INTRODUCTION
From May to July 2011, a large outbreak of haemolytic uraemic syndrome (HUS) and shiga toxin-producing Escherichia coli (STEC) infections occurred in northern Germany with a total of 855 HUS cases and 2987 STEC infections. Fifty-three people died. Untypically, the outbreak mainly affected adults with a median age of 41 years for HUS cases, although HUS is mainly a paediatric disease. Another characteristic was the high proportion of female cases with 68% women in all HUS cases [1]. The rare STEC O104:H4 serotype with characteristic virulence markers [shiga toxin (stx)1 negative, stx2 positive, intimin gene (eae) negative] and a special resistance against extended-spectrum β-lactamase (ESBL), was identified as the outbreak strain [Reference Bielaszewska2]. Investigations of public health and food safety authorities detected several disease clusters within the outbreak with a defined exposure time and location. Environmental investigations, tracing back and forward activities as well as epidemiological studies related to those clusters identified sprouts as the outbreak vehicle [Reference Buchholz3]. Sprouts could be traced back to a sprout producer in Lower Saxony and had been suspected as an outbreak vehicle since 5 June 2011 [4]. In the end all German clusters could be linked to one sprout producer. Additionally, investigations on a cluster in France [Reference Gault5, Reference Mariani-Kurkdjian6] and international food traceback activities revealed a high probability that the cause of the outbreak was sprouts of fenugreek seeds imported from Egypt [7].
One of the German clusters was notified to public health authorities on 7 June 2011, 2 weeks after the peak of the outbreak. After a family party several of the guests contracted STEC O104:H4 infection. The party was for a 70th birthday and took place on 28 May 2011 in a community centre in the south of Lower Saxony. Previously this region had not been part of the highly affected areas during the course of the outbreak. The self-service buffet with warm and cold dishes was prepared by a catering company from Hesse, no sprouts were served. During this time public and media attention was high and the outbreak was detected soon after the first cases occurred. Because of the unusual time and location, and the absence of sprouts as possible vehicle, we investigated the cluster to identify the vehicle and to prevent further transmission.
METHODS
Epidemiological investigation
We conducted a retrospective cohort study of the party guests. A complete list of all guests who attended the party was obtained from the party host. Using the same standardized questionnaire, all guests were interviewed by telephone (in Lower Saxony and Bavaria) or they completed the questionnaire, which was sent by post (in Hesse), themselves. We asked about meals consumed at the party including 36 different food items, clinical symptoms of STEC infection occurring after the party and whether any household contacts had gastrointestinal symptoms. Data was entered and stored in a database created in Microsoft Access 2003 (Microsoft, USA).
A case was defined as a party guest that developed diarrhoea, or abdominal cramps within 3 weeks after the party with a laboratory confirmation of STEC O104:H4 infection. Guests not meeting the case definition were treated as non-cases.
Associations between STEC infection and food items were calculated by univariable and multivariable analysis. We calculated risk ratios using univariable analysis. P values were estimated using χ 2 test. We built a multivariable model using logistic regression, using forward stepwise selection, due to small case numbers, including variables with P values < 0·05 in univariable analysis. To assess the fit of the model we used the likelihood ratio test. Data was analysed with Stata SE10 (StataCorp., USA).
Laboratory investigation
Stool samples of party guests were tested for STEC in the laboratories of the federal public health authorities in Hesse and Lower Saxony. Asymptomatic guests were contacted by the responsible local public health department 3–4 weeks after the party with a request to send in stool samples even if they had not developed symptoms.
The samples were screened for Stx1 or Stx2 by means of enzyme-linked immunosorbent assay (ELISA) (Biopharm, Germany). Stool samples were inoculated with enrichment medium (EHEC direct medium, Heipha, Germany; RIDA mTSB medium, R-Biopharm, Germany) and incubated under gentle shaking at 37°C overnight. ELISA was performed from the supernatant according to the manufacturers' recommendations. Each sample was also directly inoculated into ESBL screening agar (Oxoid, Germany; CHROMesbl, MAST Diagnostika, Germany) to isolate ESBL-producing E. coli. All ESBL-producing E. coli isolates were subjected to a PCR hybridization assay for the detection of stx1 and stx2 genes (Amplex, Germany). According to the recommendations of the National Reference Centre for Salmonella and other Bacterial Enteric Pathogens, isolates with the pathogenic profile stx1 negative, stx2 positive, eae negative and production of ESBL were classified as belonging to the outbreak strain O104:H4 [8].
Additionally, in Hesse, biochemical identification was performed using the Vitek-2 system (bioMérieux, France) and serotyping was performed with an O104-specific rabbit antiserum (Statens Serum Institute, Denmark).
Environmental investigation
The catering company was inspected by the local veterinary authorities on 7 and 8 June 2011 immediately after they had been informed about the first suspected STEC cases by the local public health department. Delivery documents and bills of the wholesale market where the food was purchased as well as the party menu were collected. Veterinary authorities took ten environmental swab samples from utensils and surfaces in the company and further investigated eight frozen and stored food samples which were leftover from the preparation of the food and could be collected from the caterer.
Food samples were screened for Stx1 or Stx2 after 24 h enrichment in mTSB broth at 44°C. A loop of the enrichment broth was streaked onto TBX agar and Brillance ESBL (Oxoid, Germany) and incubated for 24 h at 44°C, and 24 h at 37°C, respectively. Typical colonies were confirmed by Vitek GN and subjected to PCR according to the recommendations of the Federal Institute for risk assessment (BfR) and the European Union Reference Laboratory (Rome) for E. coli for the detection of the stx2 gene.
All ESBL-producing E. coli and stx2-positive E. coli were subjected to a real-time PCR for the detection of the O104:H4 typical wzxO104 gene. Positive results were confirmed by the National Reference Laboratory for E. coli. [9].
Veterinary authorities inspected the venue where the family party took place.
RESULTS
Epidemiological findings
We identified 71 guests that attended the party. Median age of guests was 71 years (range 15–82 years), 48% were female. We found 23 cases among the 71 guests, resulting in an attack rate (AR) of 32%. Four (17%) cases developed HUS as a complication of their STEC infection (Fig. 1). Median age of cases was 70 years, 43% were female. The proportion of cases requiring hospitalization was 71%. Date of onset of symptoms was between 30 May 2011 and 13 June 2011. Incubation period ranged from 2 to 16 days with a median of 9 days (Fig. 2). Four further guests developed diarrhoea following the party, but STEC O104:H4 infection could not be laboratory-confirmed.
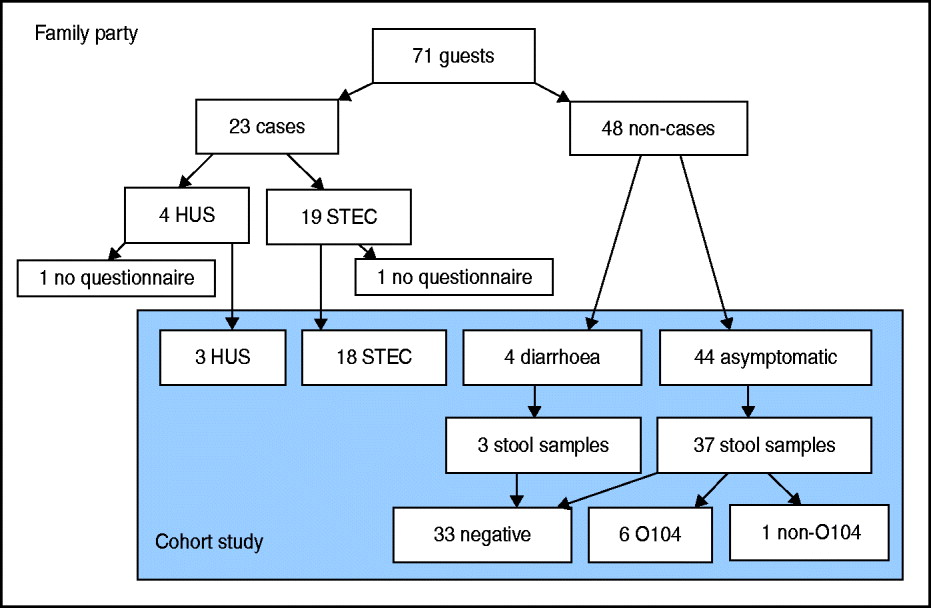
Fig. 1 [colour online]. Flowchart of guests attending a family party (n = 71) including number of cases (symptomatic guests with laboratory-confirmed STEC O104:H4 infection, n = 23) and non-cases (n = 48), and number of guests interviewed by questionnaire, that could be included in the cohort study (n = 69), with results of stool samples, northern Germany, 2011. STEC, Shiga toxin-producing Escherichia coli serotype O104:H4; HUS, haemolytic uraemic syndrome.
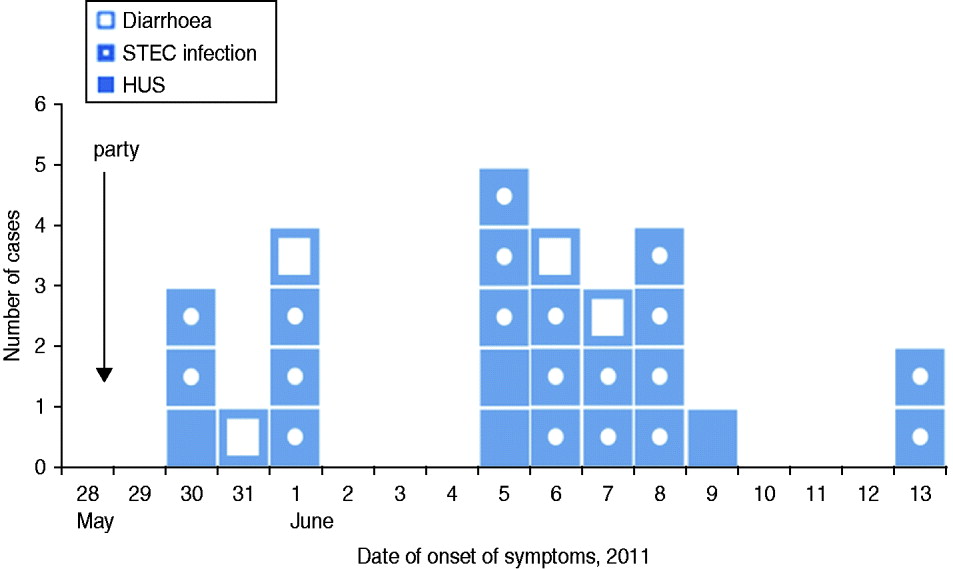
Fig. 2 [colour online]. Cases of HUS (n = 4) and STEC O104:H4 infection (n = 19), as well as guests with diarrhoea (n = 4) after a family party with 71 guests, by date of onset of symptoms, northern Germany, 2011. STEC, Shiga toxin-producing Escherichia coli serotype O104:H4; HUS, haemolytic uraemic syndrome.
With regard to secondary infections in cases and guests with diarrhoea, shown on the epidemic curve, some occurred in the same household. In seven households two persons developed gastrointestinal symptoms at the same time or one after the other. In four of these households the date of onset of first symptoms was on the same day or only 1 day apart. In the fifth household the date of onset was 2 days apart. In only two households (households A and B) did the date of onset differ by 5 days: household A [1 June (diarrhoea) and 6 June (STEC infection)], and household B [8 and 13 June (both STEC infection)] (Fig. 2). All party guests lived in three different federal states in Germany, most of them in the border region between Lower Saxony and Hesse.
Of the 71 guests, 69 (response 97%) answered the questionnaire. Two guests, one with a STEC infection and one with HUS, could not be included in the cohort study. The HUS patient could not be interviewed due to bad health, and the STEC patient refused to participate in the study.
By univariable analysis consumption of bread, salmon from the salmon plate, bean salad, herb cream and meatballs were associated with STEC O104:H4 infection (Table 1). By multivariable analysis consumption of salmon, herb cream and bean salad remained associated with STEC infection (Table 1).
Table 1. Risk factors for STEC O104:H4 infection after a family party, northern Germany, 2011 (univariable and multivariable analysis)
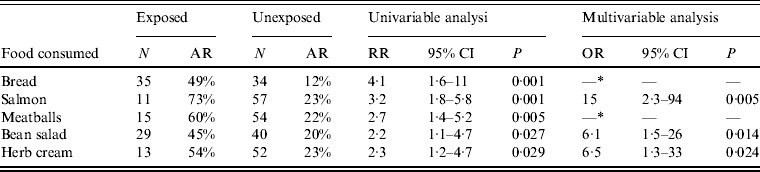
N, Total number of persons exposed or unexposed to a food item; AR, attack rate (cases exposed or unexposed/total number exposed or unexposed); RR, relative risk; CI, confidence interval; OR, odds ratio.
* Not included in final model (not significant in multivariable analysis).
Laboratory results
Stool samples of 40/48 (83%) asymptomatic guests were tested for STEC infection. Of those stool samples six (15%) tested positive for STEC O104:H4. One sample was from a family member of a known case. Another guest living in the household of a case tested positive for Stx1, and was diagnosed as being infected with a STEC not belonging to the outbreak strain.
Results of the environmental and food investigations
Of the eight food samples taken at the catering company one sample of raw bell pepper and two samples of salmon tested positive for STEC O104:H4. Environmental swab samples were all negative for STEC.
No leftover samples were available at the time of inspection of the party venue. The kitchen of the venue was not used for food preparation. All meals were prepared in the premises of the catering company and delivered directly to the venue which is ∼10 km away from the catering company. No information was available whether appropriate temperatures were maintained during the transport of the food.
Investigations at the catering company
According to the bills from the wholesale market most products were purchased fresh 2 days before the party and prepared in the kitchen of the catering company. We have no indication that pre-prepared food was used. At the time of inspection both owners of the catering company were hospitalized with a STEC infection. The woman who had prepared and served the food at the family party reported developing symptoms of diarrhoea 2 days after preparation of the food (1 June 2011) and later tested positive for STEC O104:H4. Her husband developed symptoms on 6 June 2011, but had not been involved in the preparation, transport or serving of the food. Both were carriers of STEC O104:H4 until August 2011. Further investigations revealed that their adult daughter had been hospitalized on 12 May 2011 because of bloody diarrhoea. The daughter had eaten meals in a company canteen in Frankfurt, where a cluster of STEC O104:H4 infections occurred at the beginning of May [Reference Hauri10]. This cluster was caused by sprouts originating from the sprout producer in Lower Saxony [Reference Wilking11] (Fig. 3). The woman who had prepared the food had visited her daughter in hospital and took care of her grandchild during her daughter's hospitalization [Reference Hauri10]. The son of the family who helped serve the food at the venue did not develop symptoms. He and his family all tested negative for STEC infection. Besides the woman and her son, three other waitresses served the food at the party. One of them, a 29-year-old woman, developed HUS after the party with onset of symptoms on 14 June 2011 and laboratory confirmation of STEC O104:H4 infection. We do not know whether she had eaten any of the food served. The two other waitresses did not report any gastrointestinal symptoms.
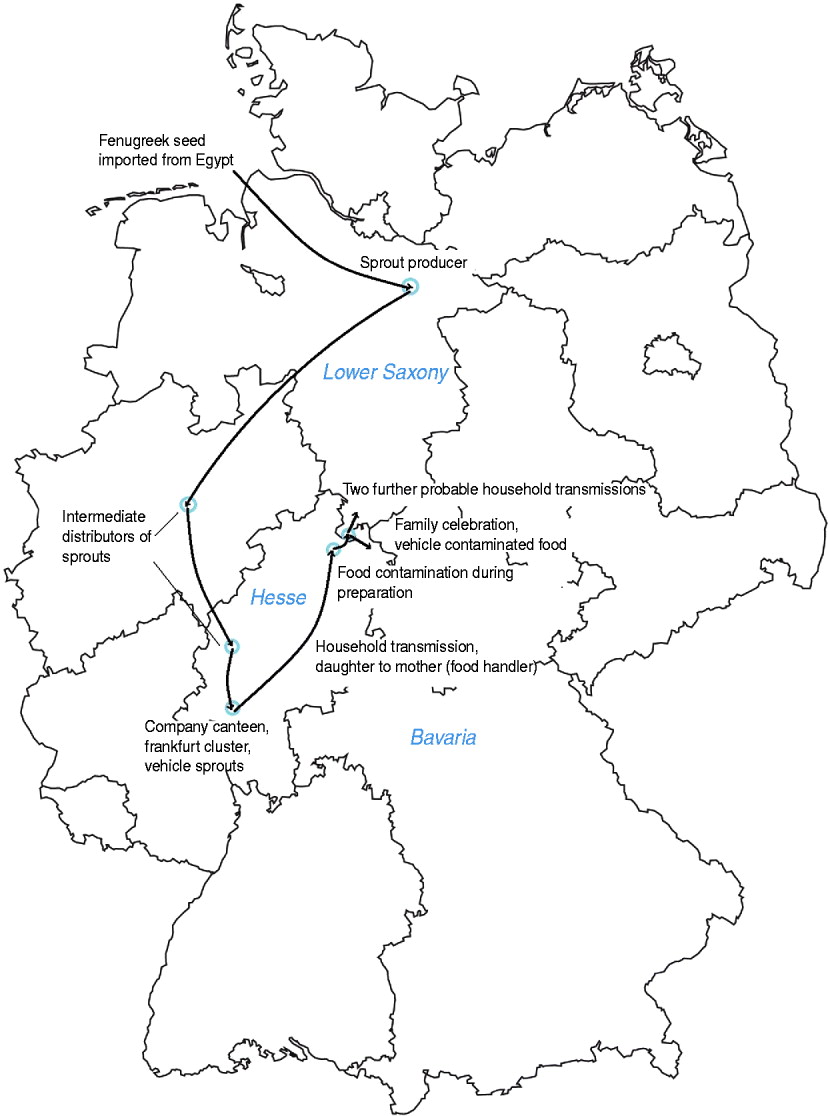
Fig. 3 [colour online]. Map of Germany and transmission chain of STEC O104:H4 from sprout producer to probable household cases occurring after a family party, northern Germany, 2011.
Control measures taken
Based on recommendations of the veterinary authorities all stored food was removed from the catering company. The premises and the utensils were disinfected and cleaned intensively. The closure of the catering company was only lifted after obtaining negative results from environmental swab samples taken 1 month later, after the disinfection. In addition, disinfection of the party venue was recommended.
DISCUSSION
The results of the epidemiological, laboratory and environmental investigations point towards different food items, i.e. salmon, bell pepper, herb cream and bean salad as vehicles in this cluster. For salmon in particular we have both epidemiological and laboratory evidence indicating it as a possible vehicle. We suggest that those food items were contaminated by the person who prepared the food. Although this person claimed to have been asymptomatic at the time of the preparation of the food she tested positive for STEC O104:H4 and could be linked as a secondary case to another STEC cluster that was caused by sprouts. Other exposures to contaminated sprouts are unlikely, as the region where the cluster occurred was not part of the highly affected areas in northern Germany during the course of the STEC O104:H4 outbreak. Furthermore the party took place 2 weeks after the peak of the STEC O104:H4 outbreak.
The role of symptomatic food handlers in transmission of disease is well known and consequently led to regulations in the German Protection against Infection Act. According to the act, food handlers suffering from gastroenteritis may not be engaged or employed in the processing of foodstuff and in kitchens of institutional caterers. Further, people who are engaged as a food handler are required to obtain an instruction and a certificate from the local health office, before they are allowed to start working in this sector [Reference Bales, Baumann and Schnitzler12]. Cases of infected, but asymptomatic food handlers involved in disease transmission have been reported for viral foodborne gastroenteritis [Reference Barrabeig13] as well as bacterial gastroenteritis outbreaks due to Salmonella [Reference Hedican14, Reference Mertens15] or presumably STEC O157 [Reference Watanabe16]. Transmission by an infected, but asymptomatic food handler has not been described for STEC O104:H4 before. In this cluster the information on onset of symptoms is based on the report of the food handler. We cannot exclude that this self-report was influenced by the awareness of the legal regulations or social desirability.
The AR of 32% is quite high. As three of eight stored food samples were positive for STEC O104:H4 a cross-contamination of food can be assumed. The eight food samples analysed had been stored at the catering company and not leftover samples from the party. This suggests that the contamination of at least three positive food items had already taken place during preparation of the food or even before. Multiple or cross-contaminations could also explain that by univariable and multivariable analysis several food items were associated with STEC infection. To adjust for effect modification and confounding we conducted a multivariable analysis. In multivariable analysis consumption of bread and meatballs was not significantly associated with STEC infection. Bread could have been consumed together with salmon or other contaminated food items and was therefore significantly associated with STEC infection by univariable analysis, but not by multivariable analysis. The other 30 food items were not associated with STEC infection. We used a very specific case definition for statistical analysis to minimize misclassification of cases. We did not exclude guests with diarrhoea or asymptomatic carriers from the non-cases in order to retain adequate sample size. Additionally risk ratios obtained in the analysis were not substantially different when excluding those individuals. Furthermore, food could have been cross-contaminated lying on the same plate with contaminated food items or during the preparation of the food using the same utensils. The salmon associated with STEC infection was served cold on a plate while the main dish, containing well cooked salmon, was not associated with STEC infection. The preparation of the salmon plate also requires a lot of contact with utensils or hands of the food handler that could have been contaminated with STEC. As most of the products used for food preparation were purchased fresh and no pre-prepared food was used, contamination before preparation in the catering company is unlikely.
The venue itself did not have a kitchen, so during the time between preparation, transportation and consumption of the food the bacterial load could have increased. Further, the meals that were served at the party were known and asked about in the questionnaire, but not all of the ingredients were known. As a consequence we did not ask about bell pepper explicitly from the beginning. We also do not know whether or not there was bell pepper in the bean salad.
This outbreak involved participants in three federal states of Germany. Good cooperation and communication supported an effective outbreak investigation. On the other hand different interview methods (telephone interview and self-administered questionnaires) were used. There also may have been a recall bias as the interviews took place 2–4 weeks after the party. This cluster also received high attention from the media which could have influenced the reporting as well.
This is the first time serotype O104:H4 with the described outbreak patterns has been identified in Germany. With confirmation of the serotype the isolates could be classified as most likely belonging to the outbreak strain. The median incubation period of 9 days in this cluster is unusually long compared to other STEC outbreaks [Reference Tarr, Gordon and Chandler17], but is consistent with the characteristics of the outbreak strain O104:H4 [4, Reference Frank18]. In the two households with two cases who had a 5-day difference between the date of onset of symptoms secondary transmission cannot be excluded. But even those 5 days are still a shorter period than the median incubation period of the other cases in this outbreak and other STEC O104:H4 clusters. Secondary cases due to household transmission of STEC O104:H4 have rarely been described [Reference Hauri10, Reference Aldabe19], but can not be excluded.
The high number of asymptomatic carriers could also be due to consumption of contaminated food at the party or in one case due to household transmission. The stool samples of asymptomatic participants were taken 3–4 weeks after the party, therefore the number of asymptomatic infections could have been even higher.
CONCLUSION
This cluster was the only cluster during the HUS and EHEC outbreak in northern Germany 2011 where STEC O104:H4 could be laboratory-confirmed in a food item. In contrast to other clusters, sprouts could be excluded as a vehicle of STEC O104:H4 infection in this cluster. The results of the investigation of this cluster strongly suggest a contamination of several food items by an infected food handler. Transmission chains of at least three generations can be demonstrated leading to this cluster. The onset of symptoms of the food handler only relies on self-reporting and can not be verified. Our results emphasize the importance of the proper implementation of hygiene and legal regulations concerning food handlers and food preparation. The instruction of food handlers by the local health authorities should be followed by regular information and education of food handlers to increase the awareness of their role in the transmission of infectious diseases.
ACKNOWLEDGEMENTS
The authors thank D. Ziehm, K. Beyrer, N. Jahn, M. Scharlach, H. Scharlach, J. Dreesman, W. R. Wienecke, D. Bode, LGL Bavaria, Local public health department Fürstenfeldbruck, Germany for data collection. Thanks are due to J. Dreesman, PAE and EPIET coordinators for scientific advice.
DECLARATION OF INTEREST
None.






