To early humans living on the coast, the oceans must have appeared as largely inhospitable expanses, difficult to access, seemingly limitless, and so virtually unexplored and unexploited. But even prehistoric cultures, especially once they became proficient seafarers, started to have substantial impacts on marine environments, such as on islands and near coastal settlements, in some cases causing local extinctions (Erlandson & Rick, Reference Erlandson and Rick2010). Within historical times, particularly within the past century, the scale of exploitation of marine living resources and degradation of marine ecosystems have become global issues raising widespread concern. An estimated 40% of the world’s oceans are now strongly affected by human impact with no area untouched (Halpern et al., Reference Halpern, Walbridge and Selkoe2008).
Efforts at nature conservation have been, and indeed still are, directed largely at terrestrial environments, as is to be expected. In our use of living space and natural resources we have wrought enormous changes to terrestrial habitats and their biotas. And increasingly we are being disadvantaged by environmental consequences associated with our burgeoning population. Aside from any moral sensibility, we need to safeguard the functioning of healthy ecosystems on which we ultimately depend. On land, the need to afford protection to vulnerable species and habitats has long been recognized, and many practices of terrestrial conservation are well established. But widespread concern about the health of the world’s oceans and use of their resources has surfaced only relatively recently, resulting in concerted moves towards marine conservation.
Marine and Terrestrial Ecosystems
Ecological concepts have stemmed largely from our experience of the terrestrial environment, and programmes for nature conservation were established on land long before the emergence of marine conservation. Marine and terrestrial ecosystems, however, call for different approaches to conservation, and the attributes of marine ecosystems must be borne in mind in developing strategies for their conservation. It is worth considering, therefore, the distinguishing features of marine ecosystems and how they differ from those on land (e.g. Carr et al., Reference Carr, Neigel and Estes2003).
The most obvious difference between terrestrial and marine systems is one of size. The oceans cover 362 million km2, or 71% of the Earth’s surface (Harris et al., Reference Harris, Macmillan-Lawler, Rupp and Baker2014). The difference, however, is far greater by volume. On land, the habitable zone is a comparatively thin veneer – generally some tens of metres in height – whereas the oceans have a mean depth of about 3.7 km, and this entire marine space is inhabited. As a result, the marine realm accounts for more than 99% of the habitable volume of the planet (Dawson, Reference Dawson2012).
For land organisms, biological tissue is far denser than the surrounding atmosphere. Water, on the other hand, some 800 times denser than air, is by comparison a very supportive medium, so many marine organisms can maintain near neutral or positive buoyancy and inhabit the water column at little energetic cost. The density of suspended particles also means that suspension feeding is of major importance in marine ecosystems, whereas the sparsity of suitable particles in air effectively rules out a comparable method of feeding for land animals.
The large thermal capacity of the ocean dampens short-term temperature variability. Most marine organisms need to contend with only small and gradual changes in temperature compared to the extremes often experienced by land organisms. So even at higher trophic levels, ectothermic, or ‘cold-blooded,’ animals predominate in the sea. However, the temporal variability in abiotic factors such as temperature differs radically between marine and terrestrial systems. Variance of temperature on land is relatively constant, at least over ecological timescales. In marine systems, on the other hand, whereas short-term variability is constrained, this variance increases over longer timescales. In other words, large, slow oceanographic processes account for more environmental variability than smaller, short-lived processes (Stergiou & Browman, Reference Stergiou and Browman2005).
Inhabitants of terrestrial ecosystems are usually close to sources of primary production, whereas such proximity is rare in marine ecosystems. Most ocean space is deep sea, remote from the euphotic zone, and where the biota largely comprises microorganisms and animals dependent upon the flux of detritus from surface waters. The euphotic zone, where photosynthesis is possible, typically extends to depths of only a few tens of metres in coastal waters and usually to little more than 100 m in the open ocean. This is of similar dimension to the height of the photosynthetic layer on land as represented by the tallest trees. To counter the unsupportive environment of the atmosphere, land plants invest heavily in structural materials, such as cellulose and lignin, to elevate their photosynthetic tissues. In this way vegetation is usually a large and important component of terrestrial communities. Many terrestrial plant communities such as tropical forests are structurally complex and contain an immense diversity of plant species. This infrastructure in turn provides for a huge diversity of niches and a corresponding richness of associated animal species, of which more than two-thirds are insects. An analogous structural dimension is provided in coastal habitats by macroalgae, seagrasses, mangroves, and saltmarsh plants (see Chapter 12), although these are restricted to shallow waters. Phytoplankton, on the other hand, although they are the main primary producers in the marine environment, provide few habitat opportunities for associated species, except perhaps for microbes.
Food Webs
Global net primary production has been estimated at about 105 x 109 tonnes C y−1, with roughly equal contributions from land and the oceans (Field et al., Reference Field, Behrenfeld, Randerson and Falkowski1998; Carr et al., Reference Carr, Friedrichs and Schmeltz2006), even though the biosphere is predominantly marine (Table 1.1). The nature of production, however, differs greatly between the two systems. Marine primary producers are mainly phytoplankton, which represent only 0.2% of the global biomass of primary producers. Phytoplankton have an average turnover time of only 2–6 days and so can achieve high rates of production. By contrast, plant biomass on land is dominated by forests, and the turnover time of terrestrial primary producers averages 19 years (Field et al., Reference Field, Behrenfeld, Randerson and Falkowski1998). For many land ecosystems, the major primary producers are the largest and longest-lived organisms (notably trees), whereas in marine systems most primary producers are microscopic and short-lived.
| Oceans | Land | |
|---|---|---|
| Total net primary production (x 109 t C y−1) | ~50 | ~60 |
| Total primary producer biomass (x 109 t C) | 1 | 500 |
| Average turnover of biomass | 2–6 days | 19 years |
Much terrestrial primary production is indigestible to herbivores and is broken down by decomposers and detritivores. This may explain why levels of herbivory in the sea can typically be 10–20 times those on land (May, Reference May1994). The classical model of marine food chains was one in which a large proportion of phytoplankton production is grazed directly by zooplankton with few subsequent trophic steps. However, pelagic food webs incorporate far more components at low trophic levels than previously realized, and traditional distinctions between autotrophic phytoplankton and heterotrophic protists are often blurred. Particularly significant is the role played by a range of planktonic organisms in the smallest size classes, including cyanobacteria (of less than 2 µm) that may account for most of the pelagic primary production in warm ocean waters. There are also minute grazers (2–5 µm) and a range of protists (5–20 µm) that in turn are consumed by larger zooplankton (Longhurst, Reference Longhurst2007).
Major food-web patterns are recognizable in the global ocean based on gross differences in nutrient supply, productivity, and trophic complexity. At one end of the spectrum are nutrient-poor open ocean waters with food webs of high trophic complexity culminating in low production of top consumers. This situation is typical of tropical and subtropical mid-ocean regions where a strong thermocline separates the warm surface layer from deeper cold water. The stability of this stratification keeps phytoplankton near the surface, meaning there is sufficient light to support their growth. However, stratification also inhibits mixing of the water column and thereby the input of deep, nutrient-rich water into the euphotic zone. In higher latitudes, as sea surface temperature falls in autumn, the difference in density between the surface and deep layers diminishes. This enables vertical mixing to occur during winter, which replenishes nutrients at the surface in readiness for a spring phytoplankton bloom. The productivity of such systems is dominated by this major seasonal event.
In some areas, notably coastal upwelling systems, the conditions favouring maximum production occur over significantly longer periods. Upwelling ecosystems occur mainly along the west coasts of continents at subtropical latitudes where there are prevailing offshore winds and strong eastern boundary currents. Here, surface waters are diverted offshore so that cold subsurface nutrient-rich water is drawn to the surface. Major coastal upwelling systems occur off Peru, Oregon and California, north-west and south-west Africa, and in the NW Indian Ocean (in this case driven by the seasonal monsoon). These nutrient-rich systems are characterized by few trophic levels and high productivities of fish, seabirds, and marine mammals. Whilst upwelling regions total only about 1% of the world ocean area, they account for about 20% of global fish landings (Mann, Reference Mann2000).
Food webs of shelf waters are generally of intermediate complexity and productivity. Here nutrients are not lost into deep water and can be returned to the surface, particularly in mid- to high latitudes when the water column loses its thermal stability in winter. Also, estuaries can supply important nutrients to coastal waters. A significant proportion of primary production in coastal areas may be contributed by fringing macrophytes rather than by phytoplankton, such as by the highly productive kelp, saltmarsh, and mangrove systems.
Differences in marine and terrestrial food webs and the susceptibility of their respective trophic levels to harvesting make for contrasting patterns of human use. On land, where food production is based on agriculture, plants such as cereals and sugar cane are the primary harvest. Crops are increasingly being grown for herbivore production as our consumption of meat rises (Tilman et al., Reference Tilman, Cassman, Matson, Naylor and Polasky2002). By contrast, our consumption of terrestrial carnivores is minuscule. An essentially inverse pattern pertains to the sea where the catch is predominantly of carnivores, in particular predatory fish. Some herbivores, such as clupeid fishes and bivalve molluscs, are also exploited, but primary producers (in this case macroalgae) have traditionally made up only a small proportion of the total marine biomass taken. With the catch coming largely from wild stocks, the trophic level of targeted marine species has not customarily been a concern. But with existing fisheries under intense pressure, there is interest in making greater use of organisms at lower trophic levels, such as herbivorous zooplankton. The difficulty is that such organisms tend to be uneconomic to harvest. In the case of agriculture, the trophic level of a harvest directly affects the energy efficiency and viability of the operation. The contribution of aquaculture to marine production is steadily increasing but will need to focus increasingly on organisms of low trophic status – algae, herbivores, and detritivores – and avoid methods that are environmentally harmful (see Chapter 5).
Ocean Basins and Circulation
The world’s land and sea areas are distributed very unequally. In the Southern Hemisphere, the marine area is four times greater than the land area, whilst in the Northern Hemisphere, it is only 1.5 times larger. In fact, the latitudinal distributions of sea and land in the two hemispheres are almost mirror images (Fig. 1.1). The temperate zone of the Southern Hemisphere is almost entirely maritime, but in the Northern Hemisphere this is where the landmasses are concentrated. This disparity has major implications for the global distribution of marine environments. Continental shelf, estuarine and brackish systems are, for instance, better represented in the Northern Hemisphere. A corresponding distribution of human population and associated scientific endeavour has meant too that marine environments of the Northern Hemisphere have in general been more intensively researched than their southern counterparts.
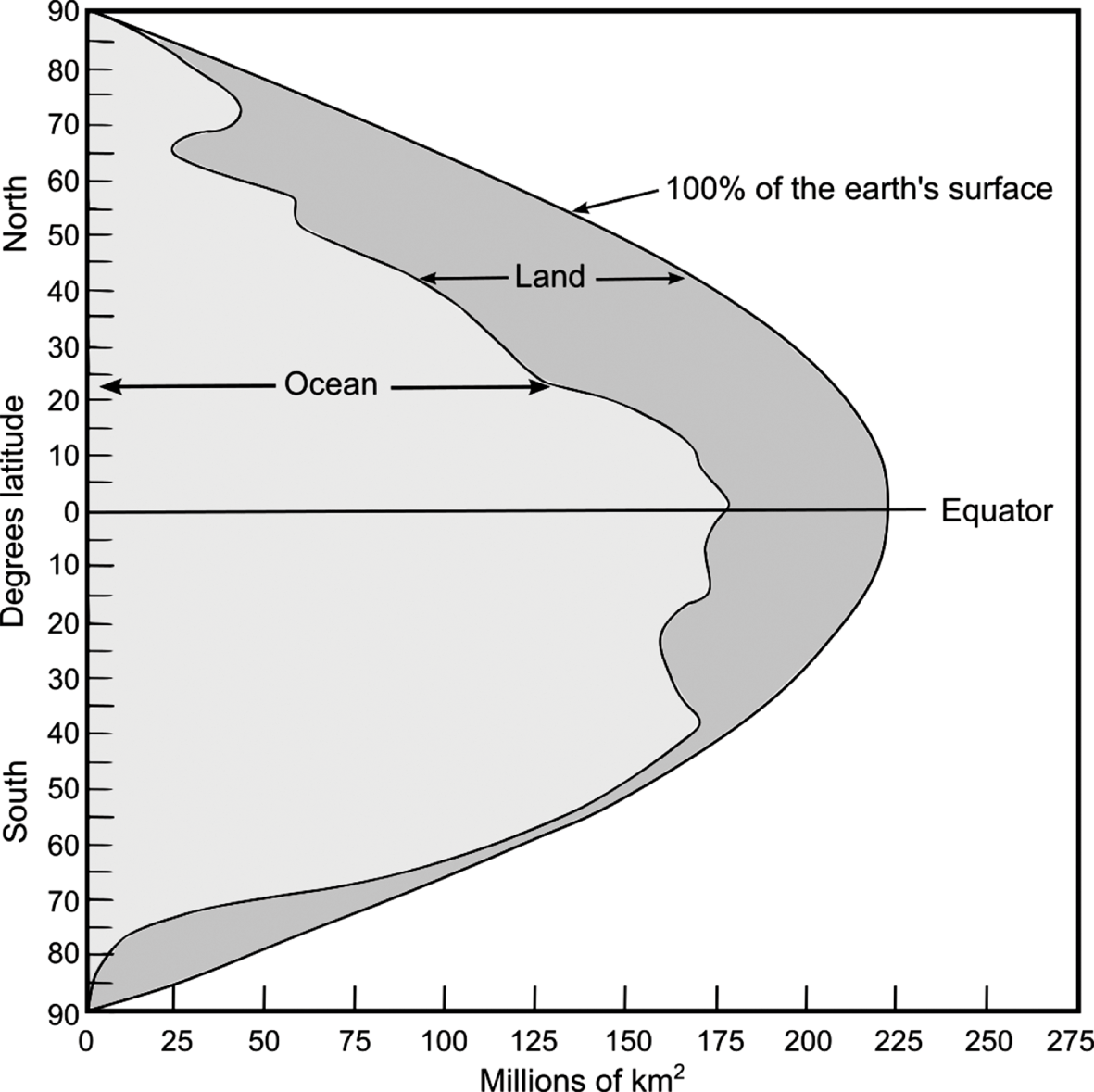
Fig. 1.1 Distribution of ocean and land by latitude. Areas are based on 5° latitude intervals. The areal extent of ocean differs markedly between the two hemispheres, particularly at mid-latitudes.
The global marine environment can be defined in terms of the major oceans and associated seas, their circulation, and bathymetric zonation. The Pacific Ocean accounts for roughly 47% of the total ocean area, the Atlantic Ocean plus Arctic Ocean and Mediterranean Sea 28%, and the Indian Ocean 20% (Harris et al., Reference Harris, Macmillan-Lawler, Rupp and Baker2014). The southern sectors of these oceans comprise the Southern Ocean at about 6%, which, although less well defined geographically, has distinctive hydrological and biological characteristics. The northern limit of the Southern Ocean is taken as the Antarctic Polar Front (at 50–60° S), a major circumglobal zone where surface Antarctic and subantarctic surface water masses meet (see Chapter 17).
Oceanic circulation is driven chiefly by the prevailing pattern of global winds and differences in the salinity and temperature (i.e. the density) of water masses. The pattern of surface currents is dominated by major gyres, huge circular flows within each of the ocean basins that move clockwise in the Northern Hemisphere and anticlockwise in the Southern Hemisphere (Fig. 1.2). An important exception is the Southern Ocean where, uninterrupted by landmasses, there is a circumglobal eastward flow. The Earth’s rotation tends to compress gyres on the western side of ocean basins to produce intense western boundary currents, such as the Gulf Stream (NW Atlantic), Kuroshio (NW Pacific), and Agulhas (SW Indian Ocean). The swift, warm flow of the Gulf Stream contributes to the North Atlantic Current, which flows across the Atlantic and has a strong moderating influence on the climate of NW Europe.
Large-scale zonal differences in the relative importance of evaporation and precipitation produce slight density differences of surface waters, and at the fronts where different water masses meet, the denser water sinks back into the interior of the ocean. Such fronts, marked by relatively abrupt changes in physical and chemical characteristics between water masses, can function as important biogeographical boundaries, at least for many pelagic species, such as the Antarctic Polar Front already mentioned and the Kuroshio Front separating subtropical and subpolar water masses (Clayton et al., Reference Clayton, Nagai and Follows2014).
The large-scale pattern of currents and frontal systems incorporates, however, a high degree of ecological complexity. Conspicuous features, at scales of up to a few hundred kilometres, include large eddies pinched off from currents. These are temporary islands of water with distinctive physical and biological characteristics. Eddies carry with them populations of organisms entrained from the parent water body and may persist for a year or so before eventually decaying and becoming indistinguishable from the surrounding water. In the vicinity of fronts, the convergence of water masses and increased nutrient availability can result in enhanced productivity and higher concentrations of pelagic organisms. As zones of increased food supply, fronts can be important feeding grounds. Apex predators such as seabirds and marine mammals are often associated with fronts (Bost et al., Reference Bost, Cotté and Bailleul2009).
The pattern of deep-water circulation is driven largely by differences in density between water masses. In particular, the sinking in polar regions of dense (colder and more saline) water sustains a convective flow, known as the global thermohaline circulation, that links the major ocean basins (Fig. 1.3). Many deep-water species have distributions consistent with features of deep oceanic circulation. The mid-slope demersal fish fauna of temperate Australia and New Zealand, for example, has obvious links with that of the temperate North Atlantic, a pattern reflecting the circulation of intermediate water masses between ocean basins (Koslow et al., Reference Koslow, Bulman and Lyle1994).
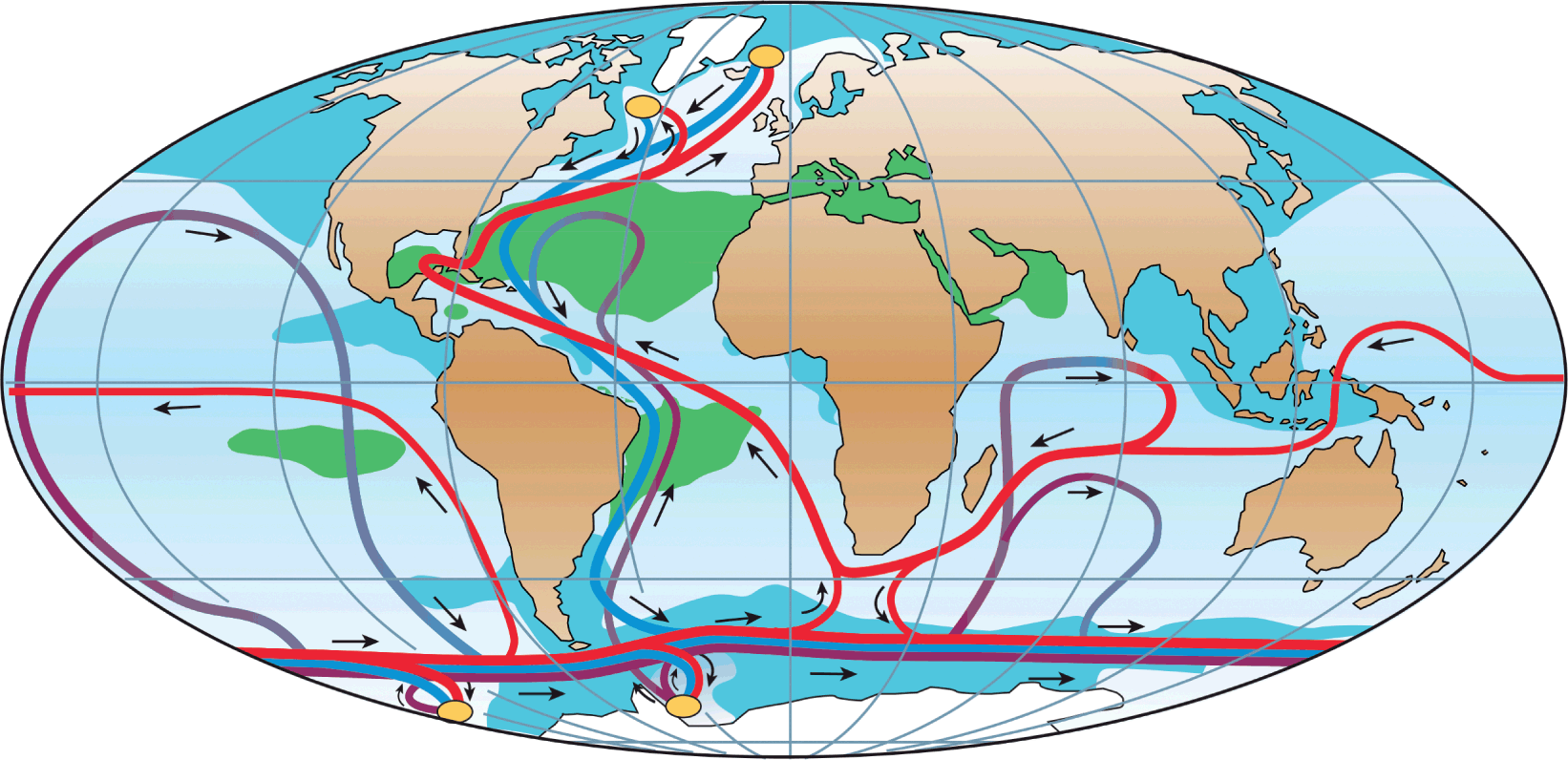
Fig. 1.3 A simplified diagram of the global thermohaline circulation. Near-surface waters (red lines) flow towards the main regions of deep-water formation (yellow ovals) – in the northern North Atlantic, the Ross Sea, and the Weddell Sea – and recirculate at depth as deep currents (blue lines) and bottom currents (purple lines). Green shading, salinity above 36; blue shading, salinity below 34.
Biogeography and Bathymetric Zones
A key requirement for conservation is a sound biogeographic framework (Lourie & Vincent, Reference Lourie and Vincent2004). Ideally, we need to understand not just the distribution of species and habitats but also regional differences in ecosystem functioning and the environmental drivers underlying these patterns. Such information is essential, for example, as a basis by which to establish networks of marine protected areas that are adequately representative (see Chapter 15) and to develop strategies for managing exploited and vulnerable species. We are still some way from a detailed understanding of the biogeography of the seas, but at least for surface waters there is broad agreement between global schemes for categorizing the marine environment for biogeographic purposes.
Broad divisions of the world’s surface ocean can be identified, related to the major climatic zones and ocean basins, and defined by physico-chemical parameters, notably temperature and salinity, wind-streams, and surface currents. On this basis various biogeographic schemes have been proposed for defining biologically meaningful areas at a range of spatial scales. A synthesis by Spalding et al. (Reference Spalding, Fox and Allen2007) provides a global biogeographic classification of the world’s coastal and shelf areas (out to the 200-m isobath) based on data from benthic and pelagic biotas (Fig. 1.4). This nested system comprises 12 realms, within which are 62 provinces and then 232 ecoregions. These realms and provinces represent the relative importance of various biotic and abiotic factors, such as taxonomic coherence and degree of endemism, and geomorphological, hydrological, and geochemical characteristics (Box 1.1). So, for example, the Temperate Northern Atlantic realm contains six provinces, each of which contains up to several ecoregions (Table 1.2). Delimiting marine areas by their characteristic ecology and living resources has been applied to coastal waters with the development of the concept of large marine ecosystems (see Chapter 17).
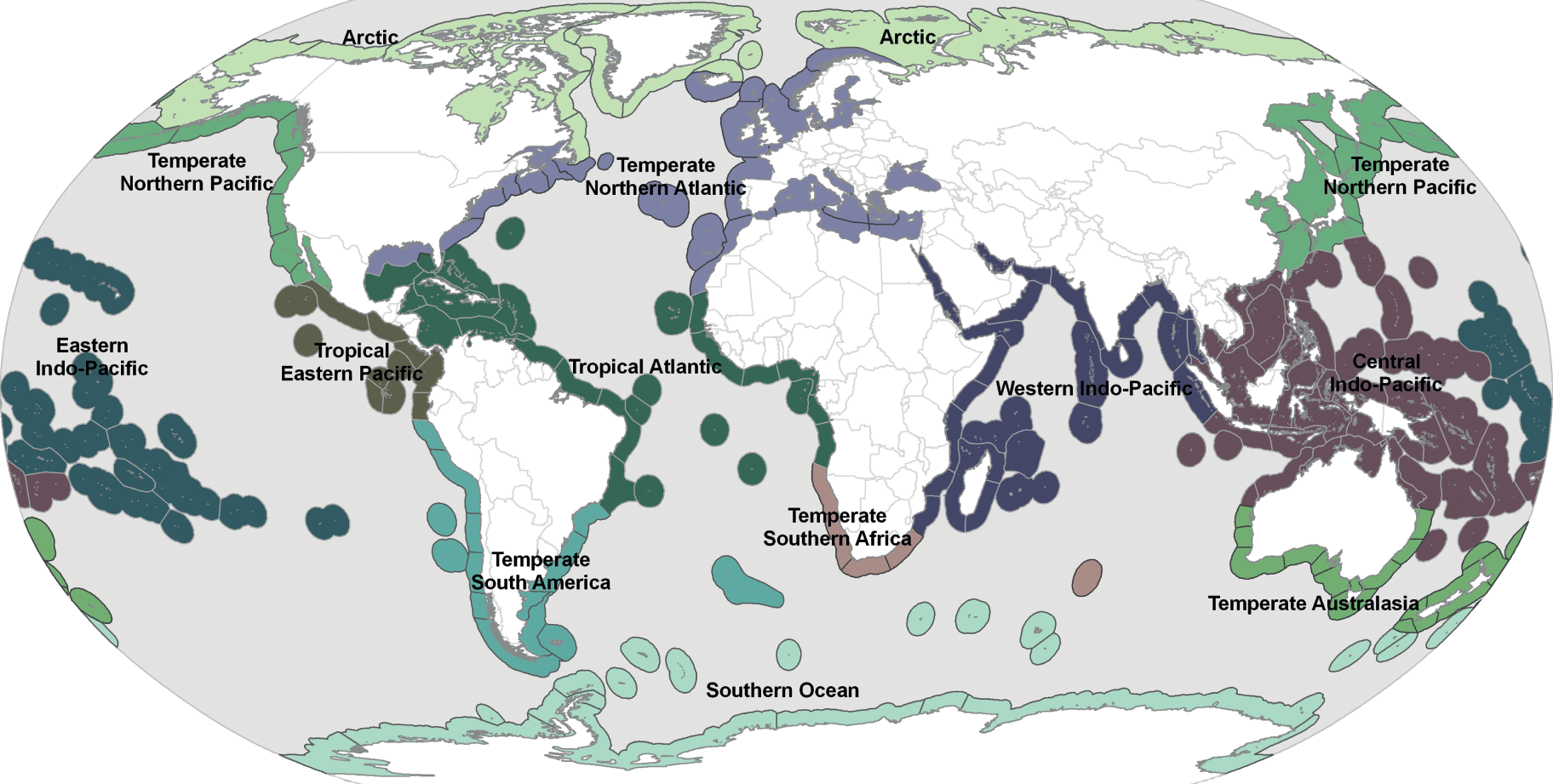
Coastal
Realms – Very large regions of coastal, benthic, or pelagic ocean across which biotas are internally coherent at higher taxonomic levels as a result of a shared and unique evolutionary history. Realms have high levels of endemism, including unique taxa at generic and family levels in some groups. Driving factors behind the development of such unique biotas include water temperature, historical and broadscale isolation, and the proximity of the benthos.
Provinces – Large areas defined by the presence of distinct biotas that have at least some cohesion over evolutionary time frames. Provinces will hold some level of endemism, principally at the level of species. Although historical isolation will play a role, many of these distinct biotas have arisen as a result of distinctive abiotic features that circumscribe their boundaries. These may include geomorphological features (isolated island and shelf systems, semi-enclosed seas); hydrographic features (currents, upwellings, ice dynamics); or geochemical influences (broadest-scale elements of nutrient supply and salinity).
Ecoregions – Areas of relatively homogeneous species composition, clearly distinct from adjacent systems. The species composition is likely to be determined by the predominance of a small number of ecosystems and/or a distinct suite of oceanographic or topographic features. The dominant biogeographic forcing agents defining the ecoregions vary from location to location but may include isolation, upwelling, nutrient inputs, freshwater influx, temperature regimes, ice regimes, exposure, sediments, currents, and bathymetric or coastal complexity.
Off-Shelf
Realms – Very large regions across which biotas are internally coherent at higher taxonomic levels as a result of a shared and unique evolutionary history. High levels of endemism, including unique taxa at generic and family levels in some groups. Distribution of individual species often does not encompass all of a realm, but coherence is often present at generic or family levels.
Provinces – Large areas of epipelagic ocean that can be defined by large-scale, spatially, and temporally stable (or seasonally recurrent) oceanographic drivers. Host distinct species assemblages that share a common history of co-evolution. Oceanographic drivers may include major ocean gyres, equatorial upwellings, upwelling zones at basin edges, semi-enclosed pelagic basins, and large-scale transitional elements. Taxonomic refinement will typically be driven by isolation at the scale of ocean basin and hemisphere.
Biomes – Groupings of provinces with common oceanographic processes (boundary current systems, mid-oceanic gyres, etc.). These may be separated by large physical distances and have very different evolutionary histories. Therefore expected to host ecosystems with comparable structural and functional properties but not the same species.
| Province | Ecoregion |
|---|---|
| Northern European Seas | South and West Iceland |
| Faroe Plateau | |
| Southern Norway | |
| Northern Norway and Finnmark | |
| Baltic Sea | |
| North Sea | |
| Celtic Seas | |
| Lusitanian | South European Atlantic Shelf |
| Saharan Upwelling | |
| Azores Canaries Madeira | |
| Mediterranean Sea | Adriatic Sea |
| Aegean Sea | |
| Levantine Sea | |
| Tunisian Plateau/Gulf of Sidra | |
| Ionian Sea | |
| Western Mediterranean | |
| Alboran Sea | |
| Cold Temperate Northwest Atlantic | Gulf of St. Lawrence – Eastern |
| Scotian Shelf | |
| Southern Grand Banks – South | |
| Newfoundland | |
| Scotian Shelf | |
| Gulf of Maine/Bay of Fundy | |
| Virginian | |
| Warm Temperate Northwest Atlantic | Carolinian |
| Northern Gulf of Mexico | |
| Black Sea | Black Sea |
Spalding et al. (Reference Spalding, Agostini, Rice and Grant2012) present a parallel biogeographic classification of oceanic epipelagic waters and semi-enclosed areas to 200 m water depth, similarly based on the distribution of taxa and major underlying oceanographic drivers, notably water movements (e.g. gyres, currents, and upwellings), nutrients, and temperature (Fig. 1.5, Box 1.1). They describe 37 pelagic provinces, large areas each with a coherent suite of oceanographic factors and distinct species assemblages. And these provinces are nested into four realms (Northern Coldwater, Indo-Pacific Warmwater, Atlantic Warmwater, and Southern Coldwater). The realms are much larger scale regions that are still distinguishable at higher taxonomic levels. The provinces can also be grouped into major biomes – systems with distinct oceanographic processes (polar, gyre, eastern boundary currents, western boundary currents, equatorial, transitional, and semi-enclosed seas). The various biomes support communities that are functionally similar but not necessarily closely related taxonomically given their often wide geographic separation. Biogeographic boundaries in such schemes are rarely sharply defined, nor are they static – there may be considerable spatial variation over various temporal scales.
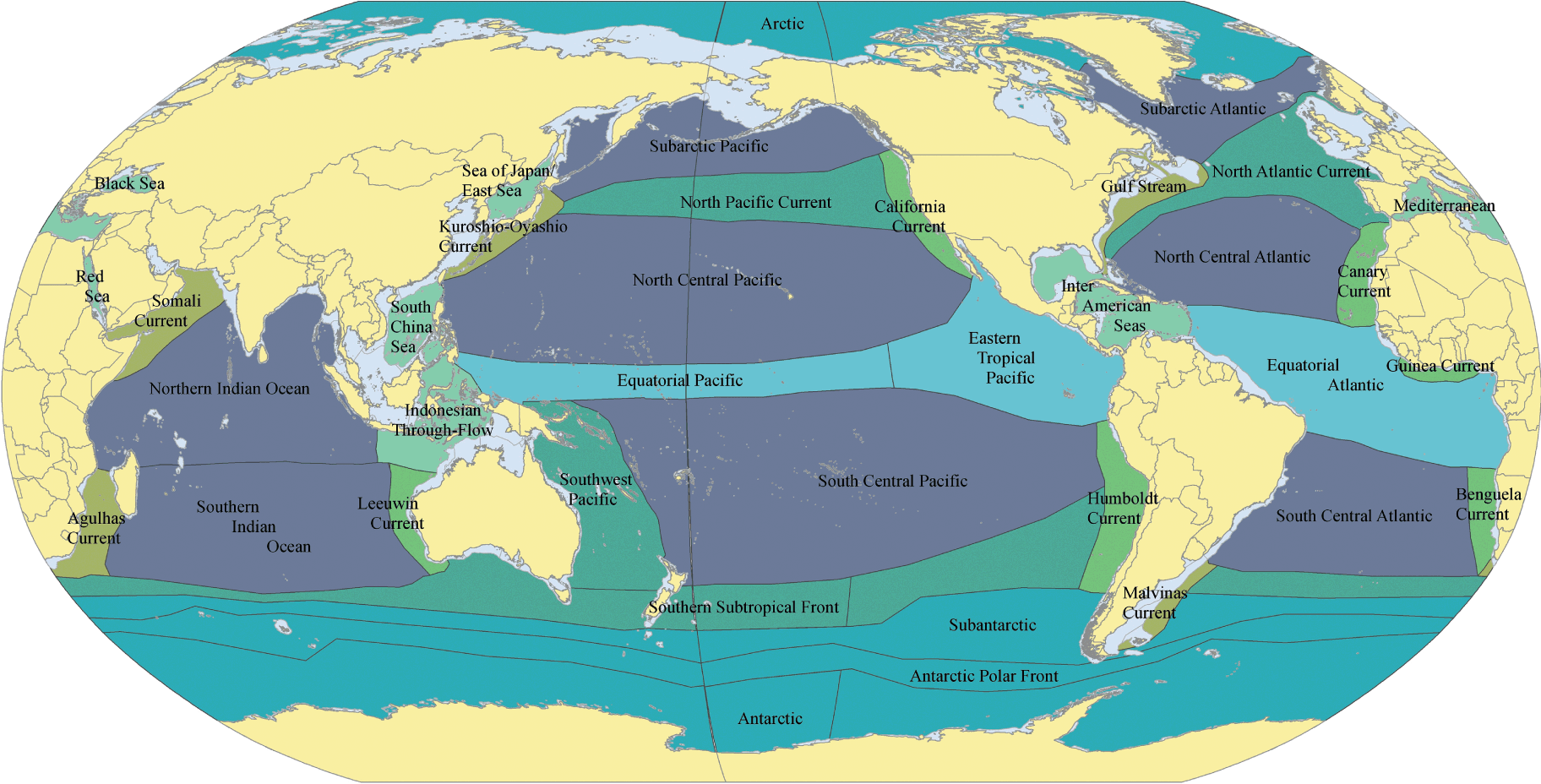
Fig. 1.5 Biogeographic provinces of surface pelagic waters. The colours represent the different biomes, for example: polar (Arctic), gyre (Subarctic Pacific), transitional (North Pacific Current), eastern boundary current (California Current), western boundary current (Kuroshio-Oyashio Current), equatorial (Equatorial Pacific), and semi-enclosed seas (South China Sea).
At the regional level, a number of classifications of marine habitats have been developed (Costello, Reference Costello2009). One of the most comprehensive for coastal benthic habitats is the marine habitat classification for Britain and Ireland, intended particularly for management and conservation (Connor et al., Reference Connor, Allen and Golding2004). It also contributes to the marine component of the European Union Nature Information System (EUNIS) habitat classification developed by the European Environment Agency. Such classifications comprise hierarchies of levels from large-scale divisions based on differences in oceanographic and geomorphic characteristics down to individual communities or biotopes (see Chapter 10).
Marine biogeographic classifications have been developed mainly for near-surface waters. Environmental conditions in the ocean differ far more dramatically vertically than they do horizontally, particularly for such factors as temperature, light, and food supply. In the tropics, the difference in water temperature at the surface and at 1 km depth could be 20°C, whereas at the surface such a temperature difference would occur over thousands of kilometres.
Major bathymetric divisions of the ocean are recognized for the pelagic and benthic realms (Fig. 1.6). The upper water column to a depth of about 200 m, known as the epipelagic zone, includes the zone that receives sufficient light for photosynthesis, the euphotic zone. A water depth of 100–200 m marks the edge of the continental shelf in most parts of the world. Shelf waters are referred to as ‘coastal’ or ‘neritic’. From about 200–1000 m, corresponding to upper continental slope depths, lies the mesopelagic zone, a region where there is still enough light for vision but not for photosynthesis. The regions below are permanently sunless. The bathypelagic zone extends from about 1000–3000 m water depth and the abyssopelagic from 3000 to 6000 m. In terms of the benthic environment, these two deeper zones correspond roughly to lower continental slope and abyssal plain depths. The deepest regions occur in the ocean trenches, the hadal zone, at some 6000–11 000 m (see Chapter 14).
As we have seen, in biogeographic classifications a number of biotic provinces are recognized for the epipelagic zone. Similar biotic distributions are recognizable in the mesopelagic zone; indeed, many species undergo diurnal vertical migration between the two zones. On the other hand, many bathy- and abyssopelagic species appear to have very broad distributions through the Atlantic, Pacific, and Indian Oceans. For bathyal (slope), abyssal, and hadal benthos, there is evidence of identifiable faunas related, for example, to ocean basins and regimes of surface production. Biogeographic provinces for deep-sea benthic systems have been proposed but still need further data on species’ distributions to test them (see Chapter 14).
There is potential for marine species to have larger geographical ranges than species on land. Apart from continental land barriers, the oceans are contiguous and lack the obvious physical boundaries that can occur between ecosystems on land. Although many marine species are sedentary, their planktonic larvae may be relatively long-lived and able to disperse widely. For example, larval durations of about a year, or in extreme examples up to a few years, have been reported for a range of taxa, providing the potential for larvae to be transported thousands of kilometres (Strathmann & Strathmann, Reference Strathmann and Strathmann2007). Even so, currents, fronts, eddies, or other oceanographic features may impede larval dispersal, and a long planktonic stage does not necessarily mean long-distance dispersal and high connectivity between populations. In fact, Weersing and Toonen (Reference Weersing and Toonen2009) found larval duration to be poorly correlated with genetic differentiation across a broad range of marine taxa. In some habitats, species with restricted ranges appear to be common. The Cape Verde archipelago off western Africa is home to 56 species of cone snail, 43 of them each restricted to a single island (Peters et al., Reference Peters, O’Leary, Hawkins, Carpenter and Roberts2013). Even the dispersal of highly mobile pelagic species may be restricted by oceanographic barriers. The harbour porpoise is widely distributed in cold coastal waters of the North Atlantic and North Pacific. In the eastern North Atlantic, there is evidence of strong barriers to gene flow between porpoises from Iberian waters and those to the north. This separation coincides with marked oceanographic changes that for this species are likely to affect food availability (Fontaine et al., Reference Fontaine, Baird and Piry2007). Questions of dispersal and connectivity are important in conservation, for example in considering the function and effectiveness of marine protected areas (see Chapter 15).
Marine Biodiversity
The Convention on Biological Diversity (CBD) defines biological diversity, or biodiversity, as ‘the variability among living organisms from all sources, including, inter alia, terrestrial, marine and other aquatic ecosystems and the ecological complexes of which they are part; this includes diversity within species, between species and ecosystems’ (Article 2). Biological diversity can thus be considered at various levels of organization. In terms of conservation effort, ecological diversity, as outlined above, may be the most effective to shape long-term strategies and goals. By conserving the diversity of healthily functioning marine ecosystems, the maintenance of other levels of diversity will follow. Taxonomic diversity is, however, easier to categorize and quantify, and it is concern over loss of species that often drives biodiversity conservation initiatives. Biodiversity is thus most often discussed at the species level.
The total number of extant species so far described is about 1.5 million (Costello et al., Reference Costello, Wilson and Houlding2012). How many remain undescribed is difficult to assess as many groups are still poorly known. Most of the genetic diversity of the oceans may reside with microbial organisms (Sogin et al., Reference Sogin, Morrison and Huber2006), yet their biodiversity has barely been explored. Conservation has so far concerned itself almost entirely with macroscopic organisms or at least eukaryote species (those with cells that have a distinct nucleus and organelles). Estimates of the number of living eukaryote species vary considerably, but recent analyses indicate a total of about 5 to 8.7 million (Mora et al., Reference Mora, Tittensor, Adl, Simpson and Worm2011; Costello et al., Reference Costello, May and Stork2013). So far, some 0.23 million eukaryotic marine species have been described, but the total number may be of the order of 0.3–1.0 million (Appeltans et al., Reference Appeltans, Ahyong and Anderson2012) to 2.2 million (Mora et al., Reference Mora, Tittensor, Adl, Simpson and Worm2011). There is considerable uncertainty around such figures. Fisher et al. (Reference Fisher, O’Leary and Low-Choy2015), for instance, estimated that coral reefs alone could support 0.8 million multicellular species. A major advance in documenting the biodiversity of the oceans has been the Census of Marine Life, an international research programme from 2000 to 2010 on marine life across the world’s seas and oceans (www.coml.org). It set up the Ocean Biogeographic Information System (www.iobis.org), which publishes millions of locations for more than 100 000 marine species online.
The above estimates indicate that up to about 20% of extant eukaryote species are marine. But a very different pattern of biodiversity emerges at higher taxonomic levels. Of the 33 or so animal phyla, 28 (85%) occur in marine habitats, and about half of these are exclusively marine, such as the ctenophores (comb jellies), brachiopods (lamp shells), and echinoderms (sea stars, sea urchins, and allies) (Box 1.2). By contrast, 16 animal phyla occur in the terrestrial-freshwater realm, only one of which is exclusive (Ray & Grassle, Reference Ray and Grassle1991). Animal diversity on land is overwhelmingly dominated by a few major taxa, in particular insects. Marine species, on the other hand, are not only spread among considerably more phyla, they are also far more equitably distributed across those phyla. Several reasons have been proposed to explain these contrasts in biodiversity between land and sea. The basic differentiation of animal phyla occurred in the seas of the Late Precambrian, Cambrian, and Ordovician before the invasion of freshwater and land in the Silurian and Devonian, and seemingly many marine groups never colonized freshwater or land for physiological or anatomical reasons. The far greater species richness on land is largely attributable to the huge diversification of insects and flowering plants.
Some group names (e.g. protists, macroalgae) are used for convenience rather than as accepted taxa.
Seaweeds (macroalgae): brown algae (Phaeophyceae), green algae (Chlorophyta), red algae (Rhodophyta)
Tracheophyta (vascular plants): mangroves, seagrasses, saltmarsh plants
Protists (unicellular eukaryotes): ciliates, foraminiferans, coccolithophores, diatoms, dinoflagellates
Porifera: calcareous sponges (Calcarea), horny sponges (Demospongiae), glass sponges (Hexactinellida)
Ctenophora: comb jellies
Cnidaria: sea anemones (Actiniaria), black corals (Antipatharia), stony corals (Scleractinia), gorgonians, sea pens (Octocorallia), hydroids, stylasterid corals, siphonophores (Hydrozoa), jellyfish (Scyphozoa)
Platyhelminthes: flatworms
Nemertea: ribbon worms
Polychaeta: bristleworms
Mollusca: chitons (Polyplacophora), bivalves, clams (Bivalvia), snails (Gastropoda), octopuses, squids (Cephalopoda)
Brachiopoda: lamp shells
Bryozoa: moss animals
Chaetognatha: arrow worms
Nematoda: round worms
Chelicerata: sea spiders (Pycnogonida), mites (Acari)
Crustacea: barnacles (Cirripedia), copepods (Copepoda), ostracods (Ostracoda), mantis shrimps (Stomatopoda), amphipods, isopods, mysids, tanaidaceans (Peracarida), krill (Euphausiacea), caridean shrimps, prawns, crabs, lobsters (Decapoda)
Echinodermata: sea stars (Asteroidea), sea urchins (Echinoidea), brittlestars (Ophiuroidea), sea lilies, feather stars (Crinoidea), sea cucumbers (Holothuroidea)
Tunicata: ascidians (sea squirts), salps
Chondrichthyes (cartilaginous fishes): sharks, rays, chimaeras (see Chapter 6)
Actinopteri: sturgeons and teleosts (bony fishes) (see Chapter 6)
Reptilia: marine turtles, sea snakes (see Chapter 7)
Aves: seabirds (see Chapter 8), waders (see Chapter 11)
Mammalia: otters, pinnipeds (sea lions, seals) (Carnivora), dugong, manatees (Sirenia), whales, dolphins, porpoises (Cetacea) (see Chapter 9)
Much of the primary production, herbivory, and predation in the sea involves smaller organisms than on land, and it has been suggested that globally there are fewer species in smaller size classes because such organisms typically have wider geographical distributions (May, Reference May1994). It is argued that free-living microbial organisms are typically ubiquitous, being so abundant, unrestricted in their dispersal, and with low extinction probabilities, such that only larger organisms have biogeographies as such (Finlay, Reference Finlay2002). However, whilst marine micro-eukaryote species may be globally distributed, they may also be genetically very diverse (Šlapeta et al., Reference Šlapeta, López-García and Moreira2006). Small phytoplankton cannot provide physical support for metazoans comparable to the role played by land plants, which may also limit marine biodiversity. Nevertheless, as the smallest size classes of planktonic organisms become better known, it is likely that a far higher microbial diversity than is currently known will be revealed.
The diversity of photosynthetic species in the sea also appears to be low compared to that on land. Of the vascular plants alone, about 308 000 living species are known, the vast majority (about 95%) being flowering plants (Christenhusz & Byng, Reference Christenhusz and Byng2016). No more than a few hundred of these can be considered truly marine, although they play key roles in coastal wetlands (see Chapter 12). There are an estimated 5000 species of marine phytoplankton (Tett & Barton, Reference Tett and Barton1995) and about 9300 species of seaweeds (www.algaebase.org). In addition, benthic microalgae can be important (or the major) primary producers in sheltered coastal habitats, such as on estuarine sediments, though their biodiversity is still poorly known.
It is convenient to recognize two major categories of marine organisms depending on their predominant adult lifestyle: pelagic organisms that inhabit the water column and benthic organisms that inhabit the sea floor. The distinction is not always clear-cut for species that live close to the sea floor, and many species have both pelagic and benthic phases in their life cycle. Even so, the great majority of marine species are predominantly benthic as adults, the sea floor being a more multifaceted environment than the overlying water mass. It is estimated that more than 90% of known marine animal species are benthic rather than pelagic (May, Reference May1994).
Genetic diversity – the ‘diversity within species’ in the CBD definition – concerns the frequency and diversity of genes and/or genomes. Gene flow might be expected to be high in the marine environment, given the relative absence of barriers and high dispersal capability of many species. For example, although total genetic diversity of marine and freshwater fishes is similar, genetic differentiation of subpopulations is significantly lower in marine fishes. This implies that, in general, more migrations occur among subpopulations of marine fish than among those of freshwater fish (Ward et al., Reference Ward, Woodwark and Skibinski1994). Various mechanisms can, however, lead to genetic differences accumulating in high-dispersal marine species (Palumbi, Reference Palumbi1994), and molecular techniques are now revealing hitherto unsuspected levels of intraspecific genetic variability in marine organisms (Bucklin et al., Reference Bucklin, Steinke and Blanco-Bercial2011).
Many taxa that in the past have been classified as single species are now known – thanks largely to DNA sequencing – to comprise two or more cryptic species: species that are more or less impossible to distinguish morphologically yet are genetically distinct. For instance, mitochondrial and nuclear DNA markers show that Ciona intestinalis, regarded as a common shallow-water ascidian of temperate to boreal regions, in fact comprises two species with largely disjoint distributions, one occurring in the Mediterranean Sea, neighbouring NE Atlantic, and Pacific and the other in the North Atlantic. Crossing experiments show the two species to be reproductively isolated (Caputi et al., Reference Caputi, Andreakis and Mastrototaro2007). Cryptic marine species are proving far more common than previously realized and may number some tens of thousands (Appeltans et al., Reference Appeltans, Ahyong and Anderson2012).
The recognition of cryptic species can have important implications for environmental assessment and conservation. Considered the most abundant and wide-ranging coral of the tropical western Atlantic, Orbicella annularis has been much used in studies of environmental degradation and global climate change. However, the taxon comprises at least three species, two of which show significant differences in growth rate and oxygen isotopic ratios, parameters that are used to assess past climatic conditions (Knowlton et al., Reference Knowlton, Weil, Weigt and Guzman1992). Similarly, morphologically similar capitellid polychaetes, originally believed to be one species, Capitella capitata, and considered a key indicator of organically polluted sediments, in fact comprise a complex of species that display wide differences in life-history features, including both benthic and planktonic larvae (Méndez et al., Reference Méndez, Linke-Gamenick and Forbes2000). Cryptic species may raise significant conservation issues. A threatened species could turn out to be more than one cryptic species, each even more vulnerable and requiring different conservation measures (Bickford et al., Reference Bickford, Lohman and Sodhi2007).
Global Patterns of Biodiversity
Biotas can differ markedly in their species richness, with some habitats and geographic regions being far more diverse than others. Being able to characterize patterns of biodiversity and understand the structuring factors is important for informing management and conservation. A range of factors might be expected to influence large-scale patterns of species richness, such as tectonic and evolutionary history, habitat area and availability, temperature, primary productivity, oxygen concentration, and environmental stability. In terms of global patterns, the most obvious trend is latitudinal, where species richness tends to be highest in equatorial regions and decline with increasing latitude north and south, a pattern very evident in the terrestrial biosphere. A similar pattern related to water temperature is seen for the marine biosphere, with species richness highest at low latitudes, at least to water depths of around 2000 m, and with peaks in the tropical Indo-west Pacific and western Atlantic regions. But for water depths greater than 2000 m, maximum richness is seen in temperate latitudes (30–50°) and regions near continental margins, corresponding to areas of high flux of particulate organic carbon (Tittensor et al., Reference Tittensor, Mora and Jetz2010; Woolley et al., Reference Woolley, Tittensor and Dunstan2016).
Diversity and availability of habitat strongly influence benthic species richness. Certain tropical habitats, such as coral reef and mangrove systems, provide for a particularly high degree of structural complexity and support a correspondingly high diversity of benthic epifauna. Witman et al. (Reference Witman, Etter and Smith2004) found the species richness of benthic epifauna of shallow (10–15 m) rock-wall habitats to peak around equatorial regions and fall away towards higher latitudes in both hemispheres. Sediment infauna, on the other hand, shows only a weak latitudinal gradient in species richness (Hillebrand, Reference Hillebrand2004). Some groups of organisms are obvious exceptions. For seaweeds, the regions with highest genus richness are in temperate latitudes, probably because of availability of large areas of suitable habitat and the role of major ocean currents (Kerswell, Reference Kerswell2006). Importantly for conservation, hotspots of species richness are often regions with medium or high human impacts (Halpern et al., Reference Halpern, Walbridge and Selkoe2008; Tittensor et al., Reference Tittensor, Mora and Jetz2010).
Variability in Marine Systems
Variability is a natural characteristic of ecological systems and occurs at a wide range of spatial and temporal scales. Population variability may depend, for example, on the responses of organisms to environmental factors, biological interactions such as predation, and a species’ life-history characteristics. At one extreme are large-scale movements and migrations of animals leading to seasonal and longer-term changes in patterns of abundance; at the other extreme are gradual shifts in the geographical distribution of species in response to changing ocean temperature patterns. Physical factors are particularly important in determining the distribution of planktonic organisms, from large-scale distributional patterns due to transport by currents to smaller-scale patchiness generated by entrainment in eddies. Organisms with short generation times, such as many planktonic species, may be able to respond rapidly to take advantage of favourable environmental conditions.
A primary environmental factor influencing benthic populations is the nature of the seabed. Even apparently subtle changes in the physico-chemical and biotic characteristics of the substratum can result in considerable differences in the distribution and abundance of benthic populations. Again this occurs across a range of scales. Many benthic invertebrates, particularly those of the more physically variable coastal and estuarine habitats, respond rapidly to environmental factors and display wide fluctuations in abundance. Benthic environments are affected by a wide variety of natural disturbances at a range of scales (Fig. 1.7). Large areas, often extending to many square kilometres, can be affected by storms, unusually low winter temperatures, salinity reductions, and oxygen depletion. Typically at the other end of the scale (at m2 or cm2) are biological disturbances, such as excavations produced by bottom-feeding fish and marine mammals and sediment reworking by infauna (Kaiser et al., Reference Kaiser, Hall, Thomas, Robinson and Brink2005).
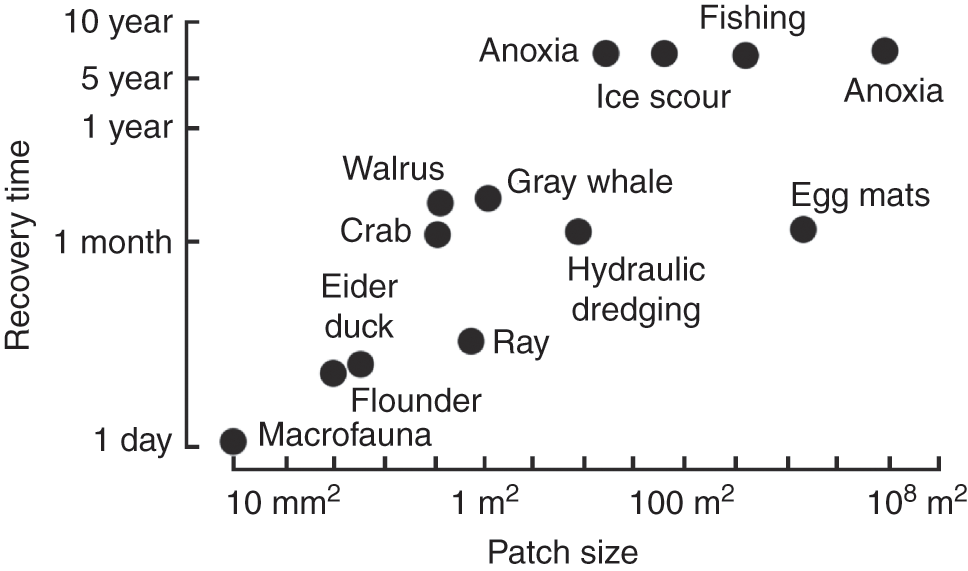
Fig. 1.7 Relationship between different scales of disturbance, both natural and anthropogenic, and their approximate recovery time.
Natural disturbances play a vital role in structuring marine communities. By creating patches, disturbances provide opportunities for other species. Rather than being homogeneous assemblages, communities are more akin to mosaics, with individual pieces at different stages of recovery after the last perturbation. The frequency, extent, and intensity of disturbances within a particular habitat are key factors affecting the composition, abundance, and diversity of its biota. Thus the benthic environment of an estuary has a regime of natural disturbance and recovery very different from that occurring in the deep sea.
Knowledge of the natural variability of wild populations is needed to assess impacts of human activities and make informed decisions about management and conservation. Natural disturbances operate over a wide range of spatial scales, and superimposed on this background may be various human-induced disturbances also operating at a range of scales (Kaiser et al., Reference Kaiser, Hall, Thomas, Robinson and Brink2005). The problem is distinguishing between the effects of human activities and natural background fluctuations in space and time, and it may not be feasible to establish an unequivocal link between cause and effect for many human activities. Some events, like disease outbreaks, harmful algal blooms, or unusually harsh winter temperatures, can remove more than 90% of a population. Such mass mortalities may often be natural phenomena but may also result from natural and anthropogenic factors occurring in concert (Fey et al., Reference Fey, Siepielski and Nusslé2015).
North Atlantic Oscillation
Temporal variability inherent in atmosphere-ocean interactions has major implications for marine ecosystems. Changes can occur at the decade-to-secular scale, such as the North Atlantic Oscillation (NAO), a periodic shift in the relative gradient between the subpolar low-pressure and subtropical high-pressure regions that drives winter westerly winds across the North Atlantic (Longhurst, Reference Longhurst2007). The NE Atlantic experiences milder winter conditions during positive NAO phases, when westerlies are stronger, and harsher conditions when the gradient weakens, allowing the inflow of cold Siberian air masses to intensify (Fig. 1.8).
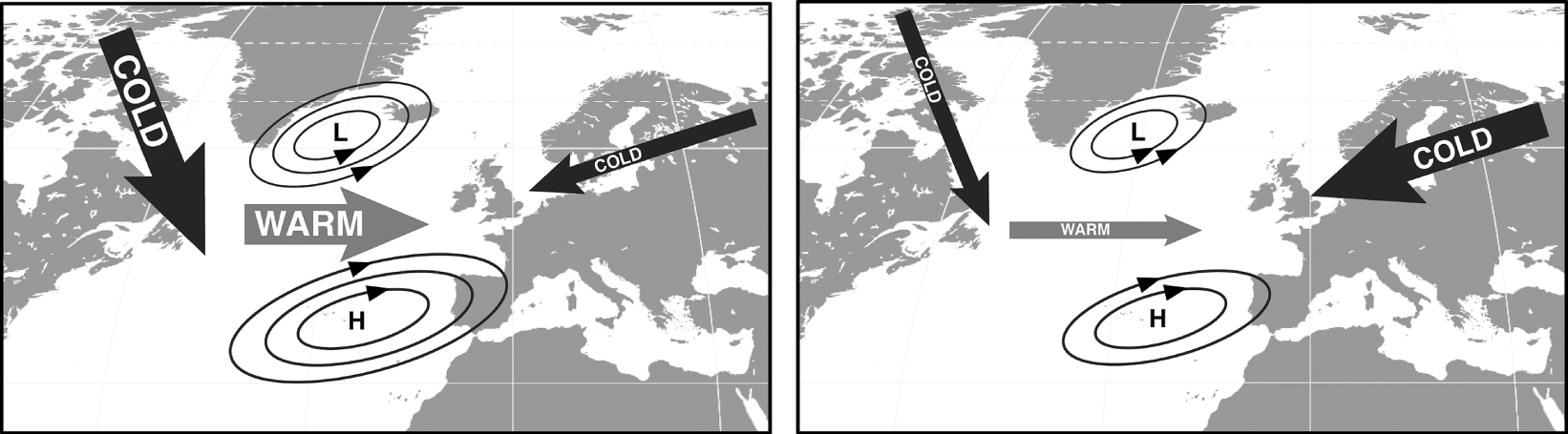
Fig. 1.8 The atmospheric circulation over the North Atlantic indicating the positive and negative modes of the North Atlantic Oscillation (NAO). The modes relate to the relative difference in air pressure between the subpolar low (L) and the subtropical high (H) and cold and warm air masses. Positive mode (left), with a large pressure difference, strong westerlies, reduced inflow of Siberian air masses, and a mild European winter. Negative mode (right), with a small pressure difference, a weaker band of westerlies, a stronger inflow of Siberian air masses, and a severe European winter.
Changes in the NAO have been linked to major changes in marine ecosystems of the North Atlantic, including shifts in the abundance of different plankton species and impacts on fish productivity (Parsons & Lear, Reference Parsons and Lear2001). A striking example is the correspondence between the prevailing wind direction (NAO index) and alternating periods of herring and sardine abundance in European waters. Herring, a predominantly arctic-boreal species, is favoured during periods of reduced westerly winds (i.e. negative NAO index), whereas the warmer water sardine or pilchard is associated with periods of intensified westerlies (positive NAO index) (Alheit & Hagen, Reference Alheit and Hagen1997; Coombs et al., Reference Coombs, Halliday, Conway and Smyth2010).
Shifts in distribution are likely to be most pronounced in populations near the edge of a species’ geographic range, in this case where the distributions of the two fish species overlap but where shifts have also been intensified by overfishing and subsequent recruitment failure (Southward et al., Reference Southward, Boalch and Maddock1988). Such data derived from sampling in the western English Channel date back more than a century and illustrate the importance of long time-series observations for our understanding of the dynamics and functioning of marine ecosystems and in forecasting effects of global climate change (Harris, Reference Harris2010).
The NAO is occasionally punctuated by marked ocean climate anomalies. Two exceptional periods reported for the North Sea in the late 1970s and the late 1980s were associated with unusual oceanic incursions of different origin. The 1970s anomaly was associated with the influx of cold, low salinity water into the North Sea and reduced inflow of warm Atlantic water, significantly decreasing the flux of nutrients from oceanic sources. This coincided with sudden changes in the abundance of macrobenthos, fish, and birds in the southern North Sea and the appearance of more cold-water species. By contrast, in the late 1980s a high NAO index and increased flow of relatively warm Atlantic water into the North Sea was accompanied by exceptionally high phytoplankton biomass, an unprecedented influx of oceanic species, and a sudden increase in macrobenthic biomass in the southern North Sea (Edwards et al., Reference Edwards, Beaugrand, Reid, Rowden and Jones2002).
Inter-decadal variability is also well documented for the monsoon zone. Off the south-west coast of India there are long-term shifts in the strength of the monsoon, upwelling intensity, diatom stocks, and landings of oil sardine (Longhurst, Reference Longhurst2007). A dozen or so low-frequency ocean-atmosphere interactions are in fact recognized from around the world, and there are probably connections between them.
El Niño-Southern Oscillation
Oceanographic variability also occurs over shorter timescales, with periodicities ranging from a few years to seasonal cycles. Particularly striking are El Niño events, the appearance from time to time of warm surface water in the eastern equatorial Pacific, notably off Ecuador and Peru. Such anomalous warmings are, however, part of a larger-scale perturbation of an ocean-atmosphere interaction centred on the tropical Pacific Ocean and known as the El Niño-Southern Oscillation (ENSO), which typically has a return interval of 2–7 years (Glantz, Reference Glantz2001; Longhurst, Reference Longhurst2007). Normally the difference between the low atmospheric pressure over Indonesia and the high in the south-eastern Pacific maintains the South-East Trades. These persistent winds mean that a thick layer of warm surface water piles up in the western equatorial Pacific, whereas in the east the thermocline is shallow, allowing cold nutrient-rich water to upwell along the coast. Every few years, however, there is a change: differences in atmospheric pressure across the Pacific diminish, the trade winds weaken, and the warm water pooled in the west surges across into the eastern Pacific. The deepening of the thermocline off Ecuador and Peru during an El Niño means that the deeper, nutrient-rich water is less available for upwelling (Fig. 1.9). Upwelling systems are noted for their high biological productivity, and the sudden decline of productivity off South America during an El Niño has dramatic impacts on the food chain, notably on the abundance of the Peruvian anchovy, seabirds, and marine mammals.
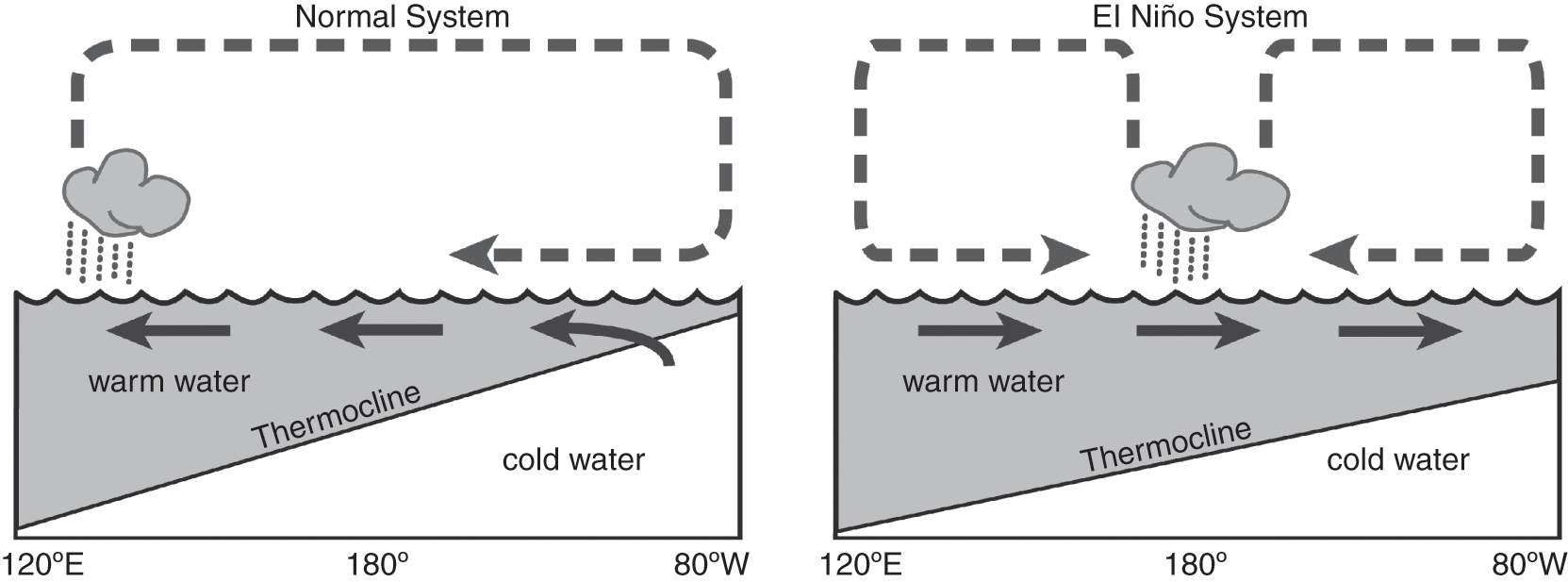
Fig. 1.9 The development of El Niño. Under normal conditions, surface water in the equatorial Pacific Ocean is pushed westward by strong trade winds, and with a shallow thermocline off the coast of Peru, upwelling occurs that supports high productivity. El Niño conditions develop when the trade winds weaken and warm surface water flows eastward, deepening the thermocline and inhibiting the Peruvian upwelling.
Depending on their intensity, ENSO events can have wide-ranging implications for marine ecosystems given their potential impact on sea surface temperatures, salinity and sea state, nutrient availability and productivity, algal blooms, and changes in the relative abundances of competing species (Glynn, Reference Glynn1988). The number of green turtles nesting at rookeries in the western Pacific has been correlated with an index of the Southern Oscillation, probably via a nutritional pathway, with turtle numbers peaking after El Niño events (Poloczanska et al., Reference Poloczanska, Limpus and Hays2009). There is a lag in the relationship such that it may be possible to forecast the size of a nesting population up to 2 years in advance, which may be of considerable value to marine turtle management. El Niño activity in the equatorial Pacific is probably linked to climate anomalies at higher latitudes, such as the eastward propagation of sea surface temperature anomalies around the Southern Ocean (White & Peterson, Reference White and Peterson1996). These have a periodicity of 4–5 years, taking 8–10 years to encircle the pole, and link with inter-annual variability in sea-ice cover, a factor of critical importance to populations of Antarctic krill and their dependent predators in the Southern Ocean (see Chapter 17).
The ENSO event of 1997–8, one of the strongest on record, was responsible for major disturbances to marine ecosystems, both locally and globally, including widespread bleaching (loss of symbiotic algae) and mortality of corals (see Chapter 13). Severe ENSO events are probably among the greatest natural perturbations known on our planet in terms of areas affected and biological consequences.
At a smaller scale are seasonal cycles, and here too we may witness occasional anomalies. An exceptional heat wave in Europe in summer 2003 resulted in unusually high sea surface temperatures. In the NW Mediterranean region, Garrabou et al. (Reference Garrabou, Coma and Bensoussan2009) reported the mass mortality of at least 25 benthic species (mainly sponges and gorgonian corals) inhabiting shallow-water (up to 40 m water depth) rocky areas. Such events are likely to occur more often given the climate warming projections, and indeed there is evidence of this for the Mediterranean Sea as a whole (Rivetti et al., Reference Rivetti, Fraschetti, Lionello, Zambianchi and Boero2014). Natural catastrophes can also occur over timescales of hours, such as tropical cyclones that cause localized devastation of coral reefs (see Chapter 13).
Spatial and Temporal Scales
Broadly speaking, because of differences in density and viscosity, physical processes of the ocean and atmosphere operate over different spatial and temporal scales. At short timescales, the ocean is less variable than the atmosphere, and for the pelagic environment, physical and biological processes can be closely coupled (Fig. 1.10). Natural large-scale changes in open sea systems typically occur over periods of years to decades, and pelagic populations have evolved reproductive strategies that can respond accordingly. As a result, marine systems can naturally undergo major shifts in species composition. By contrast, terrestrial systems can operate over much longer timescales, of the order of centuries in the case of forest growth, and land populations have adapted to cope with atmospheric variability as short-term noise. Marine systems may thus be able to respond more readily than terrestrial systems to global environmental changes imposed by human activity since these are occurring rapidly, at timescales comparable to those of natural large shifts in open sea systems (Steele, Reference Steele1991).
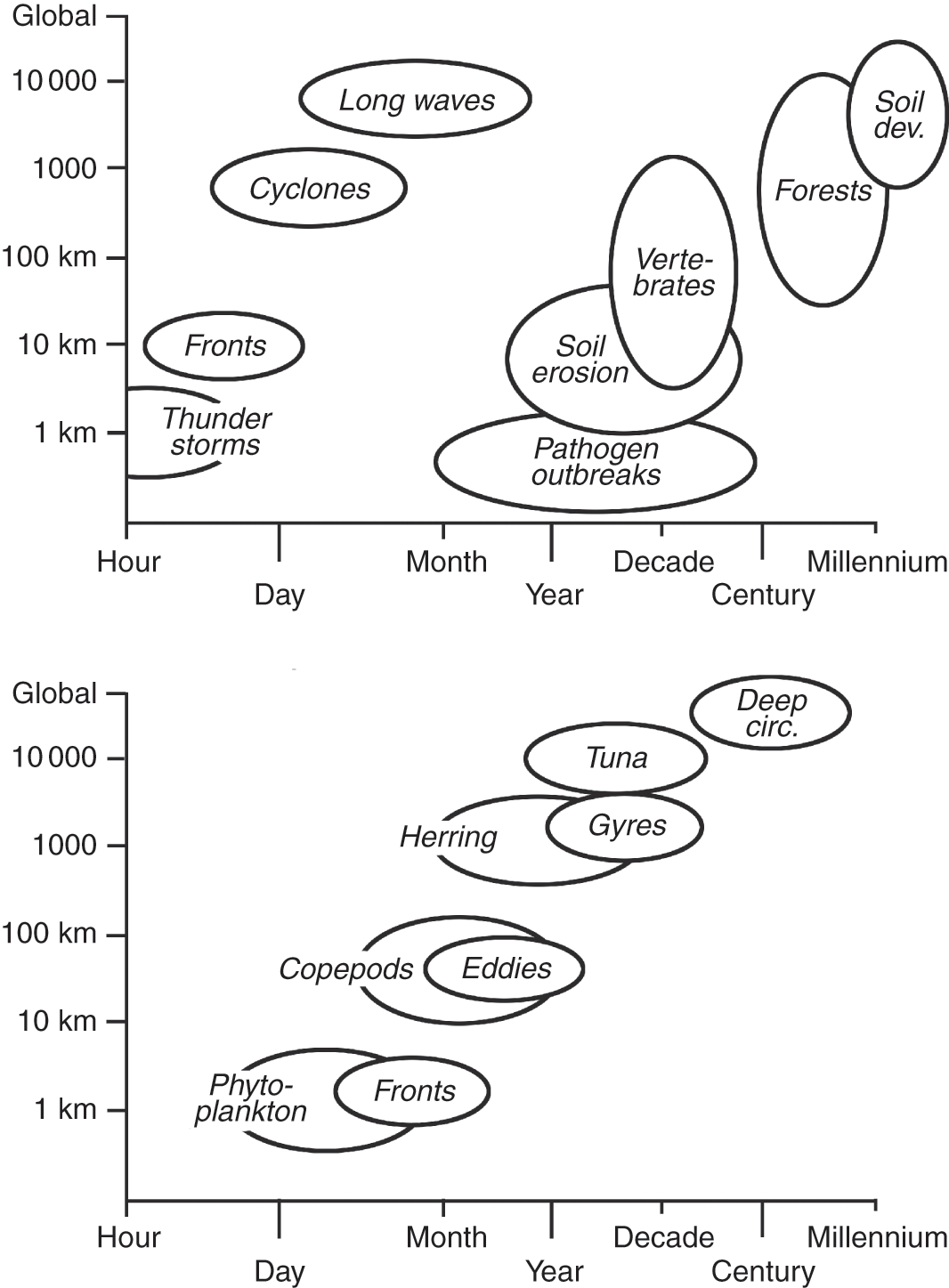
Fig. 1.10 Relationship between spatial and temporal scales for atmospheric processes and terrestrial groups showing their marked separation in time and for oceanographic processes and pelagic groups showing their marked coupling.
Reproductive Strategies
Many marine species have reproductive strategies characterized by a highly variable rate of recruitment of larvae or juveniles to adulthood. A typical strategy is to ensure that the maximum number of planktonic larvae are committed to the water column, a corollary of which is that parental investment towards the survival of individual offspring is correspondingly minimized. By contrast, many land organisms produce relatively small numbers of young to which they need to devote a degree of care before they can afford to relinquish them. There are marine species that produce small numbers of young that are brooded or otherwise protected for an extended period and others whose life-history strategy lies between these extremes of the spectrum. But many marine species produce huge numbers of eggs, commonly millions in the case of bony fish that produce pelagic eggs. A cost of this tactic is extremely high egg and larval mortality with only a few percent surviving through the juvenile period (Palumbi & Hedgecock, Reference Palumbi, Hedgecock, Norse and Crowder2005). The loss between the time of reproduction and recruitment of the new cohort to the population may be due to various physico-chemical and biotic factors, the combined effects of which are usually of far greater significance than the variability contributed by the size of the parent population. For most marine species, recruitment variability results directly from variation in mortality during early life-history stages and, within limits, is often seemingly independent of the adult population size, a point of particular importance in fisheries management (see Chapter 5). Populations of marine species are frequently, though unpredictably, dominated by a single year class when favourable circumstances lead to a recruitment peak. On occasions, larval survival may be exceptionally high and result in a population outbreak, such as those of the crown-of-thorns starfish on coral reefs (see Chapter 13). The fact that many marine species have small, pelagic dispersal stages that show high spatial and temporal variability adds to the difficulty of understanding population dynamics and forecasting recruitment events, which has important implications for marine conservation. Life-history characteristics have a major bearing on the vulnerability of species to overexploitation (see Chapter 4).
A Sea Ethic
In this chapter we have looked at major features of the marine environment and its biological diversity. These have various implications for the unique challenges of marine conservation. In that respect we also need to consider how these aspects intersect societal attitudes. In general, public regard for the marine environment is often poorly developed, and a ‘sea ethic’ that recognizes intrinsic values beyond the purely utilitarian has yet to gain wide acceptance (Dallmeyer, Reference Dallmeyer, Norse and Crowder2005). There are various reasons for this. That most of the biosphere is unseen and undiscovered has not fostered an environmental awareness of the marine realm; in fact, often the opposite, as our impacts resulting from exploitation and waste disposal have to a degree been hidden or not even considered given the obvious immensity of the oceans. Also, few marine species engage public concern compared to the many, often emblematic animals and plants on land. In the sea, virtually all organisms are seen as exploitable. There has existed, at least in Western societies, a widespread view that those who exploit marine resources should not be hampered by notions of boundary and property that we take for granted on land, a view that has exacerbated human impacts on the sea and its resources – as we shall see in subsequent chapters.















