surveyed in this study. The source of the Rio Branco is the confluence of the Takutu and Uraricoera Rivers, above the city of Boa Vista. The range of Rio Branco Antbird extends far to the south along the Rio Branco (not shown; see text).
Conservation efforts focused on gallery forests in the Rio Branco drainage face serious challenges. Ongoing environmental degradation from gold mining, irrigation for rice plantations, increasing frequency and intensity of fires, and regional population growth all impact gallery forests throughout the ranges of Rio Branco Antbird and Hoary-throated Spinetail (Bird et al. Reference Bird, Buchanan, Lees, Clay, Develey and Yépez2012; Filizola et al. Reference Filizola, Marinho, Borges, Estupiñán, Naka and Laranjeiras2020; Naka et al. Reference Naka, Laranjeiras, Lima, Plaskievicz, Mariz and Da Costa2019; Soares-Filho et al. Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006). Both species have been listed on the International Union for Conservation of Nature (IUCN) Red List of Threatened Species since 1988 (IUCN 2022), and in 2012 IUCN upgraded their threat assessments to “Critically Endangered” as both were considered likely to decline by more than 80% over 20 years due to habitat loss in Roraima. Despite this dire prediction, there have been no surveys in Brazil to assess trends in either species’ status since the original population estimates were made in 2004 and 2005 (Vale et al. Reference Vale, Bell, Alves and Pimm2007).
We report here new distributional data for Rio Branco Antbird and Hoary-throated Spinetail from the Ireng and Takutu Rivers, which form the border of Brazil and south-western Guyana. The survey by Vale et al. (Reference Vale, Bell, Alves and Pimm2007) covered a vast area within Roraima but did not include extensive tracts of gallery forest along the international border, particularly along the upper Takutu River, where both species have been known to occur at least since the mid-1990s (Robbins et al. Reference Robbins, Braun and Finch2004). These heretofore unsurveyed gallery forests have the potential to significantly augment previous global population estimates. In Guyana, development pressures are currently lower than in Roraima, providing a window of opportunity to implement effective conservation plans for these species along the Takutu and Ireng Rivers. The purpose of this survey was to provide data to serve as a baseline to assess future population changes and to inform conservation action in Guyana.
Methods
Study site
The Roraima–Rupununi savanna (often termed “Rio Branco–Rupununi” savanna; Myers Reference Myers1936) is the largest continuous Amazonian savanna, covering an area of more than 50,000 km2 in northern Brazil and adjacent Guyana, and featuring a mosaic of habitats including seasonally flooded wetlands, patches of scrubby forest, forested mountains, and open grasslands with varying amounts of tree cover (Barbosa et al. Reference Barbosa, Costa e Souza, Xaud, Barbosa, Xaud and Costa e Souza2005; Naka et al. Reference Naka, Cohn-Haft, Mallet-Rodrigues, Santos and de Fátima Torres2006; Robbins et al. Reference Robbins, Braun and Finch2004). Narrow corridors of gallery forest grow along the larger rivers in the savanna. Ranging from tens of metres to more than a kilometre in width, these linear forests support a unique biodiversity in the savanna landscape and are the focus of our work. Two rivers – the Takutu and Ireng (known in Brazil as the Rios Tacutu and Maú, respectively) – form part of the international border between Brazil and Guyana (Figure 2; we use the Guyanese names for both rivers here). The Ireng River originates in the tepui highlands and flows south-west before joining the Takutu, which flows northward; downstream from their confluence near the Brazilian town of Conceição do Maú, the Takutu bends to flow westward and is entirely within Brazil (Figure 2). Unlike the humid forest covering much of the Guiana Shield, the Roraima–Rupununi savanna ecosystem features strong seasonality. During the annual rainy season, from April to August, the landscape is transformed into a vast wetland as rivers overflow their banks and flood the surrounding savanna. During the long and intense dry season, many rivers and creeks stop flowing entirely, and fires are common.
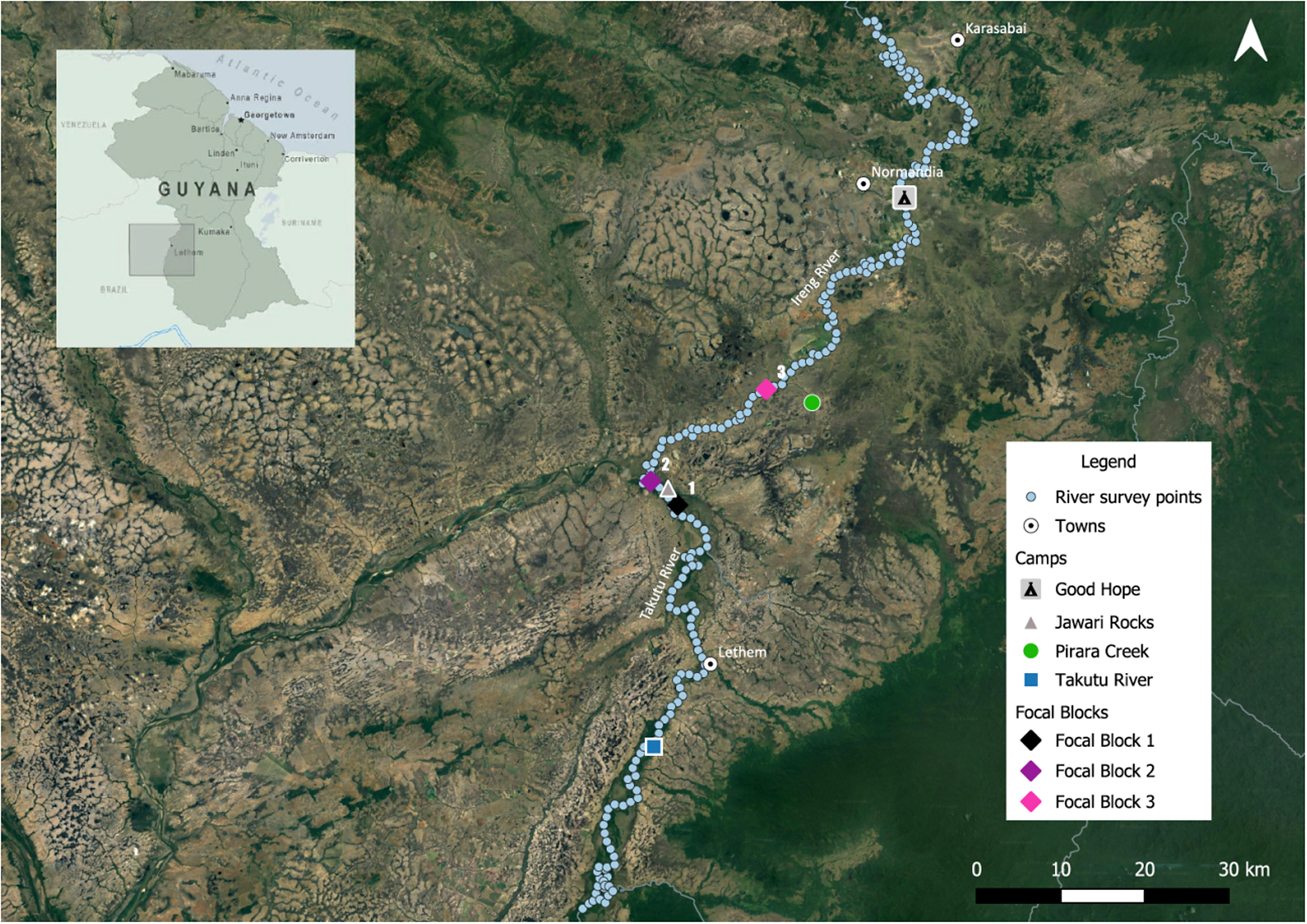
Figure 2. The study area showing survey camps, river survey points, and focal blocks for density estimation.
Our surveys were carried out by boat and on land from 21 July to 13 August 2021, during the rainy season, as this is considered to be both species’ main breeding period in Brazil (Vale et al. Reference Vale, Bell, Alves and Pimm2007). The rainy season was also chosen because Vale et al. (Reference Vale, Bell, Alves and Pimm2007) carried out their surveys at the same time of year; we thereby minimised any potential differences in results due to seasonal effects on vocal activity. River surveys were based from four camps: Good Hope and Pirara Creek, both on the Ireng River; Jawari Rocks, near the confluence of the Ireng and the Takutu; Takutu Camp, above Lethem on the Takutu River (Figure 2).
Geographical distribution
To determine the extent of each species’ range along the Ireng and Takutu Rivers, we established survey points at 1-km intervals along 219 km of river: 131 km up the Ireng from its confluence with the Takutu, and 88 km up the Takutu from the same confluence (Figure 2). Four additional points were established along the first 4 km of Sawariwau Creek upstream from its confluence with the Takutu, near the southern end of the survey area. The uppermost survey point on the Ireng River was determined in advance using satellite imagery and was positioned near to where the Ireng begins to enter large expanses of humid forest in the foothills of the tepui highlands, far upriver from the uppermost known locality for either species. The upper limit on the Takutu was roughly 10 km above the mouth of Sawariwau Creek and was determined by logistical constraints. At each survey point, we conducted playbacks for both species using recordings of vocalisations on the Merlin® bird ID app (Cornell Lab of Ornithology 2023) with an external speaker to broadcast the sound as far as possible. Both the Hoary-throated Spinetail and the Rio Branco Antbird are highly territorial, and the playback survey is a rapid and efficient method for ascertaining the presence of territorial species that inhabit dense vegetation or that may otherwise be inconspicuous (Johnson et al. Reference Johnson, Brown, Haight and Simpson1981; Vale et al. Reference Vale, Bell, Alves and Pimm2007).
All playbacks were carried out from a boat, which was held stationary at each point on or near the Guyana shore of the river. Spinetail vocalisations were played first, followed by the those of the antbird. After two minutes of waiting for a response, the sequence was repeated, followed by an additional five minutes of waiting. Each point typically took 8–10 minutes to complete. If either species was detected during the initial two-minute interval, we skipped the second playback sequence for that species. For each species, we recorded the number of birds at each point and whether they were on the Guyana or Brazil side of the river. We conducted playbacks from the early morning hours until 16h00, a window of time within which we noted little if any variation in response behaviours; we therefore did not adjust the timing of playback trials among sampling points to account for time of day. To maximise chances of finding the birds at each point, given our time constraints, we surveyed all points twice and on successive days when possible, except for the uppermost 14 points on the Ireng River, which we surveyed once each. If a species was detected at a given point on the first playback survey, we skipped playback for that species on the second survey. Surveys were not conducted under rainy or windy conditions.
Local density
To determine mean densities that could inform regional population estimates, we spot-mapped individuals in three forest blocks along the Ireng and Takutu Rivers: the first just upriver from our Jawari Rocks camp, the second at the confluence of the Ireng and the Takutu, and the third on the Ireng just downriver from the mouth of Pirara Creek (Figure 2). We overlaid a grid on satellite imagery and plotted survey points spaced 200 m apart throughout each block. Point spacing was set at 200 m to maximise coverage given the time available, and also to reduce the probability of double-counting individuals on successive points given the birds’ tendency to approach the speaker. To ensure complete coverage, we extended the grids into the savanna adjacent to each block and carried out surveys at any points that were within 100 m of the edge of the block. The focal forest blocks ranged in size from 70 ha to 451 ha, allowing us to establish a total of 171 survey points at distances of up to 1.6 km from the river (Table 1). At each point, we repeated our playback protocol from the river surveys, recording the number of birds of each species and their approximate distance and direction (compass heading) from the survey point. We then mapped all detections within each block, consolidating observations of birds estimated to be less than 30 m from each other. As both the spinetail and the antbird live in pairs that maintain permanent territories, for each territorial response, we assumed that a second bird was present even if not detected at a point, as was done by Vale et al. (Reference Vale, Bell, Alves and Pimm2007) for the antbird (but not the spinetail). For both species, but particularly for the spinetail, it was sometimes difficult to determine whether two singing birds were mates or rivals. We therefore made one high (presumed rivals) and one low (presumed mates) estimate and averaged those estimates to calculate the number of individuals in each focal block. We then divided the total number of individuals by the forested area of each block to obtain density estimates.
Table 1. Location and size of the three focal blocks where local densities were estimated

Estimation of habitat extent and population size
We defined the range of each species as the entire river distance between the uppermost points where the species was detected on each river. To estimate the amount of suitable habitat available within each species’ range, we quantified the amount of gallery forest from remote-sensing data. We used the 2019 global forest cover layer at 30-m resolution (Hansen et al. Reference Hansen, Potapov, Moore, Hancher, Turubanova and Tyukavina2013) and retained any pixels with >50% forest cover as potential habitat. As each species was closely tied to the river, we then selected the most likely habitat using a buffer based on the furthest detection of each species from the river edge: 1,490 m for the spinetail and 450 m for the antbird.
Using our estimates of available habitat for each species, we calculated maximum and minimum population estimates within each species’ distribution along the Ireng and Takutu Rivers. Maximum estimates were based on the assumption that all available habitat was occupied and were obtained by multiplying the mean densities of birds in the occupied portions of the three focal blocks by the total available habitat. Minimum estimates were obtained by multiplying the maximum estimates by the proportion of points where each species was detected along the rivers within their respective distributions, using this metric as a surrogate for the percentage of occupied habitat. Finally, to calculate global population sizes, we first used the Vale et al. (Reference Vale, Bell, Alves and Pimm2007) density estimates for both species and subtracted the number of individuals estimated to occur along the Ireng River in their study, since this zone overlapped with our study area. We then added our total estimates to the remaining range-wide estimates presented by Vale et al. (Reference Vale, Bell, Alves and Pimm2007).
Results
River playback surveys
We found Rio Branco Antbird along 119 km of river: the lower 31 km of the Ireng River, and on the Takutu River up to 86 km above the mouth of the Ireng, 2 km below the furthest upstream survey point on the Takutu (Figure 3); we also found the antbird along the bottom 2 km of Sawariwau Creek, which enters the Takutu from the Guyana side near the southern limit of our survey area. The range of Hoary-throated Spinetail was less extensive: it was found along the lower 30 km of the Ireng, and 51 km up the Takutu, from the mouth of the Ireng to 5 km above the international bridge connecting Lethem to Bonfim (Figure 3). Neither species was recorded from 31 km above the mouth of the Ireng River to roughly 11 km north-west of Karasabai, the furthest upriver point we surveyed on the Ireng (Figure 2), a distance of 100 river km. We therefore excluded this upper portion of the Ireng from subsequent calculations of habitat area and population size. A total of 83 individuals of Rio Branco Antbird were tallied at 49 of 119 survey points (41%) within the species’ distribution along the rivers. The maximum number observed from a single point was five individuals. Hoary-throated Spinetails were detected at 36 of 81 survey points (44%). Sixty spinetails were observed from the river points, with a maximum of four birds observed from a single point. Of the birds observed at our survey points, 27% of spinetails and 34% of antbirds were only recorded on the second playback attempt, indicating that the spinetails were more aggressively responsive to playback, as noted by Vale et al. (Reference Vale, Bell, Alves and Pimm2007). For both species, the centre of abundance was the area within 10 km of the confluence of the Ireng and Takutu Rivers (Figure 3).
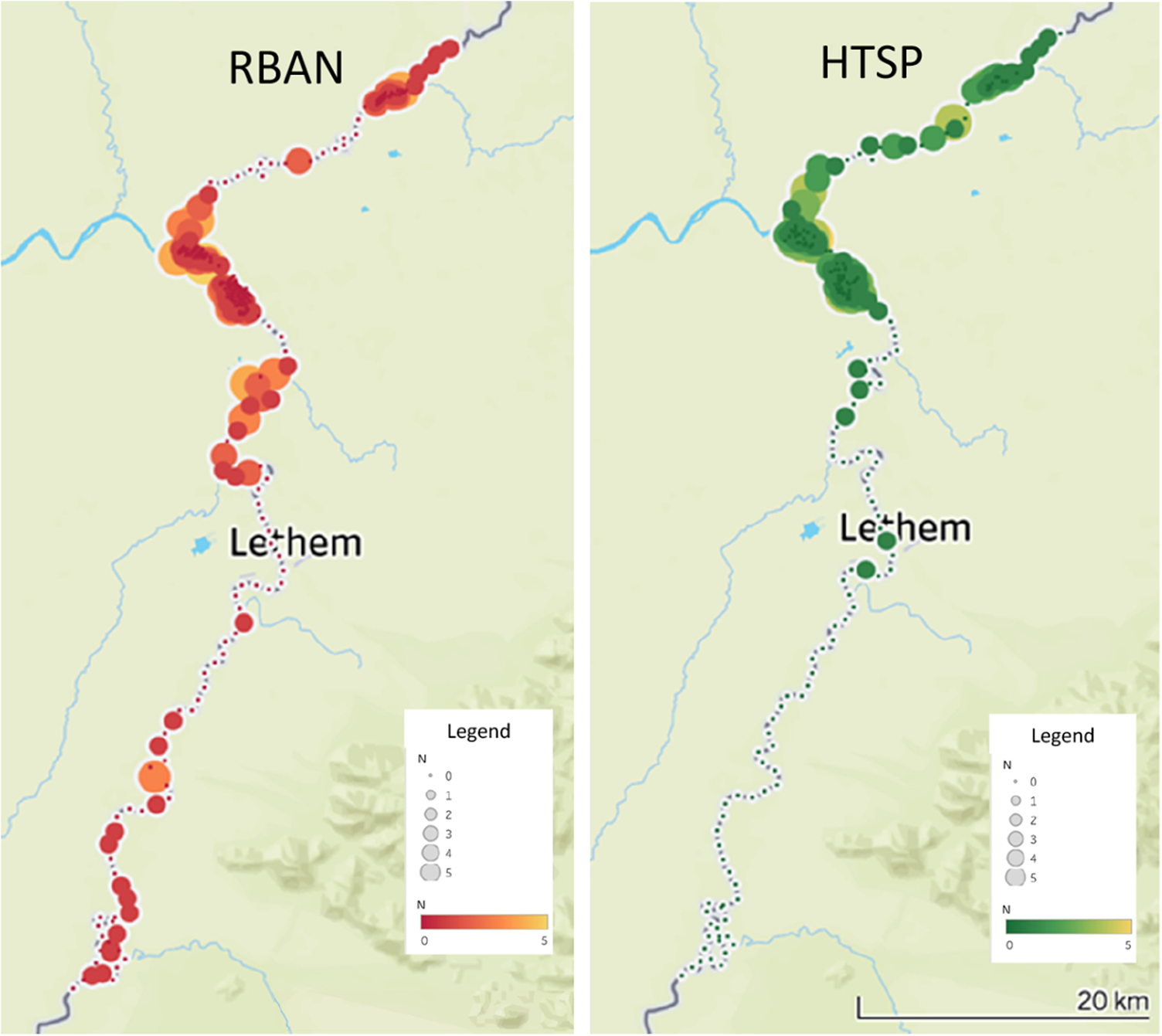
Figure 3. Distribution of Rio Branco Antbird Cercomacra carbonaria (RBAN) and Hoary-throated Spinetail Synallaxis kollari (HTSP) along the Ireng and Takutu Rivers.
Focal block surveys
A total of 116 Rio Branco Antbirds and 241 Hoary-throated Spinetails were detected from survey points in the three focal blocks. After adjusting these tallies to consolidate detections estimated to be 30 m apart or less and averaging two estimates for each species based on different assumptions regarding pair status, we derived estimates of 189 antbirds and 387 spinetails in the three focal blocks. Although both species were often detected from the same points, there were clear differences between them in the use of available habitat within each block, with Rio Branco Antbird detections tending to cluster closer to the river (Figure 4). Maximum estimated distances from the river for antbird and spinetail observations were 450 m and 1,490 m, respectively. Considering only habitat within these maxima, we calculated mean densities of 45.2 antbirds and 55.2 spinetails per square kilometre of forest.

Figure 4. Locations of Rio Branco Antbird Cercomacra carbonaria (RBAN) and Hoary-throated Spinetail Synallaxis kollari (HTSP) detection points in focal block 1. The Takutu River delimits the left and bottom edges of the block; note that the river is prone to seasonal flooding and was considerably wider than pictured here during our survey.
Population sizes
We estimated the total area of potential habitat along the Ireng and Takutu Rivers to be 6,196 ha for Rio Branco Antbird and 3,506 ha for Hoary-throated Spinetail; for both species, approximately 55% of potential habitat was on the Guyana side of the river (Table 2). Extrapolating our densities from the focal blocks and incidence data from our river surveys yielded minimum and maximum estimates of 1,147–2,798 Rio Branco Antbirds and 851–1,933 Hoary-throated Spinetails between the endpoints of each species’ observed distribution along the Guyana–Brazil border (Table 2).
Table 2. Habitat area and population estimates of Rio Branco Antbird Cercomacra carbonaria and Hoary-throated Spinetail Synallaxis kollari along the Guyana–Brazil border. “River survey incidence” is the proportion of survey points at which each species was detected within its distribution along both rivers

Global population change
We added our survey totals to the global population estimates of Vale et al. (Reference Vale, Bell, Alves and Pimm2007), the latter adjusted to exclude 2,500 ha of habitat along the Ireng River that we covered during this survey. Adding our minimum estimates of 1,147 antbirds and 851 spinetails to the adjusted global numbers yielded new global population estimates of 16,531 Rio Branco Antbirds and 5,629 Hoary-throated Spinetails.
Discussion
Additions to the global population and current range limits
We found Rio Branco Antbird and Hoary-throated Spinetail to be present in substantial numbers along the lower Ireng and upper Takutu Rivers. Our estimates constitute increases of at least 7.5% and 17.8% for global populations of the antbird and spinetail, respectively (IUCN 2022; Vale et al. Reference Vale, Bell, Alves and Pimm2007). The presence of these species in such numbers highlights the conservation value of the Ireng–Takutu gallery forests, which we consider to be a stronghold for both species considering the currently low level of threat to these forests (in both Guyana and Brazil) relative to other gallery forests in the Rio Branco drainage. We report our additions with the important caveat that existing population and distribution data from Brazil are now almost 20 years old. More current data from Brazil are urgently needed to ascertain whether recent proposals to downgrade both species’ IUCN Red List status were warranted. Indeed, we urge IUCN to delay any changes to the current status of both species until such data are available.
Our expansion of the known ranges of Rio Branco Antbird and Hoary-throated Spinetail is countered by our finding of an apparent contraction in both species’ distributions along the Ireng River since 2004, when one of us (BO) participated on an expedition that located both species near Good Hope/Moreiru (O’Shea et al. Reference O’Shea, Milensky, Claramunt, Schmidt, Gebhard and Schmitt2007). Specimen records from that expedition were the basis for the Vale et al. (Reference Vale, Bell, Alves and Pimm2007) upriver range limit for both species along the Ireng, and it was presumed that both occurred from that locality downriver to the confluence with the Takutu. In 2021, our team spent a full week along the upper Ireng River and did not encounter a single individual of either species for nearly 50 km downriver from Good Hope, despite more than 100 playback attempts supplemented by trollingFootnote 1 in what appeared to be suitable habitat. Our failure to find the birds was all the more puzzling because there was plenty of gallery forest along both sides of the river, even well upriver from Good Hope/Moreiru. When we finally did encounter them, far downriver, we were struck both by their abundance and by how easy they were to detect. This was also the point at which we began seeing spinetail nests, both active and old. For this reason, we strongly suspect that our uppermost observation point on the Ireng is the actual upriver range limit for these species at the present time, and that their ranges along the Ireng may have contracted since 2004. This suggests that both species, while locally common, remain vulnerable to local extinction, and that the suitability of gallery forest as habitat for these birds is not uniform across their ranges.
Methodological considerations
Our study design was inspired by the work of Vale et al. (Reference Vale, Bell, Alves and Pimm2007), who also used river surveys to delimit each species’ distribution and extrapolated local densities to range-wide population estimates using satellite imagery. Although our methods were similar, it is worth considering some of the differences in our methodologies and results.
We conducted our river surveys at points spaced 1 km apart, and we surveyed most points twice to increase detection probability and identify range limits with greater precision. Vale et al. (Reference Vale, Bell, Alves and Pimm2007) conducted playbacks using a similar methodology but spaced their surveys at 3 km to cover a far greater area along several different rivers. They also surveyed each point only once, recording Rio Branco Antbird at 29% of survey points, and Hoary-throated Spinetail at 44% of points. Excluding points along the 100 km of the Ireng where we found neither species, we detected the antbird and spinetail at 41% and 44% of survey points, respectively. Given the lower initial responsiveness of Rio Branco Antbird to playback, our higher detection rate for that species could be attributed to the substantial number of birds that only responded to playback after multiple attempts.
Our method for calculating local densities differed from that of Vale et al. (Reference Vale, Bell, Alves and Pimm2007), and yielded lower local density estimates for both species, particularly the antbird, for which our estimated density of 55.2 individuals/km2 was considerably less than the mean value of 76 birds/km2 calculated in their study. This discrepancy is interesting in light of the differences in distribution and habitat use of each species that we observed within our focal blocks. Vale et al. (Reference Vale, Bell, Alves and Pimm2007) calculated local density by surveying birds along point transects, 3.5 km and 4 km in length, within two strips of gallery forest 200–300 m wide.
By contrast, the forest patches that we selected as focal blocks were considerably larger, and our spot-mapping grid extended up to 1.5 km from the river. Despite this greater sampling area, we did not detect Rio Branco Antbird farther than 450 m from the river edge, and the birds tended to occur well inside that distance, being most common where the forest canopy was relatively well developed, with abundant vine growth. On the other hand, we found Hoary-throated Spinetail in forest at all distances from the river, including in areas where the canopy had been fragmented by recurrent fire, allowing dense understorey growth to flourish. Unlike the antbird, the spinetail did not seem to be any less common away from the river than close to it. This difference in habitat use between the species has implications for future efforts to identify potential habitat and assess population size.
The higher density of Rio Branco Antbird, and the roughly equal density of Hoary-throated Spinetail, reported by Vale et al. (Reference Vale, Bell, Alves and Pimm2007) may reflect their sampling focus in high-quality riverine habitats preferred by the antbird but not the spinetail. While it is possible that Vale et al. (Reference Vale, Bell, Alves and Pimm2007) underestimated the total population size of the spinetail by confining their assessment of suitable habitat to 0.5 km from the river edge, the gallery forest in their surveyed areas tended to be very narrow (Mariana Vale in litt.), rendering it unlikely that significant areas of habitat were excluded from their estimates. It is also possible that we underestimated the distribution and abundance of Rio Branco Antbird within our focal blocks because we only made one playback survey at each point.
Despite the differences highlighted above, our methods for determining range limits and local densities of Rio Branco Antbird and Hoary-throated Spinetail are broadly similar to those of Vale et al. (Reference Vale, Bell, Alves and Pimm2007), and therefore we feel justified in adding our numbers to existing global population estimates, considering that most of our study area had not previously been surveyed for these species.
Threats and conservation recommendations
Habitat destruction and contamination from small-scale gold mining is now the principal threat to gallery forests in Roraima, as is the case in riverine ecosystems throughout the Guiana Shield (Hayes et al. Reference Hayes, Voigt, Rosa, Cort, Kotlinski and Kalamandeen2023). The threat from gold mining has intensified recently as small-scale miners have been evicted from indigenous lands in western Roraima and spread across the region (Mario Cohn-Haft in litt.). This increase adds to existing pressures on gallery forests from ongoing agricultural development in Roraima, which are compounded further by the planned construction of dams along the Rio Branco and its tributaries (Filizola et al. Reference Filizola, Marinho, Borges, Estupiñán, Naka and Laranjeiras2020; Naka et al. Reference Naka, Laranjeiras, Lima, Plaskievicz, Mariz and Da Costa2019). Taken together, these current and future threats have significant potential to drastically alter the structure, extent, and connectivity of gallery forests in the Rio Branco drainage.
In Guyana, habitat destruction from mining is not currently an issue along the Takutu and Ireng Rivers, although Guyana’s accelerating developmental agenda has rekindled efforts to convert savannas to agriculture in the Rupununi region, including at sites adjacent to our study area. Within the past year alone, significant fragmentation of gallery forest has resulted from the construction of deep ditches that stretch for kilometres from the rivers through some proposed agricultural areas. This has coincided with a severe drought, exacerbating the threat from fire. As our Rupununi-based team well knows, fires have been increasing in both severity and extent over the past decade throughout the region, leading to the degradation and loss of gallery forest. The savanna–gallery forest ecotone is often abrupt, and large savanna fires can burn entirely through narrow strips of gallery forest, further disrupting their connectivity and reducing their value as dispersal corridors for birds and other animals. This has implications for the distributions of Rio Branco Antbird and Hoary-throated Spinetail. Impacts of fire on our study area were noted in 2021 and have increased substantially since then, particularly during the most recent (2023–2024) dry season (Don Melville and Leroy Ignacio personal observations). Fire-killed vegetation now dominates large areas of our focal blocks. Although we lack data on whether and how these fires may impact our study species, we note that the abruptness of both species’ range limits along the Ireng River, and the apparent range contraction since 2004, may be a consequence of changing fire regimes. We also suggest that, to the extent that fires disrupt population connectivity in gallery forests, forest cover alone may not be a reliable proxy for available habitat, particularly in landscapes where fire has burned through gallery forest and transformed this linear ecosystem into isolated fragments. If fire impacts these species negatively, reductions in both species’ distributions may occur in the near future if current fire regimes continue unabated.
Considering the current and future threats to gallery forests in both Guyana and Brazil, further fragmentation and destruction of this ecosystem seem all but assured without direct conservation action. Although indigenous reserves such as the Terra Indígena Raposa Serra do Sol offer nominal protection to gallery forests in some parts of the species’ Brazilian distributions, we feel that a multi-stakeholder approach to conservation is more suitable for the Takutu–Ireng gallery forests in Guyana and can complement efforts in Brazil to conserve these species over their entire known ranges. The Key Biodiversity Area (KBA) concept pioneered by IUCN provides clear criteria for population data to be used in the identification and delineation of areas of high biodiversity value, independent of legal or ownership status (IUCN 2016). The KBA framework is therefore ideally suited for application to our study area, which consists of state and private lands. The populations of Rio Branco Antbird and Hoary-throated Spinetail along the Guyana side of the Ireng and Takutu Rivers are more than sufficient to fulfil the criteria for delineation of a KBA. Based on the results of our assessment, we propose here a KBA encompassing most of our survey area (Figure 5). This KBA would be one of the first to be confirmed in Guyana and is contiguous with previously proposed KBAs on the Brazilian side of the Takutu River, ensuring recognition of the majority of both species’ habitats in this vital corridor.

Figure 5. Tentative proposed boundary of the Ireng–Takutu Key Biodiversity Area.
We must emphasise that a KBA designation alone simply indicates that an area has high conservation value; it does not confer formal protection, nor does it have any bearing on indigenous land claims or the rights of private landowners. Nevertheless, since August 2021, the South Rupununi Conservation Society (SRCS) has held multiple consultations with national and local stakeholders, including landowners and nearby indigenous communities, to discuss the creation of a Conservation Management Zone (CMZ) within the proposed KBA boundary. The CMZ would have a governance structure and monitoring system to provide protection for the two bird species and other threatened wildlife found within the zone. The creation of the CMZ is dependent on the interest of local landowners and alignment with the objectives of several government agencies. SRCS is therefore working with stakeholders to explore other conservation options including existing legislation that can provide “win-win” solutions for both wildlife and land users.
Although our survey has given us a much better idea of both species’ status and distribution along the Ireng and Takutu Rivers, more research on these species is urgently needed, including an update on the status of their populations in Roraima, and how each species responds to fire-induced habitat changes. In Guyana, we remain optimistic that a combination of stakeholder outreach and continued field studies will result in a collaborative and positive conservation outcome for these species.
Acknowledgements
We are thankful to the private landowners, indigenous communities, and stakeholders that participated in the consultation process and were supportive of the project. We especially thank our camp assistants, Vidia Kaitano and Berlinda Francis, for their invaluable support in the field. We also wish to thank the staff of Conservation International – Guyana for financial and logistical support, particularly Marcelle Chan A Sue, Venisha Daniels, and Sheweta Ramdhan. We thank Nina Hadley and Andrew Snyder of Re:wild for their ongoing support of our research. Mariana Vale and an anonymous reviewer provided helpful comments on a previous version of this manuscript. This work was supported by Conservation International – Guyana (grant number CI-112307) and Re:wild (grant number SMA-AG0-00013).









