Acute lung injury is the result of the host response to either inhaled or endogenous noxious agents and is characterised by the activation of nasal and bronchoalveolar-associated lymphoid tissue. Since lungs are constantly exposed to a large number of micro-organisms and provide a common entry route for pathogens into the body, lung infection is one of the most frequent causes of morbidity and death in humans(Reference Augusto, Li and Synguelakis1). The inflammation cascade is initiated by the innate immune system, in which neutrophils have a key role in the inflammatory response as they contribute to the recruitment, activation and programming of antigen-presenting cells(Reference Chaudhuri and Sabroe2). Furthermore, they generate chemotactic signals that attract monocytes and dendritic cells(Reference Nathan3).
Enteral nutrition has a role in the prevention or amelioration of inflammatory responses in systemic mucosae. Dietary lycopene, a carotenoid with a powerful antioxidant capacity, can decrease eosinophilic infiltrates and reduce Th2 cytokine responses from lymph nodes, in a mouse model of allergic airway disease(Reference Hazlewood, Wood and Hansbro4). Dietary supplementation with amino acids such as glycine(Reference Wheeler, Rose and Yamashima5) and tryptophan(Reference Melchior and Sève6) moderates lung inflammation, while dietary EPA and γ-linolenic acid reduce the risk of mortality in patients with acute respiratory distress syndrome(Reference Pontes-Arruda, Demichele and Seth7). Traditional Chinese formulae(Reference Xie, Dong and Wu8, Reference Yeh, Lin and Wang9) have also been shown to be effective in the treatment of acute inflammation in mice challenged with lipopolysaccharide (LPS).
The possible mechanisms for such a therapeutic action are not well understood. In some cases, the effects are attributed to the absorbed nutrients acting at extra-intestinal tissues, as is the case of antioxidant molecules, that once absorbed in the small intestine, reach the lung, where they prevent or reduce oxidative processes(Reference Hazlewood, Wood and Hansbro4). In others, the mechanism is indirect, involving activation of new mediators that eventually reach the target tissues. This is the case for dietary lecithin, a source of PUFA-containing phospholipids that mediate an increase in lipid peroxidation and platelet-activating factor bioactivity in the lung(Reference Muehlmann, Zanatta and Farias10). A third mechanism involves interaction of luminal contents with mucosal elements. Certain dietary components interacting with the mucosal lymphoid tissue (gut-associated lymphoid tissue; GALT) can modulate the local immune response that, in turn, can modify the immune response of other mucosal lymphoid tissues due to the interconnection of the mucosal immune systems(Reference Kiyono and Fukuyama11). This is the mechanism by which oral bioactive proteins such as colostrum-derived proteins(Reference Lilius and Marnila12) or milk-derived proteins(Reference Cross and Gill13, Reference Gill, Doull and Rutherfurd14) modify the peripheral immune responses.
In rats challenged with Staphylococcus aureus enterotoxin B, dietary supplementation with spray-dried plasma proteins (SDP) reduced the mucosal immune response in both the organised and diffused GALT, protecting the gut mucosal lymphoid tissue from excessive activation(Reference Pérez-Bosque, Amat and Polo15, Reference Pérez-Bosque, Miró and Polo16, Reference Pérez-Bosque, Pelegrí and Vicario17). SDP can also modulate the mucosal immune response in extra-intestinal tissues; for example, in an epidemiological study of Canadian swine farms, feeding plasma proteins was associated with reduced mortality due to porcine reproductive and respiratory syndrome(Reference Dewey, Johnston and Gould18). Other peripheral effects of dietary SDP involve changes in the activation of the pituitary–adrenal axis following an LPS challenge(Reference Carroll, Touchette and Matteri19) and reduced mRNA expression of TNF-α and IL-1β in the adrenal gland, spleen, hypothalamus, pituitary gland and liver(Reference Touchette, Carroll and Allee20).
Since this class of supplements also improves the survival and performance of turkeys during a respiratory challenge stimulated by Pasteurella multocida (Reference Campbell, Quigley and Russell21), we investigated the therapeutic potential of plasma supplements in a mouse model of acute lung inflammation, based on the intranasal administration of LPS. Specifically, we studied the innate immune system because this mediates the defence against pathogens and regulates tissue health and integrity(Reference Chaudhuri and Sabroe2).
Material and methods
Animals and diets
Male C57BL/6 mice were supplied by Harlan Ibérica (Barcelona, Spain) and kept under stable temperature and humidity conditions, with a 12 h light–12 h dark cycle and free access to food and water. All protocols used in the present study were approved by the Ethics Committees for Animal Experimentation of the University of Barcelona (Barcelona, Spain) and the Catalan government. The animals were weaned at day 19 after birth, distributed at random in groups of seven to eight mice (two to three animals per cage) and fed the experimental diets for 2 weeks. This time period was adequate for plasma supplements to demonstrate their effect on immune response. The mice were monitored for food intake and body weight throughout the experimental period. Dietary treatments included (1) control: mice fed the control diet; (2) SDP: mice fed a diet with 8 % SDP; (3) Ig concentrate (IC): mice fed a diet with 2 % IC (provided the same amount of Ig as the SDP diet); (4) LPS: mice fed a control diet and challenged with LPS; (5) LPS-SDP: mice fed the diet with 8 % SDP and given LPS; (6) LPS-IC: mice fed the diet with 2 % IC and treated with LPS.
SDP is a feed ingredient obtained by centrifuging blood from healthy pigs, and IC is obtained by removing most of the albumin from plasma, resulting in a fraction containing approximately 40 % Ig(Reference Lee, Sim and Al-Mashikhi22). Both ingredients were spray-dried to obtain a stable powder. Maintenance of the native structure of the protein was confirmed by immunoelectrophoresis and Western blotting(Reference Borg, Capmbell and Polo23). The diets were balanced for energy and total N, and lysine and methionine were formulated to meet the National Research Council requirements(24) for laboratory animals (Table 1). The N content of the control diet was adjusted with milk protein. All diets were prepared by Harlan Ibérica.
Table 1 Composition of experimental diets
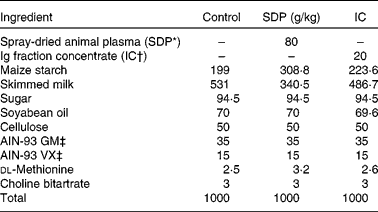
SDP, spray-dried plasma, IC, Ig concentrate.
* SDP from porcine blood (AP-820; APC-Europe S.A., Granollers, Spain).
† APC-Europe S.A.
‡ Provided by Harlan Ibérica (Barcelona, Spain).
Lipopolysaccharide challenge
LPS from Escherichia coli (O128:B12; Sigma-Aldrich, St Louis, MO, USA) was dissolved in PBS (625 μg/ml). The mice (16–18 g body weight) were anaesthetised with isofluorane on day 33 (for 24 h sampling) or on day 34 of life (for 6 h sampling), and were administered 10 μl of the LPS solution (or PBS) in each nostril. To collect bronchoalveolar lavage fluid (BALF), lung and blood for analysis of the lymphocytes (24 h after the LPS treatment) or cytokines (6 and 24 h after LPS), the mice were anaesthetised by intraperitoneal administration of ketamine (80 mg/kg) and xylazine (6 mg/kg). Blood was withdrawn by cardiac puncture. At the end of the experiment, the mice were killed by cervical dislocation. The samples used for protein and RNA determinations were immediately frozen with liquid N2 and stored at − 80°C.
Bronchoalveolar lymphocytes
Bronchoalveolar lavage was performed by the intra-tracheal instillation of 1 ml PBS(Reference Woolard, Huding and Tabor25). The BALF was recovered (0·8–0·9 ml) and centrifuged at 950 g for 10 min, and the pelleted BALF cells were re-suspended in PBS–FBS. The cells were counted, and viability was determined with a solution of acridine orange and ethidium bromide. In all cases, cell viability was higher than 95 %. The supernatant was frozen at − 80°C for further analysis.
Lung lymphocytes
Once BALF was obtained, the samples of the lung tissue were excised for protein, cell isolation and mRNA determinations. The lung lymphocytes were isolated according to Woolard et al. (Reference Woolard, Huding and Tabor25). Briefly, the lung tissue was washed and later was finely minced and incubated in Roswell Park Memorial Institute (RPMI) 1640 (Invitrogen, Carlsbad, CA, USA) with 5 % FBS, 100 000 U/l penicillin, 100 mg/l streptomycin, 10 mm-HEPES, 2 nm-l-glutamine and 150 U/ml collagenase (Invitrogen). After incubation for 90 min at 37°C in an automatic shaker, the remaining intact tissue was disrupted by passage through a 21-gauge needle. The suspension was centrifuged at 600 g for 7 min at 4°C. The supernatant was discarded, and the pellet was suspended in 40 % Percoll (GE Healthcare Bio-Sciences, Little Chalfont, Bucks, UK) layered onto 80 % Percoll and centrifuged at 600 g for 20 min at 15°C. The cells at the interface were collected, washed in RPMI-1640 with FCS, HEPES and penicillin/streptomycin, and suspended in PBS–FBS. The cells were counted, and viability was determined as described earlier. In all cases, cell viability was higher than 75 %.
Blood lymphocytes
After cervical dislocation, the blood was drawn by cardiac puncture with a heparinised syringe and mixed with 7 ml of erythrocyte lysing buffer (0·17 m-NH4Cl, 0·01 m-KHCO3, 0·1 mm-EDTA, adjusted at pH 7·3). This solution remained at room temperature for 5 min and was then centrifuged at 300 g for 5 min at 25°C. The cells obtained from the pellet were washed with PBS–FBS and centrifuged at 300 g for 5 min at 4°C. The supernatant was discarded, and the cells were suspended in the same solution.
Cell staining
The cells (3 × 104 for the BALF samples and 1·5 × 105 for the lung and blood samples) were incubated with primary mouse monoclonal antibodies for 30 min at 4°C. The primary antibodies used were anti-CD45 APC-A750 (eBioscience, Inc., San Diego, CA, USA) for leucocytes; lymphocytes and non-lymphocytic leucocytes were separated by forward/side scatter; anti-CD68 A700 (eBioscience, Inc.) for monocytes, anti-Ly6G FITC (BD Pharmingen, Franklin Lakes, NJ, USA) for neutrophils, anti-CD14 PE (eBioscience, Inc.) for activated neutrophils/monocytes; and isotype control. The samples were subsequently washed in PBS–FBS and centrifuged at 600 g for 6 min at 4°C. Finally, the washed cells were fixed with 400 μl of 4 % w/v paraformaldehyde and stored at 4°C until analysis. The samples were analysed in a BD FACSAria™ Flow Cytometer at the Cytometry Unit of the Scientific and Technical Support Services of the University of Barcelona.
Lung homogenate
The samples were homogenised in a lysis buffer containing 0·1 mm-EDTA, 0·1 mm-ethylene glycol tetraacetic acid, 1 % Triton and 2 % protease inhibitor cocktail (Sigma-Aldrich). The homogenate was centrifuged at 4°C at 14 000 g for 10 min. The IL-10 was determined in the supernatant (see later). The amount of protein in the lung homogenate and supernatant was assayed by the Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA).
Cytokine and chemokine assays
The cytokines determined were IL-1α, IL-1β, TNF-α, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte CSF (G-CSF). The chemokines CCL2, CCL3, CCL4 and CXCL1 were studied by means of the Bio-Plex Mouse Cytokine Panel (Bio-Rad) in samples of BALF supernatants from animals killed 6 and 24 h after the LPS challenge. The cytokine IL-10 was studied in samples of the lung homogenate with the Bio-Plex Mouse Cytokine Assay (Bio-Rad). The detection limits were 0·8 pg/ml for IL-1α; 5·8 pg/ml for IL-1β; 12 pg/ml for TNF-α; 1·4 pg/ml for IL-6; 8·1 pg/ml for GM-CSF; 2·3 pg/ml for G-CSF; 6·4 pg/ml for CCL2; 13 pg/ml for CCL3; 2·7 pg/ml for CCL4; and 1·6 pg/ml for CXCL1. The detection limit for IL-10 was 2·1 pg/ml.
RNA extraction and RT
Frozen samples of lung tissue taken from animals 6 h after the LPS challenge were disrupted mechanically, and total RNA was extracted with TRIzol (Invitrogen) following the manufacturer's instructions. Extracted RNA was treated with RQ1 RNase-free DNAase (Promega, Inc., Madison, WI, USA) and reverse transcribed with Superscript™ III RT (Invitrogen) using an oligo-(dT)20 primer, according to the supplier's protocol (Invitrogen).
Real-time PCR
Complementary DNA served as a template in a 20 μl reaction volume containing 0·2 μmol/l of each primer: inducible NO synthase (iNOS), forward: 5′-GGC AAC ATC AGG TCG GCC ATC AC-3′, reverse: 5′-CGT ACC GGA TGA GCT GTG AAT T-3′ (122 bp); glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward: 5′-GGC ATT GCT CTC AAT GAC AA-3′, reverse: 5′-CCC TGT TGC TGT AGC CGT AT-3′ (73 bp) and 2x SyBr® GreenER SuperMix (Invitrogen). Real-time PCR was performed in an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Carlsbad, CA, USA). The PCR conditions were as described previously(Reference Pérez-Bosque, Miró and Polo26).
Statistical analysis
All results are expressed as means with their standard errors. The effects of LPS and diets (and their interaction) on leucocyte populations and on iNOS expression were analysed by two-way factorial ANOVA, followed by the Bonferroni post hoc test. Cytokine and chemokine concentrations were analysed by three-way ANOVA (LPS challenge, time, diet and their interactions), followed by the Bonferroni post hoc test, using SPSS 14.0 software (SPSS, Inc., Chicago, IL, USA). Differences were considered significant at P < 0·05.
Results
Leucocyte counts and cell populations
Intra-nasal administration of LPS induced an immune response in BALF, lung tissue and blood that was consistent and reproducible, and with low variability. In the lung airways, there was massive leucocyte recruitment (thirty-fivefold increase in BALF; P < 0·001; Fig. 1(A)), which was reduced by 25 % by SDP (P < 0·05) and by 37 % by IC (P < 0·05). LPS administration increased the recruitment of the lymphocytes and non-lymphocytic leucocytes into the lung airways (P < 0·05), and both were significantly reduced by SDP and IC (Fig. 1(A)).

Fig. 1 Cell count in (A) bronchoalveolar lavage fluid and (B) lung tissue in control (□), spray-dried plasma (SDP, ![]() ), Ig concentrate (IC,
), Ig concentrate (IC, ![]() ), lipopolysaccharide (LPS,
), lipopolysaccharide (LPS, ![]() ), LPS-SDP (
), LPS-SDP (![]() ) and LPS-IC (■) mice. Values are means, with their standard errors represented by vertical bars, n 7–8. (A) The LPS effect, the diet effect and the interaction between diet and LPS were significant in the three cell types (P < 0·001, P < 0·05 and P < 0·05, respectively). (B) The LPS effect was significant in leucocytes and granulocytes (P = 0·018); the diet effect was significant in the three cell types (P < 0·05); the interaction between diet and LPS administration was significant only in lymphocytes (P < 0·05). a,b,c Mean values with unlike letters were significantly different (P < 0·05)
) and LPS-IC (■) mice. Values are means, with their standard errors represented by vertical bars, n 7–8. (A) The LPS effect, the diet effect and the interaction between diet and LPS were significant in the three cell types (P < 0·001, P < 0·05 and P < 0·05, respectively). (B) The LPS effect was significant in leucocytes and granulocytes (P = 0·018); the diet effect was significant in the three cell types (P < 0·05); the interaction between diet and LPS administration was significant only in lymphocytes (P < 0·05). a,b,c Mean values with unlike letters were significantly different (P < 0·05)
In control mice, the majority of BALF non-lymphocytic leucocytes was monocytes (94·3 %), while only 2·6 % were neutrophils (Table 2). This profile was reversed by LPS since the neutrophil population became several-fold higher than the monocyte population, and this cellular shift was not affected by dietary treatment. LPS also increased 3·5-fold the percentage of activated neutrophils and twentyfold the population of activated monocytes. Both SDP and IC supplements reduced the percentage of activated neutrophils, but only SDP prevented in part monocyte activation in the challenged mice (Table 2).
Table 2 Populations of neutrophils and monocytes in bronchoalveolar lavage fluid (BALF), lung and blood after 24 h administration in control, spray-dried plasma (SDP), Ig concentrate (IC), lipopolysaccharide (LPS), LPS-SDP and LPS-IC mice
(Mean values with their standard errors, n 7–8)

a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
In the lung tissue, LPS increased leucocyte recruitment by 30 % (Fig. 1(B)), and this effect was completely prevented by SDP supplementation. However, the effects of LPS on the relative distribution of leucocyte subsets (Table 2) were not modified by the plasma supplements. Interestingly, both supplements reduced the percentage of monocytes and neutrophils in the unchallenged groups. As to the degree of activation of these cells, LPS increased the percentage of activated neutrophils almost threefold and that of activated monocytes over thirtyfold, whereas dietary supplements did not modify this response.
In the blood, LPS induced a leucocyte distribution profile similar to that observed in the lung tissue, i.e. an increase in the percentage of activated neutrophils and monocytes (tenfold and threefold, respectively), which were partially inhibited by both plasma supplements (Table 2).
Chemokine and cytokine
Chemokine and cytokine expression is presented in Figs. 2 and 3, respectively. Although most of the variables showed maximal responses at 6 h, there were still significant differences 24 h after the LPS challenge. In general, the response to LPS was a several-fold increase in the expression of cytokines and chemokines, both 6 and 24 h after the challenge.

Fig. 2 Concentration of (A) CXCL1, (B) CCL2, (C) CCL3 and (D) CCL4 in the supernatant of bronchoalveolar lavage fluid from control (![]() ), spray-dried plasma (SDP,
), spray-dried plasma (SDP, ![]() ), Ig concentrate (IC,
), Ig concentrate (IC, ![]() ), lipopolysaccharide (LPS,
), lipopolysaccharide (LPS, ![]() ), LPS-SDP (
), LPS-SDP (![]() ) and LPS-IC (■) mice 6 and 24 h after the LPS challenge. n.d., Not determined. Values are means, with their standard errors represented by vertical bars, n 5–6. The LPS effect was significant in the four chemokines studied (P ≤ 0·001); the diet effect was significant for CCL2 and CCL3 (P < 0·05); the time effect was significant except for CCL4 (P < 0·001); the interaction between diet and LPS was significant for CCL2 and CCL3 (P <0·05); the interaction between LPS and time was significant for all the chemokines except for CCL4 (P < 0·005). a–f Mean values with unlike letters were significantly different (P < 0·05).
) and LPS-IC (■) mice 6 and 24 h after the LPS challenge. n.d., Not determined. Values are means, with their standard errors represented by vertical bars, n 5–6. The LPS effect was significant in the four chemokines studied (P ≤ 0·001); the diet effect was significant for CCL2 and CCL3 (P < 0·05); the time effect was significant except for CCL4 (P < 0·001); the interaction between diet and LPS was significant for CCL2 and CCL3 (P <0·05); the interaction between LPS and time was significant for all the chemokines except for CCL4 (P < 0·005). a–f Mean values with unlike letters were significantly different (P < 0·05).

Fig. 3 Concentration of (A) IL-1α, (B) IL-1β, (C) IL-6, (D) G-CSF, (E) GM-CSF and (F) TNF-α in the supernatant of bronchoalveolar lavage fluid from control (![]() ), spray-dried plasma (SDP,
), spray-dried plasma (SDP, ![]() ), Ig concentrate (IC,
), Ig concentrate (IC, ![]() ), lipopolysaccharide (LPS,
), lipopolysaccharide (LPS, ![]() ), LPS-SDP (
), LPS-SDP (![]() ) and LPS-IC (■) mice 6 and 24 h after the LPS challenge. Values are means, with their standard errors represented by vertical bars, n 5–6. The LPS effect was significant in the six cytokines studied (P < 0·001); the diet effect was significant for IL-1α, IL-1β, IL-6 and G-CSF (P ≤ 0·039) and for TNF-α (P < 0·001); the time effect was significant except for IL-1β (P ≤ 0·010); the interaction between diet and LPS was significant for IL-1α, IL-1β, IL-6 and G-CSF (P ≤ 0·05) and for TNF-α (P < 0·001); the interaction between LPS and time was significant for all the cytokines except for IL-1β (P ≤ 0·010). a–f Mean values with unlike letters were significantly different (P < 0·05).
) and LPS-IC (■) mice 6 and 24 h after the LPS challenge. Values are means, with their standard errors represented by vertical bars, n 5–6. The LPS effect was significant in the six cytokines studied (P < 0·001); the diet effect was significant for IL-1α, IL-1β, IL-6 and G-CSF (P ≤ 0·039) and for TNF-α (P < 0·001); the time effect was significant except for IL-1β (P ≤ 0·010); the interaction between diet and LPS was significant for IL-1α, IL-1β, IL-6 and G-CSF (P ≤ 0·05) and for TNF-α (P < 0·001); the interaction between LPS and time was significant for all the cytokines except for IL-1β (P ≤ 0·010). a–f Mean values with unlike letters were significantly different (P < 0·05).
The effect of LPS on chemokines at 6 h was significant, especially on the expression of CCL3 (a more than 1000-fold increase; Fig. 2). At 24 h, the response was still significant. Dietary treatment reduced chemokine production at both 6 h (CCL2, CCL3 and CCL4) and at 24 h (CCL3 and CCL4, Fig. 2).
For cytokines, the highest response was obtained for IL-6, TNF-α and G-CSF (more than 1000-fold increase; Fig. 3), and the response at 6 h, with the exception of IL-1α, was higher than at 24 h. Both dietary treatments were effective in reducing cytokine concentration, including an 80 % reduction in TNF-α and a 30 % reduction in IL-1α expression. In spite of increased variability in cytokine concentrations 24 h after LPS challenge, both dietary treatments reduced the levels of IL-1α and TNF-α (Fig. 3). The expression of IL-10 in the lung tissue is shown in Fig. 4. LPS had no effect on its concentration in the tissue, but both SDP and IC supplements stimulated its expression in the challenged mice.

Fig. 4 Concentration of IL-10 in lung tissue from control (![]() ), spray-dried plasma (SDP,
), spray-dried plasma (SDP, ![]() ), Ig concentrate (IC,
), Ig concentrate (IC, ![]() ), lipopolysaccharide (LPS,
), lipopolysaccharide (LPS, ![]() ), LPS-SDP (
), LPS-SDP (![]() ) and LPS-IC (■) mice 6 h after the LPS challenge. Values are means, with their standard errors represented by vertical bars, n 5–6. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
) and LPS-IC (■) mice 6 h after the LPS challenge. Values are means, with their standard errors represented by vertical bars, n 5–6. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
Inducible NO synthase expression
In the lung tissue, LPS increased iNOS expression by 13 % (LPS group, 1·13 (sem 0·03); control group, 1·00 (sem 0·02); P < 0·05), and this effect was removed by both the SDP and IC supplements (LPS-SDP, 0·99 (sem 0·04) and LPS-IC, 1·02 (sem 0·02); both P < 0·05).
Discussion
Dietary spray-dried plasma proteins are widely used in farm animal nutrition because they maintain normal intestinal barrier function and growth performance(Reference Campbell, Polo and Russell27). They also have anti-inflammatory effects as they attenuate the mucosal inflammatory response in the small intestine of rats exposed to a staphylococcal enterotoxin(Reference Moretó and Pérez-Bosque28, Reference Pérez-Bosque and Moretó29); in humans, bovine immunoglobulin concentrates reduce the clinical effects of cryptosporidiosis with AIDS(Reference Greenberg and Cello30).
In addition to its effects on gut homeostasis, which involve modulation of GALT(Reference Moretó and Pérez-Bosque28), animal plasma supplements can also affect lymphoid tissue populations and the expression of cytokines in peripheral tissues, such as the spleen and the liver(Reference Touchette, Carroll and Allee20), and in the lungs(Reference Campbell, Quigley and Russell21). These observations have led to the hypothesis that dietary plasma supplements may also be effective in modulating the response of the immune cells in the non-intestinal mucosal tissues such as the nasopharyngeal mucosa (nasal and bronchoalveolar-associated lymphoid tissue). This hypothesis is sustained by the existence of the common mucosal immune system that connects the inductive sites (here the GALT) with the effector sites (nasal and bronchoalveolar-associated lymphoid tissue) for the generation of T lymphocyte responses(Reference Kiyono and Fukuyama11).
The study was done during the post-weaning period, because at this stage the mucosal immune system is still immature and therefore more susceptible to infection(Reference Bailey, Haverson and Inman31); furthermore, we had demonstrated in rats that plasma supplements are already effective in this early period(Reference Pérez-Bosque, Pelegrí and Vicario17). In the mouse model of acute lung inflammation induced by LPS, the pulmonary response is characterised by lymphocyte migration and the massive release of pro-inflammatory cytokines and chemokines, which recruit monocytes and neutrophils into the lung airway and into the lung tissue(Reference Yeh, Lin and Wang9, Reference Reutershan, Basit and Galkina32). We were primarily interested in the effects of SDP and IC on the innate immune response because of its triple role: first, mediating the defence against pathogens; secondly, detecting tissue damage, and finally, regulating tissue health and integrity(Reference Chaudhuri and Sabroe2).
The main finding of the present study is that dietary supplementation with SDP or IC reduces the innate immune response to LPS inhalation. The reduction in leucocyte counts in BALF and the lung tissue, the lower concentration of pro-inflammatory cytokines and chemokines in BALF, and the lower iNOS expression in the lung tissue, all suggest a dietary-dependent reduction in the chemical mediators responsible for acute lung injury. These results agree with previous observations in rats indicating that dietary plasma proteins protect the intestine from immune overstimulation caused by the intraperitoneal administration of S. aureus enterotoxin B(Reference Pérez-Bosque, Pelegrí and Vicario17). The mechanism of action involves a reduction in the pro-inflammatory to anti-inflammatory cytokine ratios in both Peyer's patches and the mucosa(Reference Pérez-Bosque, Miró and Polo26).
In mice that were not exposed to LPS, the number of leucocytes recruited into the airway was low, with a clear predominance of monocytes, and a very low amount of neutrophils. The LPS challenge increased cell recruitment to the airway space; specifically, the percentage of neutrophils increased by 70 %. These findings are consistent with the results reported by Reutershan et al. (Reference Reutershan, Basit and Galkina32) who used a similar model. The primary function of neutrophils in the innate immune response is to contain and kill invading microbial pathogens(Reference Nathan3), and their concentration in BALF is a maximal 24 h after LPS administration(Reference Reutershan, Basit and Galkina32). In BALF and the lung tissue, LPS increased the proportion of activated neutrophils (three- and twofold, respectively) and of activated monocytes (twenty- and thirty-fourfold, respectively), as a consequence of the release of large amounts of chemokines, in agreement with the previous observations(Reference Yeh, Lin and Wang9). The effects of LPS on leucocyte counts in BALF were attenuated by SDP and IC, indicating lower cell infiltration, consistent with reduced eosinophil infiltration and lower degree of degranulation observed in the intestine of rats challenged with S. aureus enterotoxin B(Reference Moretó and Pérez-Bosque28). Both the plasma supplements reduced the percentage of activated neutrophils and monocytes in the lung airway; therefore, lower cell migration and smaller activation of inflammatory cells to the pulmonary tissue may reduce the potential damage to the respiratory epithelium and vascular endothelium associated with the inflammatory response.
The main effects of dietary supplementation with plasma proteins were observed in the lung airway, but there were also marked effects in the lung tissue and in peripheral blood. It is noteworthy that there is a reduction in the resident monocytes and neutrophils in the lung tissue, which implies a reduction in the normal pro-inflammatory state associated with the mucosal system(Reference Suzuki, Chow and Downey33). At a systemic level, both diets decreased the percentage of activated monocytes and activated neutrophils induced by LPS, consistent with the reduction in the percentage of activated monocytes and activated neutrophils in the lung airway observed in the plasma-supplemented mice. The finding that diet reduces the activation of neutrophils and monocytes is important, because these populations are involved in the acute lung injury induced by LPS(Reference Schuh and Pah34).
Inhaled LPS increased cytokine and chemokine concentration as well as iNOS expression in the lung tissue and in BALF, in agreement with the previous observations(Reference Yeh, Lin and Wang9). Moreover, pro-inflammatory cytokines can alter the structure of the respiratory and vascular epithelia, which compromise the gas exchange. For example, excess TNF-α enhances the paracellular permeability of microvascular endothelial cells leading to tissue dysfunction and shock(Reference Tracey and Cerami35). The present results show that LPS increased TNF-α concentration in BALF at 6 h, reaching a concentration 1700-fold higher than in the controls, which dropped to only sixtyfold 24 h after the challenge, consistent with the previous reports(Reference Larsson, Rocksén and Lilliehöök36). The concentration in BALF of the other pro-inflammatory cytokines studied was also dramatically increased at 6 h, confirming the observations of Yeh et al. (Reference Yeh, Lin and Wang9) and partly prevented by both SDP and IC. The effects of SDP and IC on GM-CSF expression may be of special significance, because it plays an important role in the pathogenesis of acute and chronic lung disease(Reference Puljic, Benediktus and Plater-Zyberk37).
Since chemokines attract leucocytes into tissues, they are good indicators of the inflammatory state. CCL2 recruits monocytes, lymphocytes and basophils, and activates mast cells and basophils(Reference Puneet, Moochhala and Bhatia38). The pattern of the response of chemokines in BALF was similar to that of cytokines, i.e. most of them (CXCL1 and CCL3) showed an acute response that was greater at 6 h than at 24 h, whereas CCL2 was much more evident at 24 h after the challenge. Both SDP and IC attenuated the LPS-induced increase in chemokine concentration, and this effect is relevant due to the role of chemokines as inducers of cell migration into the bronchoalveolar space during inflammation.
The mechanism of action of dietary functional proteins is not well understood. Spray-dried plasma contains biologically active peptides and functional proteins including Ig (IgA, IgG and IgM), albumin and growth factors(Reference Borg, Capmbell and Polo23). These proteins show special efficacy when the animals are exposed to environmental or immunological challenges. The IC supplement lacks most of the albumin fraction, and therefore it contains higher amounts of γ-globulins. Although it has been suggested that IgG is responsible for the SDP effects(Reference Van Dijk, Niewold and Nabuurs39), the contribution of other active plasma components to the functional effects of plasma supplements should also be considered. Plasma contains over 250 active peptides(Reference Anderson and Anderson40) that retain most of their biological activity after spray drying(Reference Borg, Capmbell and Polo23). The main effects of SDP and IC involve regulation of mucosal GALT, which may be the result of reduced mucosal binding of luminal antigens mediated by plasma glycoproteins(Reference Van Dijk, Niewold and Nabuurs39). GALT regulation can also be ascribed to changes in the profile of the gut microflora(Reference Bhandari, Xu and Nyachoti41) or to the biological activity of other plasma components either interacting with luminal extensions of dendritic cells or crossing the epithelium across M cells or through tight-junction complexes. Although the interaction of SDP and IC with the intestinal mucosa is not yet clarified, it is well demonstrated that plasma supplements modulate the expression of pro-inflammatory cytokines in the GALT of pigs(Reference Bosi, Casini and Finamore42) and rats(Reference Pérez-Bosque, Miró and Polo26). Our studies in the rat model of mild intestinal inflammation(Reference Pérez-Bosque and Moretó29) indicate that the mechanism by which plasma supplements reduce GALT activation may also involve profile modulation of cytokines(Reference Pérez-Bosque, Miró and Polo26).
Cuzzocrea et al. (Reference Cuzzocrea, Mazzon and Dugo43) have shown that IL-10 has also an important role during acute lung inflammation, inhibiting the release of pro-inflammatory mediators such as IL-6(Reference Heyen, Ye and Finck44) and iNOS(Reference Ogando, Cella and Ribeiro45). In contrast to the mild response induced by S. aureus enterotoxin B in the intestine, the effects of LPS in the lung are characterised by increased leucocyte recruitment and strongly enhanced cytokine and chemokine production. However, even in this acute inflammatory condition, both SDP and IC inhibited the immune response, indicating that dietary modulation of intestinal lymphocyte proliferation(Reference Pérez-Bosque, Pelegrí and Vicario17) and cytokine expression(Reference Pérez-Bosque, Miró and Polo26) can reduce inflammatory responses in distant regions, albeit interconnected by the common mucosal immune system. The analysis of IL-10 in the lung tissue showed that both SDP and IC supplements can significantly increase its expression, supporting the view that the peripheral anti-inflammatory effects of plasma supplements also involve modulation of the cytokine ratios.
In conclusion, dietary SDP or IC interacts with the immune cells of GALT and reduces the pulmonary response to an LPS challenge, consistent with the hypothesis that there is extensive communication between the mucosal tissues through the common mucosal immune system. The present results support the view that oral intervention with plasma protein supplements is a new strategy for preventing, managing and attenuation of lung inflammation.
Acknowledgements
The present study was supported by grant RDITCRD07-1-008 (Generalitat de Catalunya, Barcelona, Spain). The research group is also supported by grant 2009SGR0471 for Consolidated Research Groups (Generalitat de Catalunya). Mò. M., L. M. and Mi. M. reported no conflicts of interest; A. P.-B. and J. P. are employed by APC-Europe S.A. (Granollers, Spain); L. R., Joy C. and Joe C. are employed by APC, Inc., Ankelny, IA, USA; E. W. is employed by Proliant Health and Biologicals, Ankeny, IA, USA. The authors' contributions were as follows: Mi. M., J. P., L. R., Joy C., E. W., Joe C. and A. P.-B. designed the study; A. P.-B., Mò. M. and L. M. conducted the study; J. P. provided the essential materials; A. P.-B., Mò. M. and Mi. M. analysed the data and wrote the manuscript; Mò. M., J. P., L. R., Joy C., E. W., Joe C., Mi. M. and A. P.-B. discussed the results. All authors read and approved the final manuscript.








