INTRODUCTION
Conservation paleobiology aims to use knowledge of the past to make informed predictions about the future of Earth's threatened biodiversity (Dietl and Flessa, Reference Dietl and Flessa2011, Dietl et al., Reference Dietl, Kidwell, Brenner, Burney, Flessa, Jackson and Koch2015, Barnosky et al., Reference Barnosky, Hadly, Gonzalez, Head, Polly, Lawing and Eronen2017). These studies provide a baseline of what community structures looked like in the past, how extant communities might respond to impending changes, and how we should approach conservation. For example, in the Greater Everglades Ecosystem, paleoecological data from pollen and invertebrates have been used to establish pre-anthropogenic trends and cycles, predict future changes, and identify restoration targets for anticipatory resource management efforts (Wingard et al., Reference Wingard, Bernhardt and Wachnicka2017). Other similar work has documented the presence of Bison in the Grand Canyon region (Martin et al., Reference Martin, Martin and Mead2017), inspired interagency conservation and management plans for Bison today (Plumb and McMullen, Reference Plumb and McMullen2018), and encouraged restoration of threatened plant species in Hawaii (Burney and Burney, Reference Burney and Burney2007).
Increasingly xeric landscapes, such as those in the southwest US and northwest Mexico, are challenging for natural resource management (Sayre et al., Reference Sayre, McAllister, Bestelmeyer, Moritz and Turner2013). Integration of fossil sites with climate patterns may reveal potential future areas for faunal refugia and migratory corridors as fauna must shift their geographic ranges or risk extinction (Blois and Hadly, Reference Blois and Hadly2009). Additionally, studying past faunal change may reveal trends in restructuring of faunal communities, which may enhance anticipatory natural resource management decisions and strategies. In particular, large animals and plants are disproportionately important for ecosystem function (Enquist et al., Reference Enquist, Abraham, Harfoot, Malhi and Doughty2020), and changes in these communities can indicate environmental change (Blois and Hadly, Reference Blois and Hadly2009).
Paleobiology of mammal communities has commonly focused on body size and diet because they can be inferred from fossil remains. Smith et al. (Reference Smith, Smith, Lyons and Payne2018) demonstrated a widespread 95% decline in mean body mass of North American mammal communities (e.g., from 100 kg to 5 kg) associated with the loss of the Pleistocene megafauna. Stegner and Holmes (Reference Stegner and Holmes2013) investigated mammalian community structure over 16 million years and demonstrated static diversity of dietary functional groups except in times of major environmental pressures. In the Pleistocene of Mexico, mammal communities were more taxonomically diverse, with more large-bodied taxa and a more similar ratio between herbivores and carnivores as compared to modern communities (Ferrusquía-Villafranca et al., Reference Ferrusquía-Villafranca, Arroyo-Cabrales, Martínez-Hernández, Gama-Castro, Ruiz-González, Polaco and Johnson2010).
Overall, our knowledge of the fossil mammals of Mexico is biased toward taxa of larger sizes and younger geological ages, possibly because of collection methods (Montellano-Ballesteros and Jiménez-Hidalgo, Reference Montellano-Ballesteros, Jiménez-Hidalgo, Vega, Nyborg, Del Carmen Perrilliat, Montellano-Ballesteros, Cevallos-Ferriz and Quiroz-Barroso2006; Ferrusquía-Villafranca et al., Reference Ferrusquía-Villafranca, Arroyo-Cabrales, Martínez-Hernández, Gama-Castro, Ruiz-González, Polaco and Johnson2010). Of 64 Neogene sites in Sonora, Mexico, only three sites have detailed faunal records: El Golfo, San Clemente de Térapa (hereon, Térapa), and Rancho la Brisca (White et al., Reference White, Mead, Baez, Swift, Molina-Freaner and Van Devender2010). El Golfo is the only known Irvingtonian LMA site in northern Mexico (Lindsay, Reference Lindsay1984; Croxen et al., Reference Croxen, Shaw and Sussman2007; Sussman et al., Reference Sussman, Croxen, McDonald and Shaw2016). Rancho La Brisca is from the Rancholabrean LMA but has a slightly different fauna than is preserved at Térapa and other sites in the region because its record is biased toward small and medium fish, amphibians, and reptiles (Van Devender et al., Reference Van Devender, Rea and Smith1985). Additional small and isolated records in the region, including La Botana, Llano Prieto, and Chinobampo, consist of mostly large mammals, such as Bison, Equus, Mammuthus, Glyptotherium, and Camelops (White et al., Reference White, Mead, Baez, Swift, Molina-Freaner and Van Devender2010; Cruz-y-Cruz et al., Reference Cruz-y-Cruz, Sánchez-Miranda, Carpenter, Terrazas-Mata, Sedov, Solleiro-Rebolledo and Benavente-Sanvicente2018). West of Térapa, La Playa has fossils of Bison, Platygonus, Mammuthus, Equus, camelids, and other large mammals, but also has the first record of Cynomys in Sonora, which suggests more small mammals may be found at other sites (Mead et al., Reference Mead, White, Baez, Hollenshead, Swift and Carpenter2010).
Located in Sonora, Mexico, Térapa (29°41′N, 109°39′W; 605 m elevation; Fig. 1) provides an excellent case study of faunal response to environmental and climatic changes in an area vulnerable to continued alteration because of its geographic location in an ecotone between the northern Nearctic climate and the southern Neotropical climate. Modern (i.e., interglacial) Sonoran and Chihuahuan deserts are typified by scrubland ecosystems with warm tropical-subtropical climates, and at present, this area is situated on the margin of the Madrean Archipelago and the Sinaloa-Sonora Hills and Canyons (Morrone et al., Reference Morrone, Escalante and Rodríguez-Tapia2017). However, when the fossil-bearing sediments were deposited in the Térapa basin, western Sonora was more temperate, with cooler, drier summers and wetter winters than are seen today (Nunez et al., Reference Nunez, MacFadden, Mead and Baez2010), and was typified by pinyon-juniper-oak woodlands (Van Devender, Reference Van Devender, Felger and Broyles2007). Stable isotope analyses of carbon and oxygen from ungulate teeth (Nunez et al., Reference Nunez, MacFadden, Mead and Baez2010) and ostrocodes (Bright et al., Reference Bright, Orem, Mead and Baez2016) recovered at Térapa suggest that, at least in the valley, marsh and grasslands were likely present at the time of deposition. Previous studies of Térapa avifauna (Steadman and Mead, Reference Steadman and Mead2010; Oswald and Steadman, Reference Oswald and Steadman2011), crocodilians (Mead et al., Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006), bats and shrews (Czaplewski et al., Reference Czaplewski, Morgan, Arroyo-Cabrales and Mead2014), and ostracodes (Bright et al., Reference Bright, Orem, Mead and Baez2016) indicate that the environment was an elevational mosaic of temperate to tropical-subtropical marsh and savanna/grassland with a slow-moving freshwater stream flowing from north of Térapa down to the Río Moctezuma and Río Yaqui to the Gulf of California.

Figure 1. (For interpretation of the reference to color in this figure legend, the reader is referred to the web version of this article) Geographic location of study area (Left), inset figured in (Right) with Sonora, Mexico highlighted in yellow; (Right) location of Térapa (black star) within Sonora, Mexico.
Discovered in 2000, late Pleistocene (MIS/OIS 3) faunal deposits at Térapa include fossils of more than 60 taxa (Mead et al., Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006). A preliminary faunal list is provided in Mead et al. (Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006), and an updated faunal list is provided in Supplemental Table S1. Recent publications have described Mammuthus and Cuvieronius (Mead et al., Reference Mead, Arroyo-Cabrales and Swift2019), Glyptotherium cylindricum and Pampatherium cf. P. mexicanum (Mead et al., Reference Mead, Swift, White, McDonald and Baez2007), two shrews and six bats (Czaplewski et al., Reference Czaplewski, Morgan, Arroyo-Cabrales and Mead2014), and 39 species of birds (Steadman and Mead, Reference Steadman and Mead2010; Oswald and Steadman, Reference Oswald and Steadman2011). With Bison present throughout the stratigraphic profile, Térapa represents one of the few Rancholabrean North American Land Mammal Age (LMA) sites studied in detail in Sonora (Bell et al., Reference Bell, Lundelius, Barnosky, Graham, Lindsay, Ruez, Semken, Webb, Zakrzewski and Woodburne2004; Mead et al., Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006). However, the Holarctic genus Bison did not arrive in Mexico until much later than in the northern part of the continent. This biochronological delay complicates assigning LMA ages to Mexican sites, and also illustrates the need to further explore Mexico for fossil sites that can provide insight into North American fauna during the Pleistocene.
Fossils found at Térapa were deposited where the Tonibabi basalt flow dammed and diverted the Río Moctezuma and created a shallow lake at the northern extent of the Sierra Madre Occidental (Mead et al., Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006). Río Moctezuma sediments beneath the basalt dam date to 42.9 ± 3.3 ka using infrared stimulated luminescence, providing a maximum age estimate for the fossils (Bright et al., Reference Bright, Kaufman, Forman, McIntosh, Mead and Baez2010). Also using infrared stimulated luminescence, sediments 1 m above the basalt date to 40.2 ± 3.2 ka (Bright et al., Reference Bright, Kaufman, Forman, McIntosh, Mead and Baez2010). Using radiocarbon dating, charcoal near the top of the 11-m-thick sequence of medium- to fine-grained sediments dates to 41.7 ± 1.0 cal ka BP (Bright et al., Reference Bright, Kaufman, Forman, McIntosh, Mead and Baez2010). Additional dates throughout the sequence using amino-acid racemization on ostracodes and radiocarbon analysis on a bivalve confirm deposition between 40 ka and 43 ka (Bright et al. Reference Bright, Kaufman, Forman, McIntosh, Mead and Baez2010). Because of Térapa's geographic location and diverse fossil record at a time of extensive environmental change, ongoing research on this fauna will contribute to a better understanding of the late Pleistocene in northwestern Mexico.
Here, we use the fossil record at Térapa to investigate changes in large mammal community structure in response to environmental change. To do this, we explore community structure, as represented by feeding strategy and body size, by describing Térapa specimens and their traits, and discussing their geographic distributions in the North American desert region. We hypothesize that changes in this site's large mammal community structure at the end of the Pleistocene are because of a loss of herbivores and large-bodied taxa. We expect that the fauna described in this paper will help to further refine paleoecological interpretations as well as provide a long-term record of faunal change in a region that is vulnerable to ongoing climate change.
METHODS
Taxa described here were presented by Mead et al. (Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006) as: Equus, Tapirus, cf. Platygonus, Camelops-sized camelid, Hemiauchenia-sized camelid, Canis dirus, Lynx rufus, and Procyon. Mead et al. (Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006) also included cf. Odocoileus, Capromeryx, Stockoceras, and Bison on the preliminary list of fauna, but these additional artiodactyl specimens have been excluded from this analysis because they either require additional detailed revision or are part of another review.
Systematic paleontology
To determine taxonomic assignments, we primarily used comparative reference specimens from the Florida Museum of Natural History (FLMNH) and the East Tennessee Museum of Natural History (ETMNH). When possible, linear measurements and the related citations are provided within the taxonomic descriptions. Geographic occurrences were determined using The Paleobiology Database (https://paleobiodb.org) and literature. Records were downloaded from The Paleobiology Database on 27 July 2017 for: Taxonomy = “Equus, Palaeolama, Camelops, Platygonus, Canis, Lynx, Procyon, Smilodon,” Time = “Rancholabrean,” and Location = “North America.”
Species identifications for equid post-crania were determined using a quadratic discriminant analysis implemented in the R statistical computing environment using the package MASS (R Core Team, 2016). Training data used in the analyses are from Sertich et al. (Reference Sertich, Stucky, McDonald, Newton, Fisher, Scott, Demboski, Lucking, McHorse and Davis2014) and McHorse et al. (Reference McHorse, Davis, Scott and Jenkins2016). For analysis of the phalanx, training data included Equus occidentalis, E. conversidens, E. lambei, E. scotti, and a northwestern stilt-legged taxon. For analysis of the metacarpal, training data included Equus complicatus, E. conversidens, E. occidentalis, and E. scotti. R code for this analysis is given in Supplementary Materials.
Community structure
To investigate how community structure has changed at Térapa through time, the fossil community of Perissodactyla, select Artiodactyla, and Carnivora presented here was compared to the same three orders from the modern community. Modern mammal range maps were downloaded from the IUCN (2018). Range maps were sampled at the geographic point of Térapa to assemble a faunal list. Taxa were limited to those that are extant and either native or reintroduced. Antilocapridae, Bovidae, and Cervidae were excluded from both communities because, although they are known from Térapa (Mead et al., Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006), they are not within the scope of the discussion within this manuscript.
Feeding strategies were obtained from Paleobiology Database (https://paleobiodb.org) on 10 August 2020 by searching for taxonomic names. Strategies were grouped into herbivore, herbivorous omnivore, carnivorous omnivore, and carnivore. Body mass (BM; kg) averages were extracted from the MOM database (Smith et al., Reference Smith, Lyons, Ernest, Jones, Kaufman, Dayan, Marquet, Brown and Haskell2003) for each species. Mass estimates include males and females across distribution ranges and are not precise values for the southern geographic location of Térapa. However, they provide a measure to estimate temporal changes in the fauna.
RESULTS
From the mammalian fauna found at Térapa, we provide descriptions for eight species in seven genera, including Equus, Platygonus, Camelops, Palaeolama, Canis, Procyon, Lynx, and Smilodon. Six of these genera appeared on the preliminary faunal list of Mead et al. (Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006), and two of these genera (Palaeolama and Smilodon) are new additions to the known faunal community. Specimens from Térapa are housed temporarily at The Mammoth Site, Hot Springs, South Dakota, USA, and are curated with numbers prefixed with TERA. Specimens will be permanently housed at the Instituto Nacional de Antropología e Historia in Sonora, Mexico.
Systematic paleontology
Class Mammalia
Order Perissodactyla
Family Equidae
Genus Equus Linnaeus, Reference Linnaeus1758
Equus scotti Gidley, Reference Gidley1900
Material: Left distal metacarpal (TERA 313), left partial phalanx 1 (TERA 320), phalanx 2 and sesamoid (TERA 319).
Description: The left metacarpal (TERA 313) is broken transversely across the diaphysis, but the distal end is complete (Fig. 2A). The left partial first phalanx (TERA 320) is missing the lateral, distal portion (Fig. 2B). The metacarpal and phalanx articulate. The second phalanx is complete (TERA 319; Fig. 2C) and articulates with a sesamoid. The first and second phalanges do not articulate.

Figure 2. (color online) Equus scotti specimens. (A) left distal metacarpal (TERA 313); (B) left partial phalanx (TERA 320); (C) second phalanx (TERA 319). (A) and (B) articulate. Scale bar = 5 cm.
Identification: In quadratic discriminant analyses, the second phalanx (TERA 319) was identified to species level with 87.5% confidence, and the distal metacarpal (TERA 313) was identified with 100% confidence.
Remarks: Equus is widespread across the US and Mexico during the Rancholabrean (Ferrusquía-Villafranca et al., Reference Ferrusquía-Villafranca, Arroyo-Cabrales, Martínez-Hernández, Gama-Castro, Ruiz-González, Polaco and Johnson2010, Reference Ferrusquía-Villafranca, Lundelius and Ruiz-Gonzalez2017), including in Sonora (Cruz-y-Cruz et al., Reference Cruz-y-Cruz, Sánchez-Miranda, Carpenter, Terrazas-Mata, Sedov, Solleiro-Rebolledo and Benavente-Sanvicente2018). Taxonomy of Equus introduces complex questions surrounding species identifications. Equus scotti is a stout-legged horse that has been documented across the North American desert region during the Rancholabrean (Harris, Reference Harris2014), including in the Tule Springs local fauna (Scott et al., Reference Scott, Springer and Sagebiel2017). Recent efforts to revise equid taxonomy have considered E. scotti to be synonymous with E. mexicanus (see Alberdi et al., Reference Alberdi, Arroyo-Cabrales, Marín-Leyva and Polaco2014) and E. excelsus (see Priego-Vargas et al., Reference Priego-Vargas, Bravo-Cuevas and Jiménez-Hidalgo2017), but by convention we use E. scotti to be consistent with the data sources used in the analyses. Previous work identified E. excelsus at Térapa (Carranza-Castañeda and Roldán-Quintana, Reference Carranza-Castañeda and Roldán-Quintana2007), and it is likely the same as our E. scotti. However, equid taxonomy needs to be thoroughly and formally evaluated before these issues can be confidently resolved.
Equus cf. E. scotti Gidley, Reference Gidley1900
Material: Right P4 (TERA 284), right M2 (TERA 287), right M3 (TERA 285), right P4 and M1 (TERA 291), right P3 and P4 (TERA 168), left P2 (TERA 282, 289), left P4 (TERA 290), left M2 (TERA 286, 288), left M3 (TERA 283), right p4 (TERA 310), lower left tooth (TERA 308), upper left tooth and lower right tooth (TERA 266).
Description: Most of the teeth are complete with moderate wear. These teeth (Fig. 3A–D) are larger and have more complex enamel patterns than the teeth assigned to Equus sp. (Fig. 3E–H).
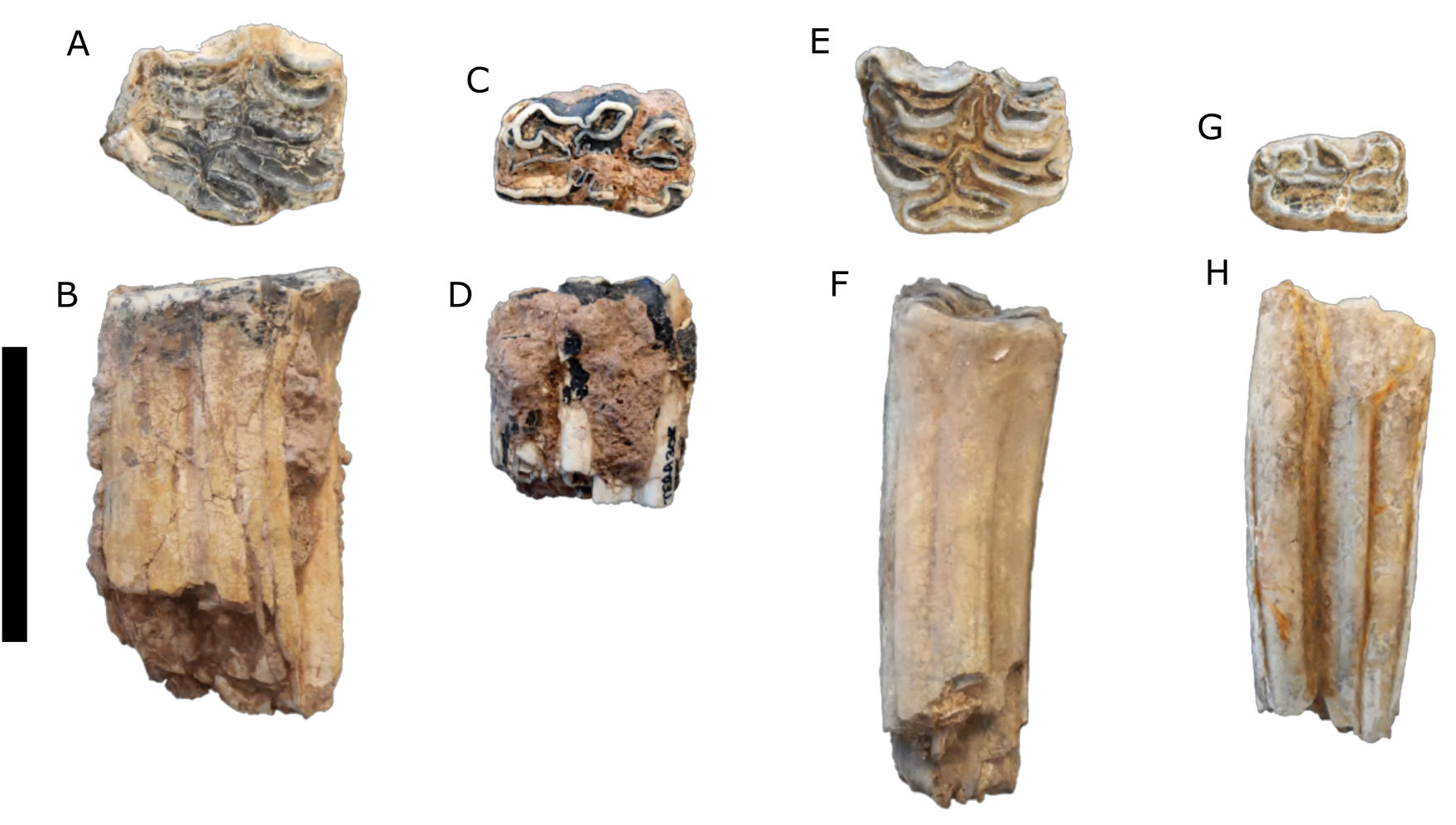
Figure 3. (color online) Equus spp. teeth. (A, B) Equus cf. E. scotti, upper left molar (TERA 286); (C, D) Equus cf. E. scotti, lower left molar (TERA 308); (E, F) Equus sp., upper left molar (TERA 303); (G, H), Equus sp., lower right molar (TERA 295). (A, C, E, G) in occlusal view; (B, D, F, H) in lingual view. Scale bar = 5 cm.
Identification: We hypothesize that the teeth are from the same species as the postcranial elements previously identified as Equus scotti because of their large size. More specific identification is difficult with isolated teeth because they lack a majority of diagnostic characters (Famoso and Davis, Reference Famoso and Davis2014).
Equus sp.
Material: Right P3 (TERA 166), right M1 (TERA 263), right M2 (TERA 157, 169, 296, 300), right M3 (TERA 298), left M2 (TERA 303), left P4 and M1 (TERA 293), left P3-M1 and right M1 (TERA 297), upper right tooth (TERA 299, 307), upper tooth (TERA 267), right m2 (TERA 295), right m3 (TERA 262, 305, 306), left m2 with fragment (TERA 312), left m3 (TERA 322), lower left molar (TERA 301, 302), lower right tooth (TERA 309), lower left tooth (TERA 311), lower tooth fragment (TERA 264, 265, 304), mandibular symphysis (TERA 294), left partial distal humerus (TERA 314), left magnum (TERA 318), right distal tibia (TERA 317), left partial calcaneum (TERA 315, 316), right cuneiform and lunar (TERA 321).
Description: The postcranial elements are slight compared to extant Equus caballus and the Térapa specimens conferred to Equus scotti, and these teeth (Fig. 3E–H) are noticeably smaller and have less complex enamel patterns than those assigned to E. cf. E. scotti (Fig. 3A–D).
Identification: It is unknown if the post-cranial elements are of the same species as the dentition. At this time, there are no morphological features to confirm species designations.
Remarks: Previous work on different specimens also identified E. conversidens at Térapa (Carranza-Castañeda and Roldán-Quintana, Reference Carranza-Castañeda and Roldán-Quintana2007), and, because of the smaller size, it is possible that our Equus sp. refers to the same taxon. Occurrence of both E. scotti and a smaller-sized horse is common at Rancholabrean sites, and the smaller horse is often identified as E. conversidens (Harris, Reference Harris2014). Again, most isolated teeth can only be identified to genus because of the lack of diagnostic characters (Famoso and Davis, Reference Famoso and Davis2014).
Mead et al. (Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006) listed Tapirus sp. among the taxa found at Térapa based on a mandibular symphysis (TERA 294; Fig. 4A). However, this specimen is now identified as Equus sp. Depth of the mandible suggests hypsodont teeth as in Equus. The narrow intermandibular space extends anterior to the second premolar as in Equus. In Tapirus, this space is closed at the anterior end of the second premolar. The mandibular foramen is located well anterior to the tooth row as in Equus. In Tapirus, the mandibular foramen is inferior to the anterior second premolar.

Figure 4. (color online) Equus spp. mandible fragments. (A) Equus sp. (TERA 294); previously identified as Tapirus by Mead et al. (Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006); (B) Equus caballus (ETMNH-Z 15462); reference specimen. Scale bar = 5 cm.
Order Artiodactyla
Family Tayassuidae
Platygonus LeConte, Reference LeConte1848
Platygonus compressus LeConte, Reference LeConte1848
Material: Molar fragments (TERA 167), deciduous upper premolar (TERA 280), right upper canine (TERA 281).
Description: The molar fragments (TERA 167) are hypsodont and zygodont (Fig. 5A). The deciduous tooth (TERA 280) is complete and more bunodont than the molar fragments (Fig. 5B). The canine is complete (TERA 281) and has an anterior occlusal surface (Fig. 5C).

Figure 5. (color online) Platygonus compressus specimens. (A) Three molar fragments (TERA 167); (B) deciduous upper tooth in occlusal view (TERA 280); (C) right upper canine in labial view (TERA 281). Scale bar = 5 cm.
Identification: This specimen was initially identified as cf. Platygonus by Mead et al. (Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006). Comparisons with fossil material at FLMNH suggest that these teeth are Platygonus because the cusps are more zygolophodont, as in Platygonus, rather than bunodont as in Mylohyus. Because Platygonus is monotypic in the middle and late Rancholabrean (Kurtén and Anderson, Reference Kurtén and Anderson1980; Wright, Reference Wright, Janis, Scott, Jacobs, Gunnell and Uhen1998), these specimens are assigned to P. compressus.
Remarks: Platygonus compressus is known from the Rancholabrean of Arizona (Murray et al., Reference Murray, Bell, Dolan and Mead2005), New Mexico, and eastern Texas, as well as the Central Plateau and Trans-Mexican Volcanic Belt (Ferrusquía-Villafranca et al., Reference Ferrusquía-Villafranca, Arroyo-Cabrales, Martínez-Hernández, Gama-Castro, Ruiz-González, Polaco and Johnson2010, Reference Ferrusquía-Villafranca, Lundelius and Ruiz-Gonzalez2017). In Sonora, Platygonus sp. is known from La Playa and Bajimari (White et al., Reference White, Mead, Baez, Swift, Molina-Freaner and Van Devender2010) and P. cf. P. vetus is known from El Golfo (Croxen et al., Reference Croxen, Shaw and Sussman2007). Térapa provides the first record of P. compressus in Sonora, but it is not unexpected.
Family Camelidae
Camelops Leidy, Reference Leidy1854
Camelops hesternus (Leidy, Reference Leidy1873)
Material: Left mandibular fragment with roots of m1-2 (TERA 278); left partial distal phalanx (TERA 279).
Description: The partial distal phalanx (TERA 279) has splayed ventral trochlea (Fig. 6A). This specimen is not a metapodial because of the lack of a condylar keel (Zazula et al., Reference Zazula, MacPhee, Hall and Hewitson2016). The mandibular fragment (TERA 278) has the roots of large selenodont molars (Fig. 6B). At its base, the m2 measures 43.28 mm.

Figure 6. (color online) Camelidae specimens. (A) Camelops hesternus, partial left distal phalanx in anterior view (TERA 279); (B) C. hesternus, left mandibular fragment, including roots of first and second molars, in occlusal view (TERA 278); (C) Palaeolama mirifica, right dentary fragment with a partial fourth premolar and three molars in occlusal view and labial view (D) (TERA 156). Black arrow indicates the infolding on the p4 that is characteristic of Palaeolama. Scale bar = 5 cm.
Identification: These specimens were initially identified as “Camelops-sized” by Mead et al. (Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006). The m2 measurement is within the range of Camelops provided by Honey et al. (Reference Honey, Harrison, Prothero, Stevens, Janis, Scott, Jacobs, Gunnell and Uhen1998) and Baskin and Thomas (Reference Baskin and Thomas2016). The mandible is also substantially larger than comparative material of Palaeolama and Hemiauchenia. The phalanx was also compared to phalanges of Palaeolama and Hemiauchenia, but it is considerably larger than specimens within both genera. Camelops is the only other Rancholabrean camelid, and it was large enough to have mandibles and phalanges of the size found at Térapa (Baskin and Thomas, Reference Baskin and Thomas2016). The most recent review of Camelops described two species: C. hesternus in the Rancholabrean and C. minidokae in the Irvingtonian (Baskin and Thomas, Reference Baskin and Thomas2016). Because Camelops is considered monotypic in the Rancholabrean and the specimens are consistent with the morphology, these specimens are assigned to C. hesternus.
Remarks: Camelops hesternus is widespread across the US and northern Mexico (Ferrusquía-Villafranca et al., Reference Ferrusquía-Villafranca, Arroyo-Cabrales, Martínez-Hernández, Gama-Castro, Ruiz-González, Polaco and Johnson2010, Reference Ferrusquía-Villafranca, Lundelius and Ruiz-Gonzalez2017), including in Sonora (Cruz-y-Cruz et al., Reference Cruz-y-Cruz, Sánchez-Miranda, Carpenter, Terrazas-Mata, Sedov, Solleiro-Rebolledo and Benavente-Sanvicente2018). In the southern US, C. hesternus is known from Rancho La Brea (Jones and DeSantis, Reference Jones and Desantis2017), Diamond Valley Lake (Springer et al., Reference Springer, Scott, Sagebiel and Murray2009), Tule Springs (Scott et al., Reference Scott, Springer and Sagebiel2017), and throughout Arizona (Mead et al., Reference Mead, Czaplewski and Agenbroad2005) and New Mexico (Harris, Reference Harris2014).
Palaeolama Gervais, Reference Gervais1867
Palaeolama mirifica (Simpson, Reference Simpson1929)
Material: Left mandible fragment with partial p4-m3 (TERA 156).
Description: The mandibular fragment (TERA 156) has a partial p4 and m1–3 with brachydont, selenodont dentition (Fig. 6C, D). The p4 has a vertical groove just posterior to the break. The m2 measures 19.18 mm at the base and 21.25 mm at the occlusal surface.
Identification: This specimen was initially identified as “Hemiauchenia-sized” by Mead et al. (Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006). The groove on the p4 is indicative of the ‘complex infolding’ seen in Palaeolama (Fig. 6C; Kurtén and Anderson, Reference Kurtén and Anderson1980; Honey et al., Reference Honey, Harrison, Prothero, Stevens, Janis, Scott, Jacobs, Gunnell and Uhen1998, p. 454). Both m2 measurements are within the range of Palaeolama provided by Honey et al. (Reference Honey, Harrison, Prothero, Stevens, Janis, Scott, Jacobs, Gunnell and Uhen1998). Because Palaeolama is monotypic in the Rancholabrean of North America (Honey et al., Reference Honey, Harrison, Prothero, Stevens, Janis, Scott, Jacobs, Gunnell and Uhen1998) and the specimens fit the morphology, these specimens are assigned to P. mirifica.
Remarks: Palaeolama mirifica is found at Rancholabrean sites in Florida, California, and Texas (Kurtén and Anderson, Reference Kurtén and Anderson1980), South Carolina (Sanders, Reference Sanders2002), Costa Rica (Pérez, Reference Pérez2011), and in the Mexican state of Puebla (Bravo-Cuevas and Jiménez-Hidalgo, Reference Bravo-Cuevas and Jiménez-Hidalgo2015). However, it has not been documented in Arizona (Mead et al., Reference Mead, Czaplewski and Agenbroad2005). Palaeolama sp. is documented at Irvingtonian sites at El Golfo in northwestern Sonora (Croxen et al., Reference Croxen, Shaw and Sussman2007) and Rio Tomayate in El Salvador (Cisneros, Reference Cisneros2005), and from the Rancholabrean of Guatemala (Dávila et al., Reference Dávila, Stinnesbeck, Gonzalez, Lindauer, Escamilla and Stinnesbeck2019). Térapa is the first Rancholabrean occurrence of P. mirifica in northwestern Mexico.
Order Carnivora
Family Canidae
Canis Linnaeus, Reference Linnaeus1758
Canis dirus Leidy, Reference Leidy1858
Material: Left mandible with c1–m2 (TERA 154), left maxilla and jugal with P3–M1, left mandible with partial c1–partial m2, right mandible with p4–m2, four incisors, two upper canines, two lower canines, one M1, one m3, and two unidentified fragments (TERA 450), distal left humerus (TERA 155).
Description: The left mandible with c1–m2 (TERA 154) and the distal humerus (TERA 155) were previously described in detail (Hodnett et al., Reference Hodnett, Mead and Baez2009); the remaining specimens (TERA 450) are described here. All teeth in the maxilla and both mandibles are in an advanced stage of wear. The left maxilla is articulated with the left jugal, and the P3 is broken between the anterior and posterior roots (Fig. 7A, B). The left mandible is missing the coronoid process and angular process, but the condyle is complete (Fig. 7C). There is no evidence of an alveolus for a p1 on the mandible. The anterior mental foramen is inferior to the anterior p2, and the posterior mental foramen is inferior to the posterior p3. The right mandible is broken between the p4 and m1 and along the inferior masseter fossa (Fig. 7D). Only the anterior root of the m2 is present and the alveolus for a single-rooted m3 is broken. Cranial and dental measurements suggest a larger than average C. dirus (Fig. 8, Supplemental Table S2; Tedford et al., Reference Tedford, Wang and Taylor2009).

Figure 7. (color online) Canis dirus specimens (TERA 450). (A) Left maxilla and jugal with P3–M1 in lateral view; (B) left maxilla and jugal with P3–M1 in occlusal view; (C) left mandible with partial c1–partial m2 in buccal view; (D) right mandible with p4–m2 in buccal view. Scale bar = 5 cm.

Figure 8. Log-ratios of measurements from Térapa Canis dirus (TERA 450) left maxilla and left mandible compared to Canis dirus from the Rancholabrean (RLB) and Irvingtonian (IRV), C. armbrusteri, C. lupus, and C. latrans. Measurements are relative to Eucyon davisi. Methods and reference data from Tedford et al. (Reference Tedford, Wang and Taylor2009), and data are available in Supplemental Table S2. Abbreviations: Left maxilla measurements: LP4 = length of P4; WP4 = width of P4; LM1 = length of M1; WM1 = width of M1. Left mandible measurements: Lp3 = length of p3; Lp4 = length of p4; Wp4 = width of p4; Lm1 = length of m1; Wm1tr = width of m1 trigonid; Wm1tl = width of m1 talonid.
Identification: Mead et al. (Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006) initially identified Canis dirus at Térapa, and Hodnett et al. (Reference Hodnett, Mead and Baez2009) agreed for TERA 154 and TERA 155. Measurements on the left mandible indicate that this specimen is too large to be a different Rancholabrean-age canid, such as C. latrans or C. lupus (Fig. 8). Canis armbrusteri was also a large canid in the late Irvingtonian (Kurtén and Anderson, Reference Kurtén and Anderson1980; Harris, Reference Harris2014) and is thought to have given rise to C. dirus (Tedford et al., Reference Tedford, Wang and Taylor2009). The Térapa teeth are too worn to examine the cusp patterns, but the upper molars have reduced labial cingula as in C. dirus rather than C. armbrusteri (Tedford et al., Reference Tedford, Wang and Taylor2009).
Remarks: Canis dirus is considered “one of the most common mammalian species in the Rancholabrean” and is found across the US and Mexico (Kurtén and Anderson, Reference Kurtén and Anderson1980, p. 171; Mead et al., Reference Mead, Czaplewski and Agenbroad2005; Harris, Reference Harris2014; Ferrusquía-Villafranca et al., Reference Ferrusquía-Villafranca, Lundelius and Ruiz-Gonzalez2017; Ruiz-Ramoni and Montellano-Ballesteros, Reference Ruiz-Ramoni and Montellano-Ballesteros2019). In the southern US, C. dirus is common in the local faunas at Rancho La Brea (McHorse et al., Reference McHorse, Orcutt and Davis2012), Diamond Valley Lake (Springer et al., Reference Springer, Scott, Sagebiel and Murray2009), and Tule Springs (Scott et al., Reference Scott, Springer and Sagebiel2017). However, Térapa is the first occurrence of C. dirus in Sonora (Hodnett et al., Reference Hodnett, Mead and Baez2009; Ruiz-Ramoni and Montellano-Ballesteros, Reference Ruiz-Ramoni and Montellano-Ballesteros2019). There are at least three individuals of C. dirus found at Térapa based on lower left canines. The maxilla and mandibles of TERA 450 are likely from the same individual because of the similar degree of wear on the teeth. The extensive wear on the teeth and the large size suggests one older individual.
Family Procyonidae
Procyon Storr, Reference Storr1780
Procyon lotor (Linnaeus, Reference Linnaeus1758)
Material: Left calcaneum (TERA 453).
Description: The calcaneum (TERA 453) is complete (Fig. 9A). There is no tubercle present on the trochlear process, which has a minimal groove, and the calcaneum does not have an accessory surface between the anterior articular surface and the cuboid facet. The latter feature has a point on its dorsal edge. The greatest length of the calcaneum is 28.03 mm and the transverse breadth of the sustentaculum is 14.49 mm.

Figure 9. (color online) Carnivora specimens. (A) Procyon lotor, left calcaneum in anterior view (TERA 453); (B) Lynx rufus, distal left radius in anterior view (TERA 451). Scale bar = 1 cm.
Identification: The calcaneum was previously identified as Procyon sp. (Mead et al., Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006). Following descriptions provided by Stains (Reference Stains1973; lack of a knob on the trochlear process), the calcaneum is now referred to P. lotor. Within Procyon, the calcaneum is assigned to P. lotor instead of P. cancrivorous because of the minimal trochlear groove, the cuboid facet, and the lack of an accessory surface between the anterior articular surface and the cuboid facet (Stains, Reference Stains1973). In addition, the length and breadth measurements are within the range of P. lotor provided by Stains (Reference Stains1973). Additional Pleistocene species of Procyon have been synonymized with P. lotor because the morphology was within the range of intraspecific variation (Kurtén and Anderson, Reference Kurtén and Anderson1980). Kurtén and Anderson (Reference Kurtén and Anderson1980) recognized only one other fossil species, P. rexroadensis, which was limited to the Blancan LMA. Emmert and Short (Reference Emmert and Short2018) recommended synonymizing P. rexroadensis with P. lotor because of a lack of distinct morphological characters.
Remarks: Pleistocene-age Procyon has been found across North America and into northern South America (Kurtén and Anderson, Reference Kurtén and Anderson1980), but the fossil record is sparse (Harris, Reference Harris2014). In Mexico, Procyon sp. is found at the Irvingtonian-age El Golfo (Croxen et al., Reference Croxen, Shaw and Sussman2007), and Procyon lotor is known from the Rancholabrean in California and New Mexico (Harris, Reference Harris2014) as well as the Chihuahua-Coahuila Plateaus and Ranges, the Sierra Madre Oriental, the Trans-Mexican Volcanic Belt and the Yucatan Platform (Ferrusquía-Villafranca et al., Reference Ferrusquía-Villafranca, Arroyo-Cabrales, Martínez-Hernández, Gama-Castro, Ruiz-González, Polaco and Johnson2010), so it is expected in the northwest of Mexico, although it is not reported from the Rancholabrean of Arizona (Mead et al., Reference Mead, Czaplewski and Agenbroad2005).
Family Felidae
Lynx Kerr, Reference Kerr1792
Lynx rufus (Schreber, Reference Schreber1777)
Material: Distal left radius (TERA 451).
Description: The radius (TERA 451) is broken transversely across the diaphysis, which is compressed anteroposteriorly (Fig. 9B). The styloid process, dorsal tubercle, and lateral tuberosity are angular and pronounced. The greatest breadth of the distal end (Bd; von den Driesch, Reference von den Driesch1976) measures 19.41 mm.
Identification: This radius was initially reported as Lynx rufus by Mead et al. (Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006), and the identification is confirmed here. The distinct features are as in Felidae rather than Canidae, and the anteroposterior compression excludes Felis (Kelson, Reference Kelson1946). As in L. rufus, there is a distinct horizontal ridge superior to the distal articulation on the posterior surface, and a lack of mediolateral constriction between the diaphysis and epiphysis. Lynx rufus is known from the Rancholabrean of Mexico (Ferrusquía-Villafranca et al., Reference Ferrusquía-Villafranca, Arroyo-Cabrales, Martínez-Hernández, Gama-Castro, Ruiz-González, Polaco and Johnson2010) and Arizona (Mead et al., Reference Mead, Czaplewski and Agenbroad2005), whereas Lynx canadensis has not been found south of Utah (Lavoie et al., Reference Lavoie, Renard and Larivière2019).
Remarks: Lynx rufus is frequently found at North American Pleistocene sites (Kurtén and Anderson, Reference Kurtén and Anderson1980), including at Rancho La Brea (McHorse et al., Reference McHorse, Orcutt and Davis2012), Diamond Valley Lake (Springer et al., Reference Springer, Scott, Sagebiel and Murray2009), and Tule Springs (Scott et al., Reference Scott, Springer and Sagebiel2017). L. rufus is known across northern and central Mexico during the Rancholabrean (Ferrusquía-Villafranca et al., Reference Ferrusquía-Villafranca, Arroyo-Cabrales, Martínez-Hernández, Gama-Castro, Ruiz-González, Polaco and Johnson2010, Reference Ferrusquía-Villafranca, Lundelius and Ruiz-Gonzalez2017). In Mexico, L. rufus is also known from the Irvingtonian of Cedazo in central Mexico (Mooser and Dalquest, Reference Mooser and Dalquest1975), and the latest Pleistocene or early Holocene of Jimenez Cave in Chihuahua (Messing, Reference Messing1986).
Smilodon Lund, Reference Lund1842
Smilodon cf. S. fatalis (Leidy, Reference Leidy1868)
Material: Fragment of right dP3 including ectoparastyle and parastyle (TERA 452).
Description: The tooth fragment (TERA 452) is mediolaterally compressed and has a distinct parastyle and ectoparastyle (Fig. 10B). The tooth fragment is lacking a distinct protocone and preserves no evidence of any lingual flaring that would indicate a protocone had been present. There is a minimal anterior cingulum on the tooth. Diagnostic measurements are not possible because of the fragmented nature of the specimen.

Figure 10. (color online) Smilodon carnassials. (A) S. fatalis adult upper right fourth premolar (UF/TRO 11) with parastyle and ectoparastyle shaded more opaquely; reference specimen; (B) S. cf. S. fatalis fragment of deciduous upper right third premolar in labial view with parastyle and ectoparastyle labeled (TERA 452). Scale bar = 1 cm.
Identification: This specimen was initially identified as Canis latrans by Mead et al. (Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006). However, the mediolateral compression and parastyle suggest this tooth is from Felidae and not Canidae. The size suggests a large cat, possibly Panthera, Puma, or Smilodon. The lack of a protocone excludes Panthera and Puma (Cherin et al., Reference Cherin, Iurino and Sardella2013; Babiarz et al., Reference Babiarz, Wheeler, Knight, Martin, Werdelin, McDonald and Shaw2018), and the ectoparastyle and cingulum are as in Smilodon (Christiansen, Reference Christiansen2013). Therefore, we confer the tooth fragment to Smilodon. Because Smilodon fatalis is common during the Rancholabrean, S. populator has not been found in North America, and S. gracilis is from the early Irvingtonian, we confer the specimen to S. fatalis.
Remarks: Smilodon is known throughout much of North America from the Irvingtonian and Rancholabrean LMAs (Kurtén and Anderson, Reference Kurtén and Anderson1980; Babiarz et al., Reference Babiarz, Wheeler, Knight, Martin, Werdelin, McDonald and Shaw2018), but this is the first record from the northwest of Mexico. In Mexico, S. fatalis has been found at Pleistocene sites across the Central Plateau, the Trans-Mexico Volcanic Belt, and the Sierra Madre Oriental (Ferrusquía-Villafranca et al., Reference Ferrusquía-Villafranca, Arroyo-Cabrales, Martínez-Hernández, Gama-Castro, Ruiz-González, Polaco and Johnson2010, Reference Ferrusquía-Villafranca, Lundelius and Ruiz-Gonzalez2017). In the United States, S. fatalis is known from the Rancholabrean of eastern New Mexico and southern California (Kurtén and Anderson, Reference Kurtén and Anderson1980; Morgan and Lucas, Reference Morgan and Lucas2001; Harris, Reference Harris2014), including at Rancho La Brea (McHorse et al., Reference McHorse, Orcutt and Davis2012), Diamond Valley Lake (Springer et al., Reference Springer, Scott, Sagebiel and Murray2009), and Tule Springs (Scott et al., Reference Scott, Springer and Sagebiel2017). The presence in northwestern Mexico is novel but not wholly unexpected, although it has not been reported from the Rancholabrean of Arizona (Mead et al., Reference Mead, Czaplewski and Agenbroad2005).
Community structure
The late Pleistocene and modern mammalian communities vary in their composition. The fossil community has two equids, two camelids, one tayassuid, one canid, two felids, and one procyonid, whereas in the present day, the Térapa region has one tayassuid, two canids, three felids, four mephitids, two mustelids, and three procyonids. The fossil community presented here consists of four herbivores from two orders of ungulates (Fig. 11). The four carnivorans are equally divided into carnivores and carnivorous omnivores. Yet, the modern community is largely dominated by 11 carnivorous omnivores, with three carnivores, one herbivorous omnivore, and no herbivores (Fig. 11). Lynx rufus (carnivore) and Procyon lotor (carnivorous omnivore) are the only taxa to occur in both fossil and modern communities.

Figure 11. (color online) Feeding strategies of large-bodied mammals at Térapa in the fossil community (n = 9 species) and the modern community (n = 15 species).
The faunal turnover of the taxa in this study corresponds with a 97% decrease in mean body mass (BM; kg) from 289 kg to 9 kg. Odocoileus virginianus is the largest ungulate at Térapa today (BM = 55 kg), but, in the past, the larger Equus scotti (BM = 555 kg), Camelops hesternus (BM = 1100 kg), Palaeolama mirifica (BM = 80 kg), and Platygonus compressus (BM = 110 kg) also occurred at the site. Similarly, with the loss of Smilodon cf. S. fatalis (BM = 400 kg), the largest carnivoran today is Puma concolor (BM = 52 kg). Canis dirus (BM = 50 kg) was nearly the same mass as P. concolor. Procyon lotor (BM = 5.5 kg) is the smallest taxon in the fossil community presented here, but the modern community has eight species of Carnivora that are smaller than P. lotor.
DISCUSSION
Térapa provides the first Rancholabrean occurrences of Palaeolama, Procyon, and Smilodon in northwest Mexico and the first records of Platygonus compressus and Canis dirus in Sonora. Equus, Camelops, and Canis are well-represented at sites across the North American desert region and, although Platygonus and Lynx are sparsely represented in northern Mexico, they have extensive records in the southwestern US, making them expected at Térapa during the Rancholabrean.
Térapa lies along the Pleistocene Sonora–Central America Pacific lowlands corridor and the Rocky Mountains–Sierra Madre Occidental corridor (Ceballos et al., Reference Ceballos, Arroyo-Cabrales and Ponce2010). The former allowed dispersal of tropical taxa north and the latter permitted the movement of temperate taxa south (Ceballos et al., Reference Ceballos, Arroyo-Cabrales and Ponce2010). Additional ‘holding pen’ areas would have been inhabited by taxa until more preferred environments allowed further migration (Ceballos et al., Reference Ceballos, Arroyo-Cabrales and Ponce2010; Woodburne, Reference Woodburne2010). With the presence of water and diverse vegetation, Térapa likely acted as an area of faunal exchange. For instance, Palaeolama originated in South America (Webb, Reference Webb and Webb1974), and researchers postulate that P. mirifica used tropical corridors along the Sonora–Central America Pacific lowlands and the Tamaulipas–Central America Gulf lowlands to move north across Mexico (Bravo-Cuevas and Jiménez-Hidalgo, Reference Bravo-Cuevas and Jiménez-Hidalgo2015).
The fauna at Térapa documents a shift in feeding strategy from a community of primarily herbivores during the late Pleistocene to one of primarily carnivorous omnivores at present. Ranges of carnivoran species and the richness of carnivoran communities have been shown to be affected by climatically driven habitat changes during the transitions of the glacial and interglacial stages of the Pleistocene (Arias-Alzate et al., Reference Arias-Alzate, González-Maya, Arroyo-Cabrales and Martínez-Meyer2017, Reference Arias-Alzate, González-Maya, Arroyo-Cabrales, Medellín and Martínez-Meyer2020). In this region of mosaic complexity and shifting ecosystems, these dynamic environmental changes may have driven faunal community change in this area, including the transition to a carnivoran-dominated community.
Previous studies have documented large declines in community average body mass at the transition between the late Pleistocene and Holocene (Stegner and Holmes, Reference Stegner and Holmes2013; Smith et al., Reference Smith, Smith, Lyons and Payne2018). The mammalian fauna presented here indicates a similar change, with a 97% decline of community average body mass from 289 kg to 9 kg. The considerable change is due to the loss of megaherbivores and the reduction in body mass of the felids present. Average body mass decline has been linked to rises in global temperature (Gardner et al., Reference Gardner, Peters, Kearney, Joseph and Heinsohn2011; Martin et al., Reference Martin, Mead and Barboza2018; Martin and Barboza, Reference Martin and Barboza2020) as well as shortages in food availability (Huston and Wolverton, Reference Huston and Wolverton2011; McNamara et al., Reference McNamara, Higginson and Verhulst2016; Westover and Smith, Reference Westover and Smith2020). Although Térapa provides a broad scope of temporal change at a single locality (with several sub-localities therein), it nevertheless captures broad environmental change as recorded by the reorganization of the terrestrial fauna.
If antilocaprids, bovids, and cervids had been included in the study of community structure, the fossil community would add four artiodactyl species (Capromeryx sp., Stockoceros sp., cf. Odocoileus, and Bison sp.; Mead et al., Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006) while the modern community would only add one species (Odocoileus virginianus). The inclusion of these taxa would only exacerbate the differences between the faunal communities during the two time periods. While it is likely additional species lived at Térapa and have not been recovered, it would require finding 59 additional species of Carnivora at Térapa to match the proportional community structure of carnivores and herbivores that is present currently. The dramatic shift from herbivores to carnivores at Térapa follows a similar pattern documented across Mexico (Arroyo-Cabrales et al., Reference Arroyo-Cabrales, Polaco, Johnson and Ferrusquía-Villafranca2010; Ferrusquía-Villafranca et al., Reference Ferrusquía-Villafranca, Arroyo-Cabrales, Martínez-Hernández, Gama-Castro, Ruiz-González, Polaco and Johnson2010).
The large mammalian fauna at Térapa aligns with previous faunal environmental analyses that described an elevational mosaic of a temperate to tropical/subtropical marsh, an adjacent semiarid savanna-grassland, a slow-moving freshwater stream, and a riparian forest area along the water (Mead et al., Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006; Nunez et al., Reference Nunez, MacFadden, Mead and Baez2010; Bright et al. Reference Bright, Orem, Mead and Baez2016). The presence of both browsing and grazing herbivores implies a diverse assemblage of trees, forbs, and grasses at Térapa in the past. Currently, there are no grazing ungulates present at Térapa, and the region lacks expansive grasslands.
Additionally, the presence of Lynx rufus suggests a more restrictive paleoenvironmental interpretation and may be indicative of a mosaic-edge habitat associated with the nearby mountain foothills (~16 km). Extant L. rufus occupy strictly temperate habitats of savanna/grassland/chaparral but also occupy relatively large home ranges (3–96 km2 for males and 1–38 km2 for females; Lariviere and Walton, Reference Lariviere and Walton1997). The diet of L. rufus includes rodents, small ungulates, large ground birds, and reptiles (Lariviere and Walton, Reference Lariviere and Walton1997), which are all present in the fossil assemblage at Térapa (Mead et al., Reference Mead, Baez, Swift, Carpenter, Hollenshead, Czaplewski, Steadman, Bright and Arroyo-Cabrales2006).
During the glacial period when the fossils at Térapa were deposited, cooler global temperatures produced a more constricted Intertropical Convergence Zone (ITCZ) and increased precipitation in northwestern Mexico (Metcalfe, Reference Metcalfe2006). As temperatures warmed into the modern interglacial period, the ITCZ expanded poleward, and shifted the mid-latitude jet stream and associated precipitation poleward (Metcalfe, Reference Metcalfe2006). The poleward shift of the ITCZ and mid-latitude jet stream also shifted the biodiversity-rich savanna habitats northwards into the southwestern US and as a consequence, left behind the present-day scrubland habitats of northern Mexico (Metcalfe, Reference Metcalfe2006). As temperatures increased by ~6°C and precipitation decreased, Sonora became more arid, and species were forced to shift their ranges, often northward (Metcalfe, Reference Metcalfe2006).
With impending climate change and anticipated warming of an additional 4°C globally by the end of the 21st century, paleoecological studies of past faunas can have great implications for wildlife conservation because of the associated biodiversity shifts (Walther et al., Reference Walther, Post, Convey, Menzel, Parmesan, Beebee, Fromentin, Hoegh-Guldberg and Bairlein2002; Foley et al., Reference Foley, DeFries, Asner, Barford, Bonan, Carpenter and Chapin2005; Lipton et al., Reference Lipton, Rubenstein, Weiskopf, Carter, Peterson, Crozier, Fogarty, Reidmiller, Avery, Easterling, Kunkel, Lewis, Maycock and Stewart2018). The Sonoran Desert is expected to continue expanding further northwards in Arizona and New Mexico as climate projections suggest an increasingly arid climate (Magaña et al., Reference Magaña, Zermeño and Neri2012). With shifting environments, species may have to alter their geographic ranges, but only 41% of natural areas (i.e., areas with low effects of human modification) in the US are suitably connected to allow for species movement (McGuire et al., Reference McGuire, Lawler, McRae, Nuñez and Theobald2016). Conservation efforts on the margins of species ranges, especially those in or adjacent to the desert southwest of North America, may also help facilitate the preservation of unique genetic adaptations to harsh climatic pressures (Plumb and McMullen, Reference Plumb and McMullen2018). Sustaining habitats and their connectivity for conservation may be difficult to facilitate, but we aim to provide a broad scope of anticipated biodiversity change in the presence of continued warming.
CONCLUSIONS
Relatively few fossil sites are well known from the Rancholabrean LMA of northern Mexico (Ceballos et al., Reference Ceballos, Arroyo-Cabrales and Ponce2010; White et al., Reference White, Mead, Baez, Swift, Molina-Freaner and Van Devender2010), and Térapa provides an extensive faunal record in an area that was once a marsh savanna but is now xeric desert chaparral. At this site, we describe mammalian community restructuring due to the loss of the large-bodied, herbivorous ungulates in response to environmental change. Whereas the fossil community was nearly evenly split between carnivorans and herbivorous ungulates, the present community is dominated by carnivorans (Fig. 11). There is also an associated 97% decrease in mean body mass through time because of the loss of the largest taxa. We postulate that this change is largely due to rising temperature and shifting precipitation regimes and the resulting climatically driven changes in vegetation, such as the loss of grasslands. As the climate continues to warm and the deserts shift north, today's fauna in the southwestern US and northwestern Mexico will be similarly affected.
Long term records of faunal change, such as that at Térapa, provide valuable information for guiding modern conservation practices. The presence of eight taxa of Perissodactyla, Artiodactyla, and Carnivora in a community that existed approximately 40–43 ka (MIS/OIS 3) provides a critical spatial and temporal record, including the first Rancholabrean occurrences of Palaeolama, Procyon, and Smilodon in northwest Mexico, and the first records of Platygonus compressus and Canis dirus in the state of Sonora. It is probable that more sites exist in this region and can contribute to a more complete understanding of the area but have yet to be found, fully analyzed, and reported upon. Future fossil recovery will provide much needed details about the fauna in and around Térapa and in Sonora, and will also enable further study of regional habitat and biodiversity shifts in the southwestern US.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/qua.2020.125
Acknowledgments
We are grateful to B. Compton (ETSU Museum of Natural History) and R. Hulbert (Florida Museum of Natural History) for access to reference specimens. We thank M. Lawing and P. Barboza for providing feedback on an earlier draft of this manuscript, S. Holte and E. Doughty for helping with species identifications, and J. Pigati, associate editor, for constructive editorial contributions during the review process. Field assistance was received from numerous colleagues and students, more than can be listed here over the 10 years, but continued help was received from M. Hollenshead, M.C. Carpenter, J.M. Meyers, C. McCracken, J. Bright, C.J. Czaplewski, G.S. Morgan, M. Imhof (Madsen), and F. Croxen. We appreciate the discussions about the Pleistocene of Sonora with J. Arroyo-Cabrales and R.S. White. F. Tapia Grijalva and E. Villalpando of Instituto Nacional de Antropología e Historia, INAH Sonora assisted with obtaining excavation and transportation permits. H. Ruiz Durazo, E.M. Aruna Moore, and family provided immeasurable logistical help throughout the many years of this ongoing project. E. Scott and B. McHorse provided equid metacarpal and metatarsal data for quadratic discriminate analyses. Support for RAS came from a Merit Fellowship and a Tom Slick Graduate Research Fellowship from Texas A&M College of Agriculture and Life Sciences, and a Dishman-Lucas Graduate Assistantship from Texas A&M legacy Department of Ecosystem Science and Management (now the Department of Ecology and Conservation Biology).














