As one of Canada’s high-volume adult epilepsy surgery centers,Reference Blume and Girvin 1 a large number of our patients at London Health Sciences Centre with drug-resistant epilepsy are subjected to intracranial electroencephalographic monitoring.Reference Dubeau and McLachlan 2 The first subdural electrodes (SE) were implanted in London in 1979 by John Girvin, who had trained at the Montreal Neurological Institute, and soon became our routinely employed invasive diagnostic standard for presurgical investigationReference Blume and Girvin 1 when surface electroencephalography could not delineate the seizure onset zone sufficiently.Reference Dubeau and McLachlan 2 With our SE implantation technique,Reference Steven, Andrade-Souza, Burneo, McLachlan and Parrent 3 complication rates were low;Reference MacDougall, Burneo, McLachlan and Steven 4 , Reference Burneo, Steven, McLachlan and Parrent 5 75% of all patients investigated with SE proceded to resective surgery for epilepsy, which resulted in seizure-freedom at 1 year (Engel I) in 47% of the cases.Reference MacDougall, Burneo, McLachlan and Steven 4
Subdural electrodes for intracranial electroencephalographic monitoring were first introduced by Herbert Jasper in the 1950s. In parallel, stereoelectroencephalography (SEEG) was pioneered by Talairach and Bancaud at Sainte Anne Hospital, Paris.Reference Dubeau and McLachlan 2 Depth electrode (DE) for SEEG were also used at the Montreal Neurological Institute in the 1970s, and, in fact, further technically refined by the introduction of digital subtraction angiography in the 1980s and double-dose gadolinium magnetic resonance imaging in the 1990s.Reference Cardinale, Casaceli, Raneri, Miller and Lo Russo 6
A paradigm shift toward SEEG has taken place at our institution since 2013, and DE have almost entirely replaced SE (Figure 1). Given the lack of high-quality studies that directly compare the superiority of one technique over the other, the use of SE versus SEEG remains a pivotal discussion and must be viewed as equipoise; the choice between the two techniques is dependent on the question of the electroencephalographic investigationReference Jayakar, Gotman and Harvey 7 and is a matter of institutional preference.
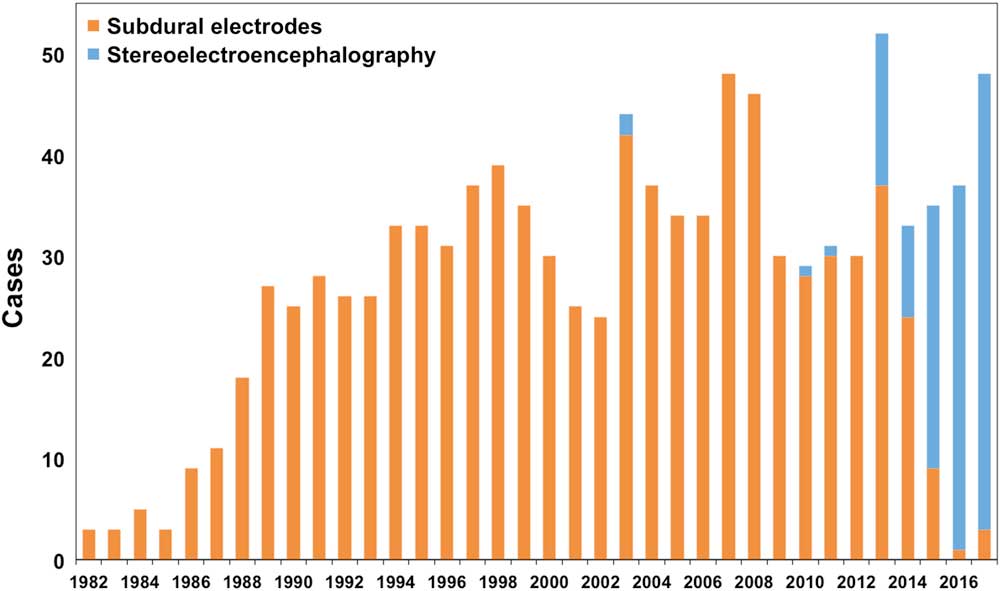
Figure 1 Absolute case numbers of subdural strip/grid electrodes and depth electrodes for stereoelectroencephalography over time at the London Health Sciences Centre Epilepsy Program in London Ontario, Canada. Over the past 3 years, the latter have evolved into the principle means of intracranial electroencephalographic monitoring in the presurgical investigation of drug-resistant epilepsy.
Although “open” combined SE and DE variants via a craniotomy were not uncommon, our first “stand-alone” stereotactic DE implantation for SEEG at our institution did not take place until 2003 (Figure 1); however, this case was challenged by the lack of technical refinements such as anchor bolts, for example, and SEEG was not resumed until a decade later. In 2013 and 2014, SEEG was performed for 28% of all our invasive electroencephalographic monitoring cases, and this percentage increased to 95% in the past 2 years (Figure 1). The considerations presented in this manuscript factored into our adopted change in practice. Our observation of (clinically silent) magnetic resonance imaging abnormalities after SE placementReference Al-Otaibi, Alabousi, Burneo, Lee, Parrent and Steven 8 and expert opinions on lower complication ratesReference Cardinale, Casaceli, Raneri, Miller and Lo Russo 6 prompted a new generation of epileptologists and epilepsy surgeonsReference Blume and Girvin 1 to give a new impetus to implementing SEEG. In the same vein, increased experience with stereotactic treatments for epilepsyReference Parrent and Blume 9 built up our confidence, allowing us to finally overcome the learning curve of Leksell frame-based stereotactic DE implantation as it is now at our institution.Reference Joswig, Benson, Parrent, MacDougall and Steven 10 It is our anecdotal experience that patients with DE suffer from fewer postoperative headaches and less discomfort than after SE implantation. Cerebrospinal fluid leakage is almost never encountered after SEEG. Last, mean operative time for SEEG is shorter by half an hour (p<0.05; unpublished data). Over the years, we have learnt to adapt to the “three-dimensional thinking” in SEEG and to appreciate the possibility of investigating deeper structures, such as the insula or deeply situated heterotopic gray matter. In fact, as previously stated,Reference Dubeau and McLachlan 2 as DE, by virtue of their longitudinal recording area, cover both deep and superficial cortical structures, “DE” is a misnomer. Our previous anatomical thinking as subdural implanters developed into a more network-based understanding of epilepsy that emphasizes semiology for planning “punctuate” DE for SEEG, which, in fact, samples less brain. On the basis of these principles and with more experience with DE extraoperative cortical stimulation, we no longer regard SE “more useful than DE to identify areas of eloquent cortex”.Reference Dubeau and McLachlan 2 Yet, we still make use—and will make future use—of SE and grids in selected cases where high-resolution extraoperative cortical simulation is warranted, as well as in very young patients or in those with contraindications to undergo magnetic resonance imaging for presurgical planning. Specific indications, strengths, and limitations of both techniques, as well as a flow-chart protocol are well summarized in a recent consensus-based expert recommendation.Reference Jayakar, Gotman and Harvey 7
With the advent of new imaging technologies, the presurgical invasive diagnostic armamentarium continues to grow.Reference Jayakar, Gotman and Harvey 7 A need for a considerate selection of old and new invasive intracranial monitoring techniques or a combination of bothReference Jayakar, Gotman and Harvey 7 to accurately localize the seizure focus remains.Reference Dubeau and McLachlan 2
Disclosures
HJ reports personal fees from UCB Canada, non-financial support from Fujirebio Europe N.V. (Ghent, Belgium), outside the submitted work; DAS, KWM, RSM, and DCD have nothing to disclose; AGP reports personal fees from Medtronic, outside the submitted work; SMS reports personal fees from UCB Pharma, personal fees from Eisai Limited, and personal fees from Sunovion Pharmaceuticals Canada, Inc., outside the submitted work; JGB reports grants and personal fees from UCB Pharma, personal fees from Eisai Limited, grants from Epilepsy Canada, and grants from Ontario Brain Institute, outside the submitted work.
Statement of Authorship
HJ: study conceptualization, data collection, manuscript drafting/finalization, literature research, and creation/edit of Figure 1. DAS, AGP, KWM, SMM, RSM, and DCD: data collection and manuscript review. JGB: study conceptualization and supervision, data collection, creation/edit of Figure 1, and manuscript review.



