INTRODUCTION
Japanese encephalitis (JE), a viral disease with a high case-fatality rate (5–20%) and an appreciable fraction of neurological sequelae in survivors (30–50%), is endemic in virtually all countries of Asia [Reference Endy and Nisalak1]. JE is transmitted from animals to humans through mosquito bites. Culex tritaeniorhynchus, the major mosquito vector, breeds by laying eggs in rice fields and the pig is considered the most common amplifying host of Japanese encephalitis virus (JEV) [Reference Halstead and Jacobson2]. Large JE outbreaks have occurred in temperate and subtropical Asian regions where rice cultivation and pig farming are considered two major risk factors [Reference Endy and Nisalak1–Reference Burke, Leake and Monath4]. However, the epidemiological patterns of the disease are less clear in tropical Asia. Recently, we found high JE incidence in children in Bali, Indonesia [Reference Kari5], that was comparable to those of other endemic regions in Japan, Korea, Thailand, and Taiwan before the introduction of JE vaccine [Reference Endy and Nisalak1, Reference Hsieh, Wang and Rasmussen6, Reference Wu7]. The novel setting of JE endemicity in Bali provided a unique opportunity to examine the environmental and behavioural risk factors for acquiring JE in tropical Asia. The majority of Balinese grow rice and are Hindu. Raisings pigs and the consumption of pork are common as in most Asian populations. The monthly average temperature in Bali is >25°C in all seasons and the mosquito population is active all year round.
Methods
Selection of cases and controls
In July 2001, we established a case referral system that included all 10 hospitals that provided in-patient paediatric service in Bali [Reference Kari5]. Patients aged 0–11 years, presenting with a disorder clinically identified as acute viral encephalitis or aseptic meningitis were enrolled in the study as suspected JE cases. Cerebrospinal fluid (CSF) and serum specimens were tested for IgM antibody by use of an IgM capture enzyme-linked immunosorbent assay (ELISA) [Reference Burke8–Reference Burke, Nisalak and Ussery10]. Because dengue virus (DENV) was known to circulate in the study area [Reference Rodhain11] and because there is cross-reactivity between antibodies to JEV and DENV, the specimens were tested for IgM antibodies to both JEV and DENV simultaneously [Reference Burke8]. All specimens were first tested at the Central Public Health laboratory in Bali and later at the reference laboratory at the Armed Forces Research Institute of Medical Sciences (AFRIMS) in Bangkok for confirmation of the test results. A ‘confirmed’ case of JE required an anti-JEV IgM of ⩾40 units by the IgM capture ELISA in the CSF. A ‘probable’ case of JE needed a positive result for IgM anti-JEV antibodies in sera only, but with a ratio of the titre of serum IgM anti-DENV antibodies to the titre of serum IgM anti-JEV antibodies of <1·0. Those who failed to meet the laboratory criteria were defined as ‘non-JE acute encephalitis.’ Only confirmed JE and non-JE cases were enrolled as cases and controls, respectively. The study was approved by the Institutional Review Board (IRB) of Udayana University, Bali and the International Vaccine Institute (IVI), Seoul. Written informed consent was obtained from parents/guardians of all study subjects.
Data collection
Data on potential risk factors for JE were collected by interviewing the parents/guardians of the enrolled patients. Interviewers were trained to use a structured questionnaire which provided predefined answers to the following variables: sociodemographic characteristics such as age, sex, ethnic group and residency; behaviours affecting exposure to the peri-domestic mosquito population such as outdoor activities after dinner, use of mosquito bed nets, mosquito window screens, use of repellent and spraying; ownership of swine by family or next-door neighbours; and proximity of household to rice field (<100 m). Exposure to mosquito repellent or spraying was determined if parents recalled the use of repellent or spraying at least once a day in the previous month. The incubation period for JE is usually 5–15 days [Reference Halstead and Jacobson2]. Exposures to the potential risk factors were taken into consideration only when they occurred within 1 month prior to disease onset for both cases and controls. All the information was collected during the first 1–3 days of hospitalization, a time when the JE/dengue serological diagnoses had not yet become available.
Statistical analysis
The baseline characteristics were evaluated by overall means of continuous variable such as age, or by frequencies of categorical variables such as sex, ethnicity, and residency. Unconditional logistic regression analyses were used to evaluate the univariate and multivariate associations between the potential risk factors and occurrence of JE. Maximum-likelihood estimates of the odds ratio (OR) were calculated with the unconditional logistic regression model [Reference Hosmer and Lemeshow12], using the logistic procedure in SAS software version 9.1 (SAS Institute Inc., USA). Factors that differed between cases and controls at a significant level of P⩽0·2 in the univariate analyses were included in the multivariate logistic analyses. The final model was fitted using a stepwise selection procedure that included all significant variables with a P value of <0·05. The goodness-of-fit of this model was assessed according to Hosmer & Lemeshow [Reference Hosmer and Lemeshow12]. Two-sided statistical tests were used. Coefficients for independent variables were exponentiated to estimate ORs, and standard errors for the coefficients were used to estimate 95% confidence intervals (CIs) for ORs. P values <0·05 were considered statistically significant.
Table 1. Comparison of features between 94 Japanese encephalitis (JE) cases and 163 non-JE acute encephalitis controls in Bali, Indonesia (July 2001–June 2004)
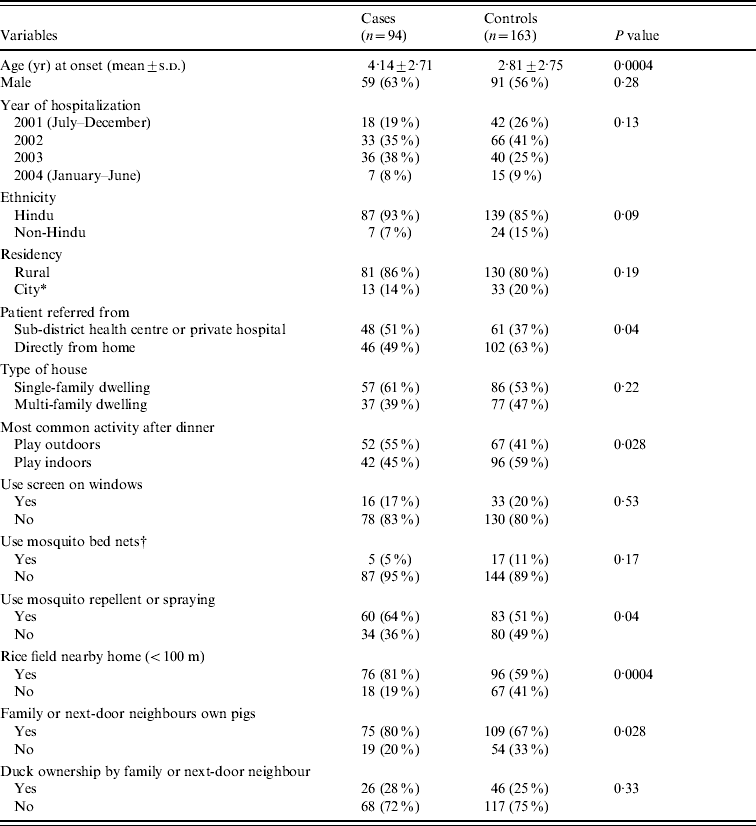
* City is defined as living at the Denpasar district.
† There are three missing data from each group.
RESULTS
During 1 July 2001 to 30 June 2004, 264 clinically suspected patients were enrolled in the hospital-based surveillance. Of these, 94 patients were diagnosed as ‘confirmed JE’, seven patients as ‘probable JE’, and 163 as ‘non-JE acute encephalitis.’ All 94 JE cases and 163 controls were included in the analyses. Most of the cases occurred in children aged <10 years. The boy:girl ratio was 1·7:1 in our study. Unlike in other temperate JE-endemic countries, transmission of disease in Bali appeared to be year round.
In the univariate analyses, cases and controls were similar in sex (63% male cases, vs. 56% controls), religious group (93% Hindu cases vs. 85% controls), residency (86% living in rural area in cases, vs. 80% controls), year of hospitalization, and type of house (39% living in multi-family dwelling house in cases, vs. 47% controls). The last variable correlates with family income, as people with lower income usually live in much less expensive multi-family houses with multiple piggeries. JE patients were more likely than controls to report ownership of pigs by their family or next-door neighbours (OR 1·96, 95% CI 1·07–3·56), and close proximity of the household to rice fields (OR 2·95, 95% CI 1·62–5·37). JE patients were more frequently referred than controls from sub-district health centre and small private hospital (OR 1·75, 95% CI 1·04–2·92). Staff at these facilities had been trained to recognize and refer suspected JE patients to the 10 surveillance hospitals. Of the other factors, statistically significant differences were found for age (mean age, 4·14 years in cases vs. 2·81 years in controls), playing outdoors after dinner (55% of cases played outdoors vs. 41% of controls), and use of mosquito repellent or spraying (64% of cases vs. 51% of controls). No association was found between JE illness and use of screens on windows (17% cases vs. 20% controls), use of mosquito bed nets (5% cases vs. 11% controls), and duck ownership (28% cases vs. 25% controls) (Table 1). In the multivariate analysis, pig ownership by the family or next-door neighbours (OR 2·24, 95% CI 1·17–4·26), close proximity of household to rice field (OR 2·93, 95% CI 1·57–5·45), and older age (OR 1·21, 95% CI 1·09–1·33) remained independently associated with JE illness (Table 2). Inclusion of interaction terms between age, residency and playing outdoors after dinner did not improve the fit of the model. Based on the final predictive model, collinearity diagnostics were performed by checking if tolerance was close to 1 (or variance inflation factor was close to 1) for each predictor. We found there was no collinearity problem with the predictors in the final regression model.
Table 2. Multivariate analysis of the association between environmental risk factors and Japanese encephalitis (JE) infection in 94 JE cases and 163 non-JE acute encephalitis controls in Bali, Indonesia (July 2001–June 2004)

OR, Odds ratio; CI, confidence interval.
* Other variables adjusted in the model include: ethnicity (Hindu vs. non-Hindu), patient referred (from sub-district health centre or private hospital vs. directly from home), most common activity after dinner (play outdoors vs. play indoors), use mosquito repellent or spraying (yes vs. no).
DISCUSSION
Our multivariate analyses found only three variables to be associated with the risk of JE in children in Bali: pig ownership by their family or next-door neighbours, close proximity of household to a rice field, and older age. JE has been reported to have a higher incidence in males than in females [Reference Endy and Nisalak1, Reference Halstead and Jacobson2]. Gender, however, was not significantly associated with JE in the current study. That the average age of controls was significantly younger than that for JE cases (2·81 vs. 4·14 years, P=0·0004) may reflect the fact that younger children might be more vulnerable to and at higher risk of other causes of acute viral encephalitis such as measles, mumps, enteroviruses [Reference Chokephaibulkit13, Reference O'Meara and Ouvrier14]. There was no association between age and the annualized incidence rates of JE in Bali in a cohort study of children aged <7 years [Reference Kari5]. We found no association between JE infection and use of mosquito bed nets, and mosquito screens on windows in the analyses. In endemic areas, especially in tropical regions, exposure to mosquito bites is consistent and intensive; temporary and intermittent protections does not work sufficiently to interrupt disease transmission.
In JE-endemic areas of Asia, pigs are often the major animal host for viral amplification due to higher viraemia with JE infection and higher attractiveness to the mosquito vector, C. tritaeniorhynchus, than other domestic animals such as duck, cattle, horse, dog and sheep [Reference Halstead and Jacobson2]. The pig population density is high in Bali: the population ratio of humans to pigs is 2:1. In addition, the seasonal curves of JE infection in pigs and JE disease in humans are parallel [Reference Kari5]. C. tritaeniorhynchus breeds on rice paddies that are widely distributed across Bali. A survey confirmed that this mosquito vector is predominant in Bali [Reference Lee15]. Hence, the ecology of JE in Bali is probably the same as in most other endemic countries of Asia: a large pig population as amplifying host for JEV and large areas of rice fields for breeding of the mosquito vector, C. tritaeniorhynchus. The specific pattern of the disease in Bali differs from that for temperate and subtropical endemic areas in that there is year round transmission of the virus [Reference Kari5].
We considered two potential sources of bias, but doubt that any could have created an artificial association between the risk factors and acquisition of JE illness. Firstly, JE patients might be aware of their JE illness and were thus more likely than controls to report exposures to mosquitoes and pigs. However, such recall bias was unlikely, because the interviews were done at the hospital admission before the specific diagnosis of JE was made. For the same reason, biased acquisition of histories by interviewers was also unlikely.
Second, the specificity of IgM capture ELISA is close to 100% for JE [Reference Jacobson16], but the sensitivity of the test is lower when the specimens are collected within the first 3 days of the disease [Reference Han17]. We cannot rule out false-negative results because two-thirds of the study subjects provided only an acute CSF specimen at the time of admission, not a convalescent CSF specimen. However, when we restricted controls to those patients whose serology was negative in both acute- and convalescent-phase specimens, our results were unchanged. Moreover, a false-negative result or misclassification of JE cases into the control group would have only made the two comparison groups, cases vs. controls, more similar with respect of exposure to the potential risk factors and would have thus led to underestimation of the associations between the putative risk factors and JE infection.
The study has the limitation of using children with non-JE acute neurological outcomes as the control group, rather than healthy children from the community. All detected JE cases between 2001 and 2004 were included in the study, therefore selection bias for JE cases was unlikely. No anti-dengue IgM antibodies were detected in CSF or serum specimens from either cases or controls [Reference Kari5], although exact aetiologies of infection in controls were unknown. It is difficult to know whether the non-JE acute encephalitis cases were representative of the population with respect to exposure to potential risk factors under study, and it is possible that risk factors shared between JE and the causes of illness in the control group could have masked some associations.
Because rice cultivation and pig farming are basic to the Balinese economy, it is not possible to modify these practices, which are associated with the risk of JE in foreseeable future. Bed nets, screens, and repellent do not provide reliable protection against JE, and prevention of human disease by vector control is impractical [Reference Solomon18, Reference Tsai19]. Immunization of pigs is not feasible because of the high birth rate of pigs, the rapid turnover of susceptible animals, and the short window between the waning of maternal antibody (2–4 months) and age of pigs at slaughter (6–8 months) [Reference Igarashi20]. Current JE vaccines are effective and cost-effective in reducing disease burden due to JE in humans [Reference Tsai19, Reference Ding21]. Therefore, efforts to control JE infections in Bali should be focused on immunization of children with JE vaccine.
ACKNOWLEDGEMENTS
This study received financial support from the Bill and Melinda Gates Children's Vaccine Program (CVP) through the Program for Appropriate Technology for Health (PATH), Seattle, WA, USA (contract no: 00-GAT.770-790-01149-LPS) and from the Korean International Cooperation Agency (KOICA contract no: 2003-001). We thank Wayan Yastini, I. Gst Ayu Joni and Panor Srisongkram for their assistance in performing diagnostic testing, and Dayu Ratna Dewi and Dewa Gede Sura Dharma for their assistance in specimen transportation and coordination with paediatricians in Bali. W. Liu is currently affiliated with Department of Epidemiology, Harvard School of Public Health, Boston, MA.
DECLARATION OF INTEREST
None.




