Up to 2–5 % of males and 8–21 % of females sustain a stress fracture during initial military training (IMT)( Reference Jones, Thacker and Gilchrist 1 ), which can lead to increased rates of attrition. Recent trials have demonstrated that supplemental Ca and vitamin D during IMT have positive effects on bone health and reduce stress fracture risk( Reference Lappe, Cullen and Haynatzki 2 – Reference Gaffney-Stomberg, Lutz and Rood 4 ). For example, one study documented a decreased relative risk (up to 20 %) of fracture in female recruits supplemented with 2000 mg Ca and 20µg vitamin D/d during navy IMT( Reference Lappe, Cullen and Haynatzki 2 ). Most recently, a randomised controlled trial demonstrated that supplementation with 2000 mg Ca and 25µg vitamin D/d during Army IMT increased circulating ionised Ca (iCa), maintained parathyroid hormone (PTH) levels, increased the circulating osteoprotegerin:receptor activator of NF-κB ligand (OPG:RANKL) ratio and improved several peripheral quantitative computed tomography measures of bone health( Reference Gaffney-Stomberg, Lutz and Rood 4 ). Although the protective effects of supplemental Ca and vitamin D have been demonstrated, poor Fe status remains a threat to physical and cognitive performance during IMT, as the Fe status of male and female soldiers declines throughout the course( Reference McClung, Marchitelli and Friedl 5 – Reference Yanovich, Karl and Yanovich 8 ). Up to approximately 33 and 21 % of female soldiers develop Fe deficiency or Fe-deficient anaemia during training, and the deleterious effects of poor Fe status on physical and cognitive function during military training and operationally demanding tasks have been characterised( Reference McClung, Marchitelli and Friedl 5 – Reference Yanovich, Karl and Yanovich 8 ). Thus, interventions to prevent bone injury, while improving or maintaining Fe status, are important for optimising the health and performance of military trainees and for the successful completion of training and entry into the armed forces.
Despite the benefits of supplemental Ca on bone health, several studies indicate that Ca may interfere with Fe absorption in animals and humans( Reference Kletzien 9 , Reference Barton, Conrad and Parmley 10 ). For example, early studies in animals documented 57, 86 and 90 % decreases in liver, blood and carcass Fe contents in rats fed a CaCO3-supplemented diet compared with a basal diet for 5 weeks( Reference Kletzien 9 ). Interestingly, the liver Fe content of the Ca-supplemented rats was lower compared with anaemic controls, suggesting that the addition of Ca to the diet may have detrimental effects on Fe status. In humans, single meal studies with added Ca documented approximately 30–80 % reductions in non-haeme and haeme Fe absorption( Reference Monsen and Cook 11 – Reference Hallberg, Brune and Erlandsson 14 ), although, longer-term, longitudinal studies( Reference Gleerup, Rossander-Hulten and Hallberg 15 , Reference Reddy and Cook 16 ) and studies where Ca was given separate from meals( Reference Ilich-Ernst, McKenna and Badenhop 17 ) have not reported an effect on Fe absorption or status. Further, the effects of providing supplemental Ca and vitamin D in a fortified food product on Fe status have not been explored in a military population during physical training. As such, the objective of the present study was to determine whether providing 2000 mg/d Ca in a fortified food product during 9 weeks of military training affects Fe status in young adults. We hypothesised that supplemental Ca as a fortified food product would further exacerbate declines in Fe status during IMT.
Methods
Volunteers
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and approved by the Human Use Review Committee at the US Army Research Institute of Environmental Medicine. The investigators have adhered to the policies for protection of human subjects as prescribed in DoD Instruction 3216.02, and the study was conducted in adherence to the provisions of 32 CFR Part 219. Human subjects participated in these studies after giving their free and informed voluntary consent.
The present study was conducted using a subset of volunteers enrolled in the previously published parent study, which examined the effects of Ca/vitamin D supplementation on bone health during IMT( Reference Gaffney-Stomberg, Lutz and Rood 4 ). In brief, volunteer recruitment, enrolment and study completion occurred between February and April 2013 at Fort Sill, OK (34·7°N latitude). Male and female subjects between the ages of 18 and 42 years who entered US Army basic combat training (BCT) during February 2013 were eligible to volunteer. Exclusion criteria included the following: <18 years of age, pregnant or lactating women, history of kidney disease or renal calculi or allergy to any component of the food product bars. A total of 152 volunteers (ninety-eight males and fifty-four females) with complete pre- and post-BCT panels for all analytes were included in this sub-analysis.
Intervention
Volunteers were block randomised by race and sex to one of the two intervention groups: a placebo bar or a Ca/vitamin D-fortified bar. Volunteers were then assigned a volunteer ID. Volunteers and all research personnel conducting data collection and/or analysis were blinded to the group assignment. Bars were specially manufactured by the Combat Feeding Directorate at the Natick Soldier Systems Center and labelled with a three-letter code to indicate the intervention group and the study key was maintained by the manufacturer. The nutrient composition of the bars is included in Table 1. Ca was added to the bars in the form of calcium carbonate and vitamin D was added as D3. Concentrations of Ca and vitamin D in the bars were selected based on previous reports demonstrating efficacy in reducing stress fracture incidence in military personnel( Reference Lappe, Cullen and Haynatzki 2 ). The placebo and Ca/vitamin D bars were identical in taste and appearance and conformed to all ration standards for safety and stability.
Table 1 Composition of placebo and calcium/vitamin D (Ca+Vit D) bars
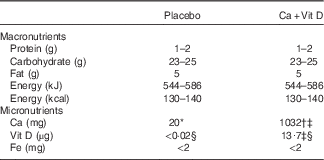
* Placebo bars contained 20 mg of incidental Ca.
† Ca as calcium carbonate.
‡ Biochemical analysis (Covance Laboratories) completed on a composite of five bars.
§ µg of Vit D3.
The bars were individually labelled with volunteer ID numbers and packaged into 1-week allotments (fourteen bars each). Volunteers were provided the bars weekly and instructed to consume 2 bars/d: one during mid-morning and the other during mid-afternoon. Empty wrappers and uneaten bars were collected from each volunteer during the weekly bar exchanges in order to monitor compliance. Compliance was 88 % in the placebo group and 81 % in the Ca/vitamin D group.
Basic combat training
The BCT course consists of 9 weeks of physical and military-specific training. Physical training requirements include aerobic activities such as foot marching with weighted packs, obstacle courses, distance running and sprinting as well as muscle strength training and calisthenic exercises. Military training includes activities such as rappelling, weapons training, prolonged standing in formation and didactic classroom instruction. Estimates of physical activity levels during BCT at Fort Sill have been reported previously( Reference Knapik, Sharp and Darakjy 18 , Reference Simpson, Redmond and Cohen 19 ). Soldiers consume 3 self-selected meals/d in a dining facility during BCT and are not permitted to consume dietary supplements.
Anthropometrics
All anthropometric measures were determined pre- and post-BCT, with the exception of height, which was measured pre-BCT to the nearest 0·1 cm using a stadiometer (Creative Health Products). Weight was determined to the nearest 0·1 kg on a calibrated digital scale (Befour Scales; Befour, Inc.) and BMI was calculated as body weight (kg)/height (m2). Skinfold thickness was measured at the tricep, suprailiac and abdomen for women and at the tricep, suscapula and chest for men. Measurements were made in duplicate to the nearest millimetre. If the measurements differed by >2 mm, a third measurement was taken. Body fat percentage was estimated using the three-site skinfold Jackson–Pollock equation( Reference Nindl, McClung and Miller 20 – Reference Pasiakos, Karl and Lutz 22 ). Calculations were sex specific as previously reported( Reference Jackson and Pollock 23 , Reference Jackson, Pollock and Ward 24 ).
Dietary intake
Pre- and post-BCT dietary intakes were estimated using a self-administered validated FFQ (Block 2005 FFQ; NutritionQuest) under the supervision of Registered Dietitians. The FFQ contained food lists developed from the National Health and Nutrition Examination Survey (NHANES) 1999–2002 dietary recall data. Nutrient intake data were excluded from the analysis if the energy intake was implausible (<1255 or >18 828 kJ (<300 or >4500 kcal) for females; <3347 or >20 920 kJ (<800 or >5000 kcal) for males).
Blood collection and circulating biomarkers
Fasting blood samples were collected by antecubital venepuncture into vacuum tubes (Vacutainer; Becton Dickinson). Serum and heparinised plasma were isolated, frozen and shipped to the Pennington Biomedical Research Center (PBRC) for assessment of indicators of Fe status. Serum ferritin and high-sensitivity C-reactive protein (CRP) levels were measured using an automated immunoassay instrument (Siemens Medical Solutions USA Inc.). Serum Fe and total Fe-binding capacity was measured using the Beckman Coulter DxC 600 Pro System (Beckman Coulter), and transferrin saturation was calculated by dividing the serum Fe by total Fe-binding capacity. Soluble transferrin receptor (sTfR) concentrations were measured using a commercially available immunoassay (Quantikine IVD; R&D Systems Inc.). Intact PTH was measured by immunoassay (Siemens Immulite 2000; Siemens Medical Solutions USA Inc.). A small aliquot of whole, heparinised blood was used at the time the blood samples were obtained to determine iCa and Hb utilising a handheld iSTAT® System point-of-care device and Chem8+ Cartridges (Abbott Laboratories). PBRC follows good clinical practices and is accredited by the College of American Pathologists. All assays were run with standards and appropriate quality control material. In addition, PBRC runs external proficiency samples and results are compared with other laboratories across the country.
Statistical analyses
Data are reported as means and standard deviations. Normality was determined using the Kolmogorov–Smirnov test. Analyses were carried out using Student’s t test or two-factor repeated-measures ANOVA with time as the within-subjects factor and treatment group and sex as the between-subjects factors. When a significant interaction was observed, post hoc analyses with Bonferroni correction were carried out to identify those differences. Significance was demonstrated at P<0·05. Data were analysed using SPSS version 21 (IBM Corp.) and graphed using GraphPad Prism 5.04 (GraphPad Software Inc.). Implausible serum ferritin values from one volunteer were not included in the final analysis; exclusion of these data did not affect study outcomes.
Results
A total of 152 volunteers with complete pre- and post-BCT panels for all Fe status indicators were included in this sub-analysis: fifty males and twenty-five females in the placebo group and forty-eight males and twenty-nine females in the Ca/vitamin D group. Volunteer demographics pre- and post-BCT are included in Table 2. No differences in sex, race or age were observed between treatment groups. Weight and BMI did not differ between groups or pre- and post-BCT. Similar to previous reports, body fat percentage decreased significantly during training (P<0·05)( Reference Lutz, Karl and Rood 3 , Reference Pasiakos, Karl and Lutz 22 ); however, no difference was observed between treatment groups.
Table 2 Volunteer demographics and body composition pre- and post-basic combat training (BCT)Footnote * (Numbers and percentages; mean values and standard deviations)
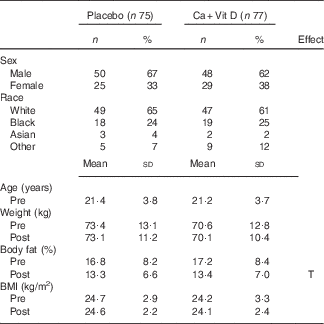
Ca+Vit D, Ca/vitamin D; T, time.
* Analyses were computed using Student’s t test or repeated-measures ANOVA with time as the within-subjects factor and treatment group as the between-subjects factor. The Bonferroni correction was used for post hoc comparisons. No differences between treatment groups were observed. Time indicates significant difference between pre- and post-BCT (P<0·05).
Dietary intake of Ca and vitamin D did not differ between treatment groups; however, with the inclusion of the intervention bars, total Ca and vitamin D intake increased approximately 3- and 5-fold, respectively, in the Ca/vitamin D group from pre- to post-BCT (P<0·05; Table 3). The level of dietary Fe consumed per day during BCT was above the RDA for males (8 mg/d) but below the RDA for females (18 mg/d; Table 3). Separated by sex, males consumed 18·0 (sd 6·6) mg Fe/d and females consumed 14·3 (sd 5·6) mg Fe/d. The level of energy and dietary protein, fat and Zn consumed per day decreased significantly (P<0·05) during training.
Table 3 Dietary intake pre- and post-basic combat training (BCT)† ‡ (Mean values and standard deviations)

Ca+Vit D, Ca/vitamin D; T, time; G, group.
* Significantly different between pre- and post-BCT (P<0·05).
† Data were analysed using repeated-measures ANOVA with time as the within-subjects factor and treatment group as the between-subjects factor. Significant effects of time and treatment group are shown (P<0·05). Post hoc Bonferroni correction was used for time-by-group comparisons.
‡ Nutrient intake data were excluded from the analysis if energy intake was implausible (<1255 or >18 828 kJ (<300 or >4500 kcal) for females; <3347 or >20 920 kJ (<800 or >5000 kcal) for males).
§ RDA for 19–30-year-old males and females.
Similar to the parent study, circulating concentrations of iCa and PTH did not differ at pre-BCT( Reference Gaffney-Stomberg, Lutz and Rood 4 ); however, volunteers consuming the Ca/vitamin D snack bar had higher serum iCa (P<0·05) and lower PTH (P<0·05) at the completion of BCT compared with volunteers consuming the placebo snack bar (Fig. 1). Circulating 25(OH)D increased (P<0·05) in both groups and 1,25(OH)2D did not change (P>0·05) in either group during BCT (data not shown), as reported previously( Reference Gaffney-Stomberg, Lutz and Rood 4 ). There were no sex differences in circulating 25(OH)D or 1,25(OH)2D in either treatment group (data not shown). Pooled data from males and females demonstrated that indices of Fe status were affected by BCT independent of the intervention (Fig. 2). Transferrin saturation, an indicator of early-stage Fe depletion, was reduced >35 % (P<0·05; Fig. 2(a)) at the completion of the BCT; however, no differences were observed between placebo and Ca/vitamin D groups. Serum ferritin decreased by approximately 50 % (P<0·05; Fig. 2(b)) and sTfR increased by approximately 28 % (P<0·05; Fig. 2(c)) post-BCT compared with pre-BCT, reflecting reduced Fe stores and tissue Fe deficiency, respectively, although no differences were observed between treatment groups. Hb levels decreased by approximately 8 % (P<0·05; Fig. 2(d)) at the completion of BCT, although no differences in Hb levels were observed between placebo and Ca/vitamin D groups. When separated by sex, Fe status declined in both male and female volunteers at the completion of BCT independent of the intervention (Table 4). However, Ca/vitamin D supplementation preserved transferrin saturation and Hb concentrations in female volunteers in the intervention group compared with the placebo group. Similar to a recent report( Reference Yanovich, Karl and Yanovich 8 ), the magnitude of change of Fe status indicators from pre- to post-BCT was greater in female volunteers compared with male volunteers. This was reflected in a larger decrease in transferrin saturation (male: 37·0 (sd 24·4) %, female: 20·7 (sd 69·6) %; P<0·05) and serum ferritin (male: 52·0 (sd 20·1) %, female: 44·5 (sd 34·7) %; P=0·095) and an increase in sTfR (male: 35·4 (sd 21·5) %, female: 47·9 (sd 27·6) %; P<0·05). CRP increased (P<0·05) throughout training but did not differ between groups (placebo: pre 2·69 (sd 6·22) mg/l, post 7·43 (sd 21·5) mg/l; Ca/vitamin D: pre 3·73 (sd 14·5) mg/l, post 6·65 (sd 10·0) mg/l).

Fig. 1 Indicators of calcium status pre- and post-basic combat training (BCT) in male and female soldiers receiving placebo or calcium/vitamin D (Ca+Vit D) bars. (a) Ionised calcium (iCa) and (b) parathyroid hormone (PTH). Values are means and standard deviations, and two-factor repeated-measures ANOVA with Bonferroni correction was utilised for comparisons represented by vertical bars. * Significantly different: P<0·05. Placebo: n 75; Ca+Vit D: n 77. ![]() , Pre-BCT;
, Pre-BCT; ![]() , post-BCT.
, post-BCT.

Fig. 2 Iron status indicators pre- and post-basic combat training (BCT) in male and female soldiers receiving placebo or calcium/vitamin D (Ca+Vit D) bars. (a) Transferrin saturation, (b) serum ferritin, (c) soluble transferrin receptor (sTfR) and (d) Hb. Values are means and standard deviations, and were analysed using two-factor repeated-measures ANOVA with time as the within-subjects factor and treatment group as the between-subjects factor. The Bonferroni correction was used for post hoc comparisons. No differences between treatments were observed. * Significantly different between pre- and post-BCT (P<0·05). Placebo: n 74–75; Ca+Vit D: n 77. ![]() , Pre-BCT;
, Pre-BCT; ![]() , post-BCT.
, post-BCT.
Table 4 Indices of iron status in male and female soldiers pre- and post-basic combat training (BCT)Footnote † (Mean values and standard deviations)
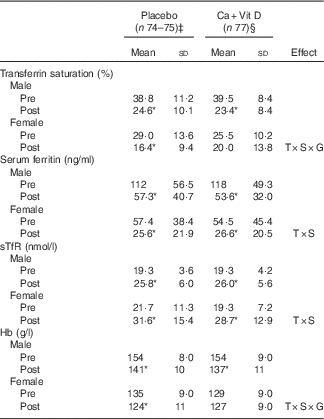
Ca+Vit D, Ca/vitamin D; T, time; S, sex; G, group; sTfR, soluble transferrin receptor.*Significantly different between pre- and post-BCT (P<0·05).
† Data were analysed using two-factor repeated-measures ANOVA with time as the within-subjects factor and sex and treatment group as the between-subjects factors. Post hoc Bonferroni correction was used to identify interaction differences. No differences between treatment groups were observed.
‡ Placebo: male, n 49–50; female, n 25.
§ Ca+Vit D: male, n 8; female, n 29.
Discussion
The objective of this randomised, double-blind, placebo-controlled trial was to determine whether providing 2000 mg/d Ca in a fortified food product during 9 weeks of military training would negatively affect Fe status in young adults. These data indicate that consumption of a Ca/vitamin D-fortified snack does not further exacerbate the decline in Fe status that occurred in both male and female soldiers. These data coupled with the results from the parent study( Reference Gaffney-Stomberg, Lutz and Rood 4 ) indicate that Ca/vitamin D supplementation provides benefits to bone health without negatively affecting Fe status.
The timing and duration of Ca/vitamin D supplementation relative to Fe intake may explain why supplementation did not impact Fe absorption/status. In the present study, the Ca/vitamin D bar was provided twice daily between meals rather than at meal time. Most studies in humans that have demonstrated a reduction in Fe absorption with supplemental Ca have been single meal studies using the dual radioisotope method. For example, Hallberg et al.( Reference Hallberg, Brune and Erlandsson 14 ) reported a dose-dependent decrease of 50–60 % in Fe absorption in men and women given 40–600 mg Ca added to a test meal. Similarly, in a three-period cross-over study, premenopausal women consuming a 500 mg Ca supplement absorbed approximately 55 % less Fe compared with the placebo( Reference Dawson-Hughes, Seligson and Hughes 12 ). Thus, Ca given separate from meals may limit interference from inhibitors such as phytate or may limit the direct effects of Ca on Fe transport( Reference Ilich-Ernst, McKenna and Badenhop 17 ).
Longitudinal studies examining the effects of Ca over time have found a reduced effect or no effect of Ca on Fe absorption compared with single meal studies( Reference Gleerup, Rossander-Hulten and Hallberg 15 , Reference Reddy and Cook 16 ) and no effect of Ca on the Fe status of men or women( Reference Ilich-Ernst, McKenna and Badenhop 17 , Reference Kalkwarf and Harrast 25 , Reference Minihane and Fairweather-Tait 26 ). This suggests that absorptive mechanisms may have adapted in the presence of high Ca intakes to maintain Fe homoeostasis over the longer study period. Mechanistically, Ca is thought to transiently inhibit Fe transport in the small intestine by interacting with divalent metal transporter 1 (DMT1) and/or ferroportin, thereby reducing Fe assimilation. However, over time, adaptive mechanisms may restore Fe absorption. Shawki & Mackenzie( Reference Shawki and Mackenzie 27 ) reported that Ca is not transported by DMT1, but that Ca blocks DMT1-mediated Fe uptake (55Fe) in Xenopus oocytes. Using differentiated Caco-2 cells grown on Transwell inserts, Lönnerdal( Reference Lönnerdal 28 ) demonstrated a significant reduction in basolateral transfer of Fe when the apical chamber was incubated with medium containing 1 µm-iron sulphate labelled with 59Fe and 100 µm-calcium chloride compared with cells with no calcium chloride. Interestingly, a greater reduction in Fe transport was observed at 1·5 h compared with 4 h( Reference Lönnerdal 28 ). Moreover, DMT1 expression was slightly reduced and surface-bound Ferroportin was significantly decreased at 1·5 h, whereas expression of both transporters increased at 4 h( Reference Lönnerdal 28 ). Using a similar model, it has been reported that DMT1 becomes internalised with high Ca concentrations( Reference Thompson, Sharp and Elliott 29 ). Collectively, these results suggest that Ca likely inhibits Fe transport by signalling for the internationalisation of DMT1 and/or ferroportin; however, adaptive mechanisms may preserve Fe status in the event that Ca and Fe are consumed together for longer periods of time.
Fe status should also be considered when examining the effects of added Ca on Fe absorption. Previous studies have reported no effects on functional Fe or Fe stores in Fe-depleted individuals when given supplemental Ca( Reference Ilich-Ernst, McKenna and Badenhop 17 ). This is likely due to heightened absorptive mechanisms for Fe when status is low( Reference Bezwoda, Bothwell and Torrance 30 , Reference Taylor, Martinez-Torres and Leets 31 ). In the present study, decrements in Fe status indicators occurred in male and female soldiers, regardless of treatment group; however, the Fe status of female soldiers was reduced compared with that of male soldiers. This is in agreement with recent reports noting a sharper decline in Fe status in female soldiers compared with male soldiers during BCT( Reference Yanovich, Karl and Yanovich 8 ). Dietary Fe intake in both the present study and previous studies( Reference Yanovich, Karl and Yanovich 8 ) was >200 % and approximately 90 % of the RDA for men and women, respectively (Table 3). Thus, women may be more susceptible to poor Fe status during BCT due to dietary intakes below the RDA. Further, women may experience increased Fe losses through menstruation( Reference Hallberg and Rossander-Hulten 32 ). The relatively reduced Fe status of female soldiers compared with male soldiers and potential adaptive mechanisms to increase Fe absorption in response to sustained Ca intakes could be one possible explanation for the observed preservation in transferrin saturation and Hb in female soldiers consuming the Ca/vitamin D snack bars.
Military trainees encounter many obstacles including musculoskeletal injury that may affect the successful completion of BCT. Nutrition is one factor that can be modified to mitigate the risk of injury while optimising physical and cognitive performance. Countermeasures to combat stress fracture are important for maintaining the health of military trainees and increasing the likelihood of successful completion of training, especially for female military personnel. This is the first study to examine the effects of Ca/vitamin D supplementation on measures of Fe status in the military population. Strengths of this study include the repeated-measures design, sample size, multiple measures of Fe status, dietary intake data and the lack of dietary supplements during BCT. A limitation of the study was the inability to measure apparent Fe absorption during BCT. Data from the present study and the parent study( Reference Gaffney-Stomberg, Lutz and Rood 4 ) indicate that Ca/vitamin D supplementation provided as a snack through the use of a fortified food product provides benefits to bone health without negatively affecting Fe status. Future studies should determine an effective means to protect bone health throughout BCT while preserving or improving Fe status.
Acknowledgements
The authors acknowledge the study volunteers and the study research team.
Approved for public release; distribution is unlimited. The opinions or assertions contained here are the private views of the authors and are not to be construed as official or as reflecting the views of the army or the Department of Defense. Any citations or commercial organisations and trade names in this report do not constitute an Official Department of the Army endorsement of approval of the products or services of these organisations.
Supported in part by the US Army Medical Research and Materiel Command and appointment to the US Army Research Institute of Environmental Medicine administered by the Oak Ridge Institute for Science and Education (to S. R. H.) through an interagency agreement between the US Department of Energy and the US Army Medical Research and Materiel Command.
E. G.-S., A. J. Y. and J. P. M. designed the study; E. G.-S., L. J. L., S. J. C., S. M. P. and J. P. M. executed the study; S. R. H., E. G.-S., L. J. L. and J. P. M. analysed the data and wrote the manuscript. J. P. M. had primary responsibility for the final content, and all the authors read and approved the final version of the manuscript.
None of the authors has any conflicts of interest to report.









