During the Late Permian the semi-enclosed Zechstein Sea was connected to open ocean through the modern territory of Norway and Greenland – a seaway in the northern Pangea (Maystrenko et al. Reference Maystrenko, Bayer, Brink, Littke, Littke, Bayer, Gajewski and Nelskamp2008) (Fig. 1a). The palaeogeographical distribution of this epicontinental sea extended from the eastern part of England and Greenland through the northern part of Denmark, Netherlands and Germany into Poland up to the western part of Lithuania and the southern part of Latvia (Van Wees et al. Reference Van Wees, Stephenson, Ziegler, Bayer, McCann, Dadlez, Gaupp, Narkiewicz, Bitzer and Scheck2000; Weissflog et al. Reference Weissflog, Elansky, Kotte, Keppler, Pfennigsdorff, Lange, Putz and Lisitsyna2008; Raczyński & Biernacka Reference Raczyński and Biernacka2014) (Fig. 1a). The Zechstein Sea reached its widest area during the first carbonate–evaporite cycle (Werra transgression) (Raczyński & Biernacka Reference Raczyński and Biernacka2014). The sea water rapidly flooded a depression and due to prevalence of the arid climate, in total seven carbonate–evaporite cycles were deposited there (Peryt et al. Reference Peryt, Geluk, Mathiesen, Paul, Smith, Doornenbal, Stevenson, Doornenbal and Stevenson2010).
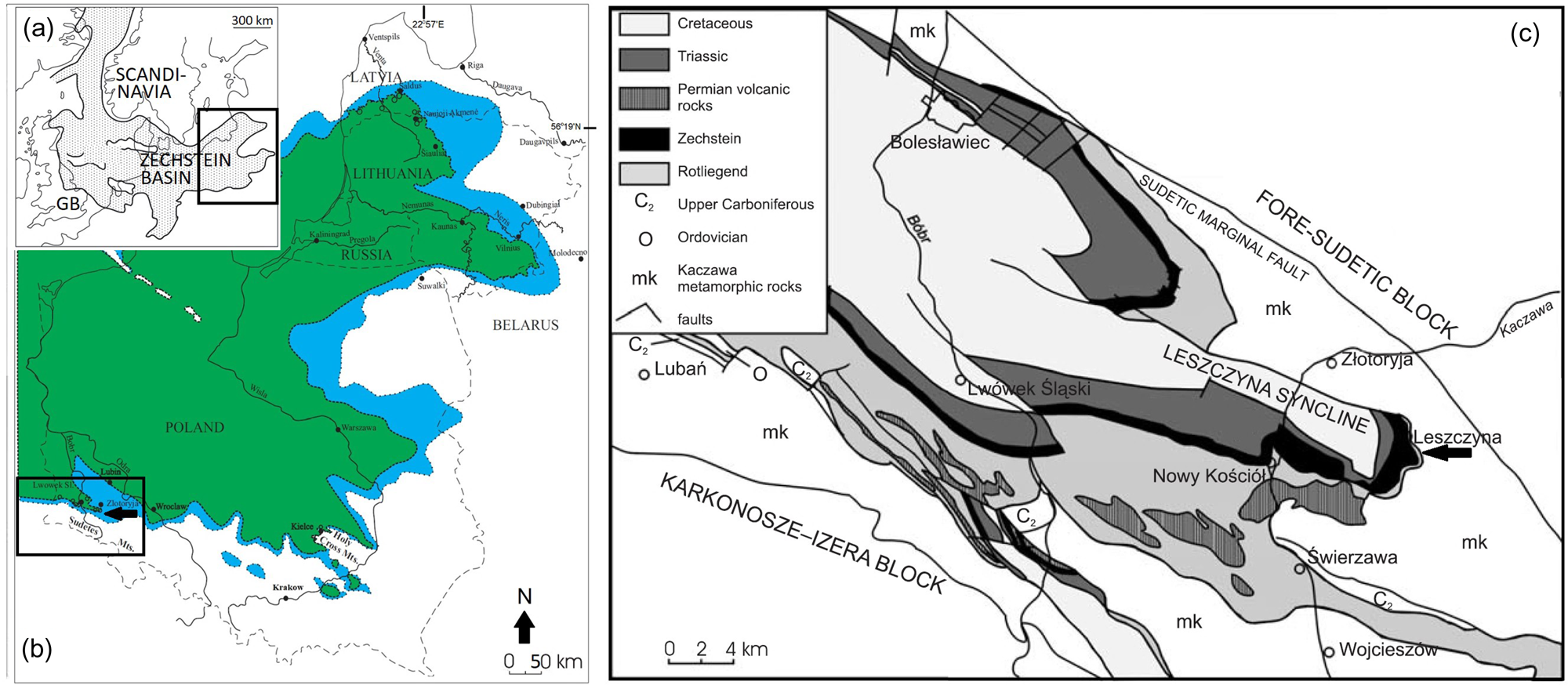
Figure 1 (a) Palaeogeography of the European Zechstein Basin (Becker & Bechstaedt Reference Becker and Bechstaedt2006). (b) The map of Eastern Europe showing Leszczyna locality (black arrow). A blue colour represents the original distribution of Zechstein sediments and green is current distribution of Zechstein sediments in Poland, Russia, Lithuania and Latvia (Raczyński & Biernacka Reference Raczyński and Biernacka2014). (c) The geological map of North-Sudetic Basin showing location of the studied site (black arrow) (after Biernacka et al. Reference Biernacka, Borysiuk and Raczyński2005).
Osteichthyes and Chondrichthyes were the most common vertebrates in the late Palaeozoic marine communities (Vázquez & Clapham Reference Vázquez and Clapham2017). Late Permian fossils of fishes are widely known from marine and freshwater ecosystems of Pangea (Koot Reference Koot2013; Romano et al. Reference Romano, Koot, Kogan, Brayard, Minikh, Brinkmann, Bucher and Kriwet2016) including the Zechstein Sea (King Reference King1850; Nielsen Reference Nielsen1952; Diedrich Reference Diedrich2009; Dankina et al. Reference Dankina, Chahud, Radzevičius and Spiridonov2017, Reference Dankina, Spiridonov, Raczyński and Radzevičius2021a, Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičiusb).
In this study, we report new chondrichthyans and osteichthyans remains (teeth, dermal denticles and scales) collected from the south-eastern part of the Zechstein Basin in Leszczyna quarry, South-West Poland (Fig. 1b, Table 1). These ichthyofaunal remains are the first record of vertebrates here. However, these fossils are not attributed to any fish genus or species with an exception for two specimens, which were taxonomically identified as ?Listracanthus sp. dermal denticle and ?Omanoselache sp. tooth. According to this reason, we divided the new material into several morphotypes based on their morphological characteristics. The same division of the ichthyofaunal remains referred to the previous works based on the Late Permian fish material from the Nowy Kościół in South-West Poland (Dankina et al. Reference Dankina, Spiridonov, Raczyński and Radzevičius2021a), Karpėnai quarry in Lithuania (Dankina et al. Reference Dankina, Chahud, Radzevičius and Spiridonov2017) and Kūmas localities in Latvia (Dankina et al. Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b). Here, we continue using the numeration of morphotypes (M1–M8) which was determined and described in the works mentioned above (Table 2). Thus, a detailed studied fish assemblage increases its distribution and diversity, partly differing from the previously found assemblage in Nowy Kościół locality which was farther offshore of the Zechstein Basin. The quantitative data of found fish fossils in these two localities (Leszczyna and Nowy Kościół quarries) do not show a significant difference. Nevertheless, according to the palaeogeographical location and palaeoenvironmental conditions of studied deposits, the new fish remains reported here add important information especially for the understanding of the relationship between Late Permian sharks’ finds from the north-eastern and south-eastern offshore of Zechstein Basin. Additionally, we tried to find a connection between the ecomorphology of Late Permian ray-finned fish teeth and the relation to their diets. Most actinopterygian teeth can be classified as belonging to small predators of soft bodied prey, which fed on small animals (both invertebrates and vertebrates, for example, fish larvae, conodonts, etc.). Rare finds of some actinopterygian teeth may belong to a specialised durophagous forms, which fed on shells and molluscs (Esin Reference Esin1997; Purnell & Darras Reference Purnell and Darras2015).
Table 1 A quantitative (vertical) distribution of fish remains through the studied samples collected from the Leszczyna quarry in South-West Poland.
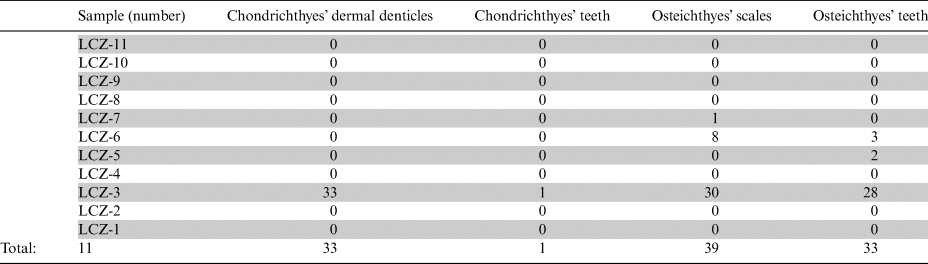
Table 2 A short description of Late Permian fish remains’ morphotypes (M) and their distribution in Poland, Lithuania and Latvia.

1. Geological settings
The Zechstein strata formed a relatively narrow zone in the outer part of the North-Sudetic Basin in Poland. The studied Leszczyna section is situated in the Leszczyna syncline (Fig. 1c). It is represented by a minor tectonic unit formed during the Late Cretaceous (Biernacka et al. Reference Biernacka, Borysiuk and Raczyński2005). This section is limited by the Kaczawa Metamorphic Unit from the north, east and south directions while from the west it connects to the North-Sudetic Basin. As a result, it has the shape of an elongated ‘pan’ (Fig. 1c).
The Zechstein marine deposits of the Upper Permian in South-West Poland are famous, in terms of the occurrence of ore-bearing deposits and exploitation of the copper metal. For example, an open-air museum of mining and metallurgy presents here facies of the limestone-marl alternations’ deposits which are exposed in an abandoned quarry in the Leszczyna village (Biernacka et al. Reference Biernacka, Borysiuk and Raczyński2005; Hara et al. Reference Hara, Słowakiewicz and Raczyński2013; Kaczawskie Association 2019). The studied profile from this locality can be divided into two parts. The lower part of this profile is exposed at the base of the quarry in a narrow trench while the upper part could be described as the profile with an extensively cropping outwall. In the lower part of the Leszczyna profile the alternating grey limestone and brown-grey marl can be observed. Within this so-called mottled (spotted) marl gold enriched (0.3 g t−1) deposits were discovered. Also, the high content of copper occurred in the form of malachite and azurite coating there. The amount of copper in these rocks exceeds 1% (Kaczawskie Association 2019). The first stage of ore formation occurred during the sedimentation based on the equilibrium law principle in the aqueous solutions (Serkies et al. Reference Serkies, Oberc and Idzikowski1967). The mottled (spotted) marls are covered by thicker layers of limestone with the thinner intercalations of copper-bearing and lead-bearing marls (Kaczawskie Association 2019) (Fig. 2). The local designation of the three units mentioned above has been in use for almost 100 years (Scupin Reference Scupin1931), although, this designation can only reflect the ore content and does not reflect the sedimentological character of the rocks (Biernacka et al. Reference Biernacka, Borysiuk and Raczyński2005). However, these rocks can be described as distal tempestite (Hara et al. Reference Hara, Słowakiewicz and Raczyński2013). The marls were formed on the bottom of the Zechstein Sea without any effect of waves. On the other hand, limestones were deposited with crushed mollusc shells in the lower part of layers as the result of the bottom currents (bearing a washed-out material from the sea coast) (Kaczawskie Association 2019). The complete and incomplete fossils of molluscs, brachiopods, ostracods and bryozoans (mostly fenestellids) were previously found in the Leszczyna locality (Raczyński Reference Raczyński1997; Hara et al. Reference Hara, Słowakiewicz and Raczyński2013). Remains of the vertebrates have not been described in the studied area.

Figure 2 The lithostratigraphic profile of Upper Permian strata in the Leszczyna area.
2. Material and methods
The studied samples were collected from three outcrops located in the Leszczyna quarry of South-West Poland [51°5′29.65″N, 15°58′42.55″E; 51°5′47.93″N, 15°58′31.69″E; 51°5′55.11″N, 15°58′24.66″E]. The samples were each taken 1.5–2.0 m throughout a vertical cross-section of these outcrops. Three samples were collected from Lower Zechstein (LCZ-1, LCZ-2 and LCZ-3) and eight samples from Middle Zechstein (LCZ-4–LCZ-11) strata. The total weight of 11 samples from the Leszczyna quarry reached 111.8 kg of carbonate material.
The microfossils were chemically extracted from collected samples using the carbonate dissolution in formic acid technique described by Jeppsson et al. (Reference Jeppsson, Anehus and Fredholm1999). The sample preparation was done in the Micropalaeontological laboratory (Vilnius University) by mixing 23.3 l of 60% formic acid and 116.7 l of tap water in a 70 l vessel. The vessel with a chemical solution and a sample were left for about 48 h till the reaction stopped. The laboratory procedure for one sample was repeated till the carbonate material was totally dissolved. The undissolved residue was dried at room temperature and sieved using the dry sieving technique (Green Reference Green2001) for the size range between 0.063 and 3.0 mm to extract it. Later, sorted micromaterial was studied using a binocular microscope.
In total, 106 isolated fish remains were found in the collected samples. The counting was done for each sample and presented in Table 1 for further comparative analyses with the Nowy Kościół quarry section in South-West Poland. All ichthyofaunal finds were divided into four groups (Chondrichthyes dermal denticles, Chondrichthyes teeth, Osteichthyan teeth and Ostichthyes scales) based on the various fossilised remains. These groups were divided into 6–8 morphotypes (M1–M8) according to their morphological characteristics for more detailed studies, with an exception of the Chondrichthyes teeth group, since only a single specimen was found. This concept of division of the ichthyofaunal material into morphotype groups follows our previous works based on the Upper Permian materials from the Nowy Kościół quarry in South-West Poland (Dankina et al. Reference Dankina, Spiridonov, Raczyński and Radzevičius2021a), Kūmas quarry in southern Latvia (Dankina et al. Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b) and Karpėnai quarry in northern Lithuania (Dankina et al. Reference Dankina, Chahud, Radzevičius and Spiridonov2017). In this study, we continue the use of the morphotypes’ numeration which was determined and described in the works mentioned above (Table 2).
Figures 2 and 3 were done using AutoCAD software to visualise lithological columns, Adobe Photoshop for adding textual information and Microsoft Excel for a quantitative distribution of fish fossils in the studied outcrops. These fossils were micrographed using a FEI Quanta 250 scanning electron microscope (SEM) at the Nature Research Centre (Vilnius, Lithuania) and presented in Figs 4, 5. The ichthyofaunal material described here is housed in the Geological Museum at the Institute of Geosciences of Vilnius University (Vilnius, Lithuania).
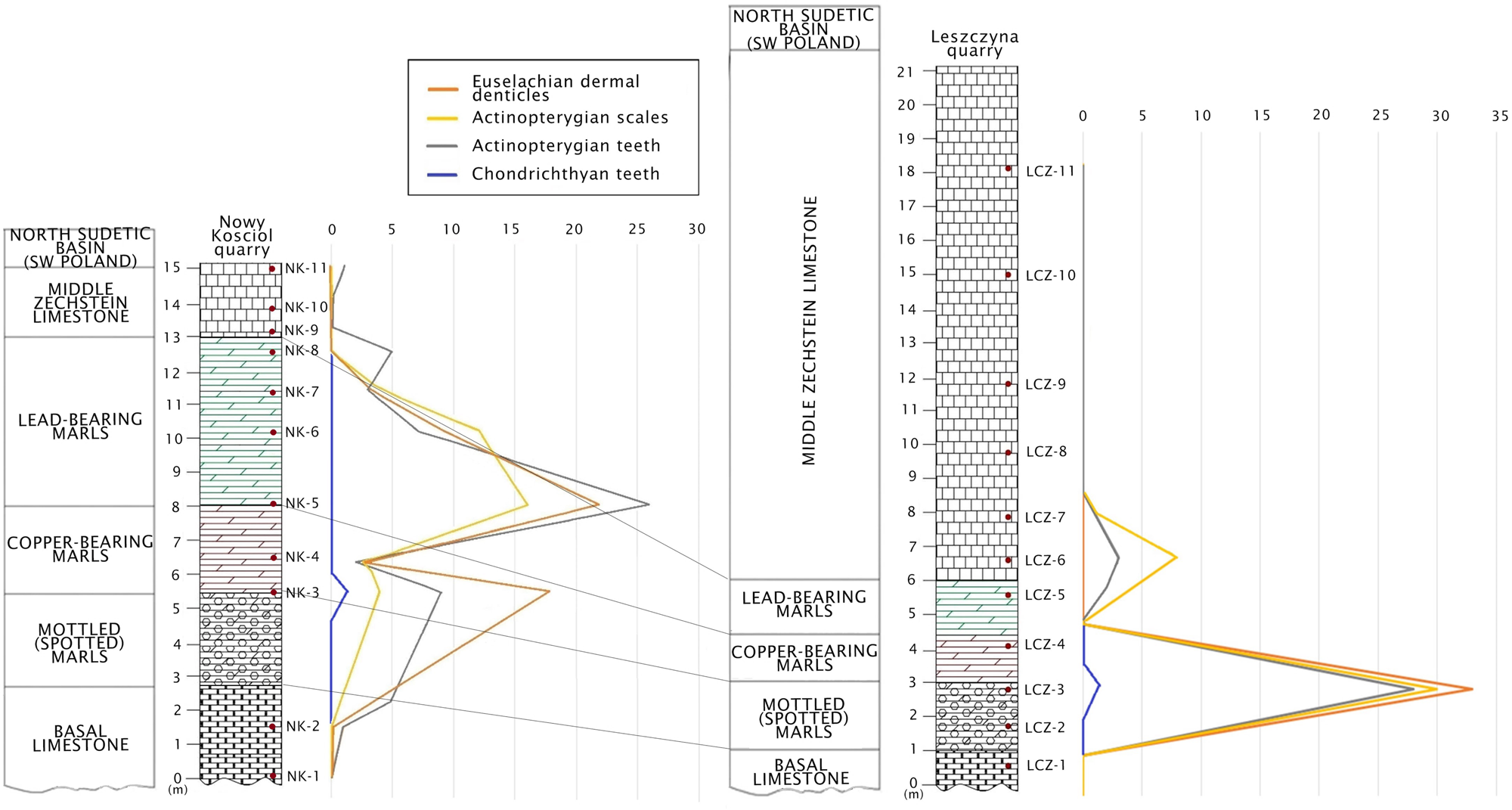
Figure 3 The lithostratigraphic correlation of Upper Permian strata between Nowy Kościół and Leszczyna quarries in South-West Poland with a quantitative (vertical) distribution of fish remains.

Figure 4 Scanning electron microscope photomicrographs of the Late Permian chondrichthyan remains from the Leszczyna quarry. (a) ?Omanoselache sp. tooth: (a1) in occlusal view, (a2) in lateral view [UOICH-LCZ-01]. (b) ‘Listracanthus’ type dermal denticles in crown view [UO-ICH-LCZ-02]. (c) Euselachian type dermal denticles of morphotype 2 in crown view [UO-ICH-LCZ-03]. (d) In crown view [UO-ICH-LCZ-04]. (e) In crown view [UO-ICH-LCZ-05]. (f–h) Euselachian type dermal denticles of morphotype 5. (f) In basal view [UO-ICH-LCZ-06]. (g) In crown view [UO-ICH-LCZ-07]. (h) In crown view [UO-ICH-LCZ-08]. (i, j) Euselachian type dermal denticles of morphotype 6. (i) In crown view [UO-ICH-LCZ-09]. (j) In crown view [UO-ICH-LCZ-010]. Abbreviation: DE = euselachian type dermal denticles of morphotype 3.

Figure 5 Scanning electron microscope photomicrographs of the Late Permian actinopterygian remains from the Leszczyna quarry. (a–c) Actinopterygian teeth of morphotype 1: (a1) in lateral view [UO-ICHLCZ-011], (a2) the elongated proximo-distally microtubercles. (b) In lateral view [UO-ICHLCZ-012]. (c1) In lateral view [UO-ICH-LCZ-013], (c2) the distinct acrodin apex. (d) Actinopterygian teeth of morphotype 7 in lateral view [UO-ICH-LCZ-014]. (e, f) Actinopterygian scales of morphotype 1: (e1) in external view [UO-ICH-LCZ-015], (e2) the roundish-shaped microtubercles: (f1) in external view [UO-ICH-LCZ-016], (f2) the roundish-shaped microtubercles. (g) Actinopterygian scales of morphotype 3: (g1) in external view [UOICH-LCZ-017], (g2) the roundish-shaped microtubercles.
3. Systematic palaeontology
Class Chondrichthyes Huxley (Reference Huxley1880)
Subclass Elasmobranchii Bonaparte (1832)
Order Hybodontiformes Patterson (Reference Patterson1966)
incertae familiae
Genus Omanoselache Koot et al. (Reference Koot, Cuny, Tintori and Twitchett2013)
?Omanoselache sp.
(Fig. 4a)
Material
Incomplete tooth [UO-ICH-LCZ-01].
Description
The shape of tooth is robust, elongated and slightly but clearly asymmetrical (Fig. 4a1). It reaches 1.6 mm mesio-distal, 0.7 mm labio-lingual and 1.0 mm in height. The central transverse part of the crown has a moderate main cusp with rounded apex (Fig. 4a2). A small labial peg is indented (Fig. 4a1). The crown surface is smooth without any ornamentation. The neck is massive and broad, with some canal openings filled by sediment (Fig. 4a2). The basal plate is elliptical in shape.
Remarks
Similar morphological features (asymmetrical crown, smooth surface with the rounded blunt apex of the main cusp, small labial intended peg and the base with randomly located foramina) were found for a comparison with an elongate tooth three times bigger in size from the Knuff Formation of Middle Permian in the Oman (Koot et al. Reference Koot, Cuny, Tintori and Twitchett2013). Moreover, the analogous specimen with similar parameters, shape and size, was previously found in the Naujoji Akmenė Formation from Upper Permian of the southern Latvia (Dankina et al. Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b).
Subclass Elasmobranchii Bonaparte (Reference Bonaparte1838)
Order incertae sedis
Family Listracanthidae Martill et al. (Reference Martill, Del Strother and Gallien2013)
Genus Listracanthus Newberry & Worthen (Reference Newberry and Worthen1866)
?Listracanthus sp.
(Fig. 4b)
Material
Incomplete dermal denticle [UO-ICH-LCZ-02].
Description
The stud-like crown is compound, elongate, flattened, and posteriorly recurved. It is covered by vertical ridges along the whole specimen. The crown is perpendicularly attached to the narrow and flat base (Fig. 4b).
Remarks
A similar dermal denticle referred to ‘Listracanthus’ type denticles which are know from Early Permian of the Middle and South Ural (Ivanov Reference Ivanov2005). The highly notable ridges are incompletely preserved in the studied material. However, these ridges are easy recognisable on the lower part of denticle which are attached to the base.
Cohort Euselachii Hay (Reference Hay1902)
Euselachii indet.
(Fig. 4c–j)
Material
Isolated euselachian dermal denticles were found in the Leszczyna quarry in South-West Poland (Fig. 4c–j). These fossils were divided into four morphotypes (M2, M3, M5 and M6) based on their morphological characteristics, such as shape of the crown and its ornament. The dermal denticles are represented here by SEM micrographs of remains UO-ICH-LCZ-03–UO-ICH-LCZ-010.
Description
The dermal denticles of morphotype 2 [UO-ICH-LCZ-03] have a trident or nearly trident shape of the crown with a low, slender and narrow neck, hidden under the crown in apical view (Fig. 4c). The exterior part of the crown is smooth without any ornamentation. The crown is obtained to be slightly obliquely up from the neck. The shape of base is poorly-preserved. The crown of the denticle is 0.3–0.4 mm in length and width, respectively. The dermal denticles of morphotype 3 [UO-ICH-LCZ-04–UO-ICH-LCZ-05] have a roundish (Fig. 4d) and semi-roundish shape of the crown (Fig. 4e). This type of denticle has a slightly elongated blunt cusp in the posterior margin of the crown (Fig. 4d). The crown surface is smooth without any ornamentation. It has some short ridges and furrows on the anterior margin (Fig. 4d, e). The crown is placed horizontally or evidently obliquely up from the neck. The neck is relatively high. The shape of the base is incompletely preserved. The crown is 0.3–0.4 mm in width and length, respectively. The dermal denticles of morphotype 5 [UO-ICH-LCZ-06–UO-ICH-LCZ-08] have an outline of drop-like crown of different width (Fig. 4f). The crown surface is smooth with some short ridges on the anterior margin (Fig. 4g) or one long furrow in the central part of the crown (Fig. 4h). The crown is placed horizontally or evidently obliquely up from the neck. The outline of the base is curved, multipetaloid in shape, with some concave canal openings in the proximal view (Fig. 4f). The denticles are 0.3–0.5 mm in width and length, respectively. The dermal denticles of morphotype 6 [UO-ICH-LCZ-09–UOICH-LCZ-10] have a vertical oriented compound shape (Fig. 4i, j). The crown is smooth and covered by short, narrow ridges along the posterior part of the denticle. The central part of the crown is strongly concave (Fig. 4j). The neck is poorly developed. The base is flat and low. The denticles are 0.4–1.0 mm in width and 0.8–1.2 mm in length.
Remarks
Dermal denticles of the morphotype 2 have strong morphological similarities (shape of the crown, the neck features and size of the fossils) to other euselachian-type dermal denticles which have already been described from the Upper Permian in Lithuania (Dankina et al. Reference Dankina, Chahud, Radzevičius and Spiridonov2017), Latvia (Dankina et al. Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b) and Poland (Dankina et al. Reference Dankina, Spiridonov, Raczyński and Radzevičius2021a). The main difference between a new material described here and material which already has been found is the absence of crown ornamentation. Dermal denticles of the morphotype 3 are referred to euselachian-like dermal denticles which were found in Permian strata of Russia (Ivanov & Lebedev Reference Ivanov and Lebedev2014) and euselachian dermal denticles in Upper Permian strata of Latvia (Dankina et al. Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b). Dermal denticles of the morphotype 5 resemble euselachian-type denticles from the Upper Permian in Lithuania (Dankina et al. Reference Dankina, Chahud, Radzevičius and Spiridonov2017), Latvia (Dankina et al. Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b) and Poland (Dankina et al. Reference Dankina, Spiridonov, Raczyński and Radzevičius2021a). Also, morphologically similar euselachian denticles are known from Permian strata of Russia (Ivanov & Lebedev Reference Ivanov and Lebedev2014). Moreover, these denticles have similarities to hybodontiform scales from Upper Triassic strata of Germany (Reif 1978) and Middle Triassic strata of Spain (Manzanares et al. Reference Manzanares, Plá, Martínez-Pérez, Rasskin and Botella2014). Dermal denticles of the morphotype 6 have strong similarities (shape of the crown and its ornament) to euselachian-type denticles which are known from Upper Permian strata of Poland (Dankina et al. Reference Dankina, Spiridonov, Raczyński and Radzevičius2021a). Furthermore, similar euselachian dermal denticles to the studied M2, M3, M5 and M6 here were found in Middle Permian strata of the United States (Ivanov et al. Reference Ivanov, Bakaev, Nestell and Nestell2021).
Superclass Osteichthyes Huxley (Reference Huxley1880)
Class Actinopterygii Cope (Reference Cope1887)
(Fig. 5)
3.1. Teeth
Material
Isolated actinopterygian teeth were found in the Leszczyna quarry in South-West Poland (Fig. 5a–d). These fossil remains were divided into two morphotypes (M1 and M7) based on their morphological characteristics of teeth shape. The teeth are represented here by SEM micrographs of the remains of UO-ICH-LCZ-11–UO-ICH-LCZ-014.
Description
The teeth of morphotype 1 [UO-ICH-LCZ-11–UO-ICH-LCZ-13] have a conical, thin, straight (Fig. 5a1, b) or slightly curved shape (Fig. 5c1). These teeth have an acrodin cap (Fig. 5c2) or lack of it in some fossils because of poorly-preserved material (Fig. 5b). Teeth surface is smooth with no distinct visible ornament (Fig. 5a–c) but with well-developed microtubercles for some teeth (Fig. 5a2). The microtubercles are proximo-distally elongated, narrow and blend together in oblique rows. These teeth are 0.3–1.1 mm in length. The teeth of morphotype 7 [UOICH-LCZ-14] are roundish in the lateral outline, short and slightly convex in the central part (Fig. 5d). The surface of these teeth is smooth without any ornament. These teeth are about 1.2 mm in width/length and 0.6–0.8 mm in height.
Remarks
The strongly resembling teeth material of morphotype 1 is known from Upper Permian strata of Lithuania (Dankina et al. Reference Dankina, Chahud, Radzevičius and Spiridonov2017), Latvia (Dankina et al. Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b) and Poland (Dankina et al. Reference Dankina, Spiridonov, Raczyński and Radzevičius2021a). Also, the teeth of this morphotype have similarities (shape of the tooth and its microstructure) to Elonichthys sp. teeth from Upper Carboniferous strata of the Czech Republic (Štamberg Reference Štamberg2016). The analogous teeth of morphotype 7 are previously described and are known from Upper Permian strata of Poland (Dankina et al. Reference Dankina, Spiridonov, Raczyński and Radzevičius2021a).
3.2. Scales
Material
Isolated actinopterygian scales were found in the Leszczyna quarry in South-West Poland (Fig. 5e–g). These fossils were divided into two morphotypes (M1 and M3) based on their morphological characteristics of the scales’ shape. The scales are represented here by SEM micrographs of the microremains of UO-ICH-LCZ-15–UO-ICH-LCZ-017.
Description
The scales of morphotype 1 [UO-ICH-LCZ-15–UO-ICH-LCZ-16] are thick and rhombic-shaped (Fig. 5e1, f1). These scales have numerous small, roundish-shaped microtubercles in the outer ganoine-covered field (Fig. 5e2, f2). The surface is smooth and flat. It has some fine and slightly diagonally oriented ridges which were developed in the posterior parts. These ridges are separated by narrow grooves (Fig. 4e1, f1). Scales are 0.6–1.8 mm in anteroposterior and 0.5–1.0 mm in dorsoventral profile. The scales of morphotype 3 [UO-ICH-LCZ-17] are thick, square-shaped, with a slightly twisted posterior corner (Fig. 5g1). The ganoine tissue is represented here by roundish-shaped and rise-shaped microtubercles in the anteroposterior direction (Fig. 5g2). Scales of this morphotype are about 0.7 mm in anteroposterior profile and about 0.4 mm in dorsoventral profile.
Remarks
The strongly resembling scales collection is known from Upper Permian strata of Lithuania (Dankina et al. Reference Dankina, Chahud, Radzevičius and Spiridonov2017), Latvia (Dankina et al. Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b) and Poland (Dankina et al. Reference Dankina, Spiridonov, Raczyński and Radzevičius2021a).
4. Discussion
A new fossil fishes’ assemblage from the Lower and Middle Zechstein sequences of the Leszczyna quarry in South-West Poland comprised low abundance microremains of isolated material from Chondrichthyes and Osteichthyes (Fig. 3). The chondrichthyan remains are taxonomically described as euselachian-type dermal denticles, including identification of single specimens of ?Listracanthus sp. Dermal denticle and ?Omanoselache sp. Tooth while osteichthyan remains are represented by actinopterygian teeth and scales. Genus Listracanthus is extremely rare throughout the entire Permian and has never been found in the Zechstein Basin before. However, the Listracanthus denticles are known from the Early Permian strata of the Urals (Ivanov Reference Ivanov2005). A new specimen has strong morphological affinities to this genus, such as a compound crown with vertical ridges which ‘appeared’ from the small and flat base. Omanoselache taxon is rare in the Palaeozoic fossil record. Although, this genus was discovered comparatively recently by Koot et al. (Reference Koot, Cuny, Tintori and Twitchett2013) based on the new ichthyofaunal finds from the Late Permian of Oman, it apparently had a wider distribution in the Triassic, ranging from Oman (Koot et al. Reference Koot, Gilles, Orchard, Richoz, Hart and Twitchett2015), China (Chen et al. Reference Chen, Cuny and Wang2007), to Spain (Manzanares et al. Reference Manzanares, Plá, Ferrón and Botella2018). Nevertheless, a morphologically similar ?Omanoselache sp. Tooth was previously found in the north-eastern part of the Zechstein Basin (Dankina et al. Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b). A similar Late Permian ichthyofaunal assemblage with the various morphotypes of euselachian-type dermal denticles, actinopterygian teeth and scales is already known from the eastern part of the Zechstein Basin (Dankina et al. Reference Dankina, Chahud, Radzevičius and Spiridonov2017, Reference Dankina, Spiridonov, Raczyński and Radzevičius2021a, Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičiusb).
A detailed comparison of the Late Permian fossils of fishes between the previously studied Nowy Kościół quarry (Dankina et al. Reference Dankina, Spiridonov, Raczyński and Radzevičius2021a) and new material described here revealed a slight difference (Table 2). In the Nowy Kościół quarry section five morphotypes of euselachian type dermal denticles (M2–M6) were found and described while only four morphotypes of euselachian type denticles (M2, M3, M5 and M6) were found in the Leszczyna quarry. Also, the actinopterygian scales of M1 and M2 were previously described in the Nowy Kościół quarry section, while M1 and M3 were found in the Leszczyna quarry. Moreover, three morphotypes of actinopterygian teeth (M1, M7 and M8) are known from the Nowy Kościół quarry section, while only M1 and M7 were found in the Leszczyna quarry (Table 2). Overall the faunal similarity is evident, and the differences could be partially explained by possible failure of sampling of common taxa because of low absolute abundances of micoremains in comparatively large rock samples (low density of finds per unit mass of a bulk sample). The correlation of lithological profiles including quantitative distribution of microremains of fishes between the Nowy Kościół and Leszczyna localities revealed marls as the richest fossils bed (Fig. 3). It is possibly related to formation of marls in the Zechstein Basin where sediments accumulated on the bottom of the basin during a calm state of the water (without any effect of waves) (Kaczawskie Association 2019) which suggest transgressive conditions, milder climate, runoff of nutrients from highlands and possibly greater productivity of marine ecosystems. A minor difference in the diversity of apparent morphotypes of fishes between the Nowy Kościół and Leszczyna quarry sections is possibly related to the palaeogeographical location of the studied area, which was much closer to the shoreline than the Nowy Kościół locality, which was further offshore (Fig. 1c). Therefore, the taxonomic differences at least partially could be accounted for by differences in the position of localities with respect to a major environmental (depth) gradient.
Omanoselache finds from the Leszczyna (South-West Poland) and Kūmas (Latvia) localities in the eastern part of the Zechstein Basin point out to the occurrence of normal marine conditions at a given area, probably facilitated by the input of fresh waters from the surrounding terrains (Dankina et al. Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b). This pattern of higher abundance and diversity of fishes in near-shore settings was determined earlier based on the stratigraphic and geographical comparison of distribution of fish micoremains in nearby Zechstein sections from north Lithuania and south Latvia (Dankina et al. Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b). Acrodus and Helodus teeth were previously reported in the north-eastern part of the Zechstein Basin (Dankina et al. Reference Dankina, Chahud, Radzevičius and Spiridonov2017, Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b) where a coastal zone was much wider compared with the south-eastern part of this basin in South-West Poland (Raczyński & Biernacka Reference Raczyński and Biernacka2014). Here, the lithological profile shows prevalence of limestones and dolostone pointing to the shallow marine shoreline nature of the sequence of the Zechstein Sea, which appearance was caused by the first and the strongest transgression which formed the overall successions of the basin (Raczyński & Biernacka Reference Raczyński and Biernacka2014; Dankina et al. Reference Dankina, Chahud, Radzevičius and Spiridonov2017, Reference Dankina, Spiridonov, Stinkulis, Manzanares and Radzevičius2021b).
Two morphotypes (M1 and M7) of Late Permian actinopterygian teeth were found and described in the Leszczyna quarry section. Their morphology revealed that ray-finned fishes occurring in the southeasternmost part of the Zechstein Sea had less different feeding modes than in the nearby areas of Poland (Dankina et al. Reference Dankina, Spiridonov, Raczyński and Radzevičius2021a). Esin (Reference Esin1997) described major trophic groups of fishes based on their teeth shape. Moreover, other scientific works are known based on actinopterygian jaw apparatus (lower jaw) which related to a feeding mode of herbivory and grazing in the marine palaeoenvironment (Bellwood Reference Bellwood2003) as well as the earliest discovered durophagous among actinopterygians according to new information about jaws including their palate and dentition (Sallan & Coates Reference Sallan and Coates2013; Friedman et al. Reference Friedman, Pierce, Coates and Giles2019). Durophagy among ray-finned fish appears in the early Carboniferous (Friedman et al. Reference Friedman, Pierce, Coates and Giles2019), and appears independently in several Carboniferous and Permian groups of Actinopterygii (Fracasso & Hovorka Reference Fracasso and Hovorka1987; Zidek Reference Zidek1992; Sallan & Coates Reference Sallan and Coates2013). In this study, the morphotype 1 is represented by canine-like shape teeth and their holders should have had feeding styles of small predators, while morphotype 7 is described as rounded teeth in lateral outline and their holders should have belonged to specialised durophagous forms. Fish with teeth shape of morphotype 1 could have fed on the soft-bodied invertebrates (fish larvae and polychaetes) and vertebrates (conodonts). Conodonts are common fossils in the Zechstein deposits of Werra cyclothem from Poland (Szaniawski Reference Szaniawski1969) and England (Swift Reference Swift, Harwood and Smith1986). Also, soft-bodied invertebrates such as polychaetes are known from the Upper Permian strata of Poland (Szaniawski Reference Szaniawski1969). Rare finds of teeth from morphotype 7 point out that their holders processed tough-solid food items, including shelled crustaceans and molluscs (Esin Reference Esin1997; Purnell & Darras Reference Purnell and Darras2015). The foraminifera and ostracods are known from and widely distributed in the Zechstein marine deposits of the Upper Permian in Poland (Woszczynska Reference Woszczynska1987).
5. Conclusion
Late Permian chondrichthyan and osteichthyan material from the Leszczyna quarry section in South-West Poland presented here add new data to the scarce ichthyofaunal fossil record in the eastern margin of the Zechstein Basin.
The palaeontological study of Zechstein carbonates in the Leszczyna quarry discovered the relatively diverse but low abundance of isolated remains identified and described as four morphotypes of euselachian dermal denticles, two morphotypes of actinopterygian teeth, two morphotypes of actinopterygian scales, ?Omanoselache sp. tooth, and ?Listracanthus sp. dermal denticle. Previously Omanoselache was known from Middle Permian strata of Oman and Upper Permian strata of Latvia while Listracanthus was only known from Lower Permian strata of the Ural Mountains. Thus, the new findings of the latter two genera are significantly extended in their palaeogeographical distributions at the end of the Paleozoic.
The correlation of lithological profiles and comparison of ichthyofaunal assemblage from the Leszczyna and Nowy Kościół quarries show that marly layer is the richest fossils bed in this area. The probable reason for increase in diversity was the prevalence of a more humid climate and runoff of nutrients to the basin.
The teeth of ray-finned fishes demonstrate the prevalence of taxa with two predatory lifestyles in the studied area. The conical teeth of morphotype 1 belonged to the small predators of soft-bodied prey, such as small vertebrates and invertebrates, while crushing teeth of morphotype 7 belonged to durophagous forms who fed on shell-encased prey. The analysis of actinopterygian teeth indicates that, in the eastern part of the Zechstein Sea, this taxon was represented mostly by small pelagic and epibenthic predators.
6. Acknowledgements
The authors thank Dr Alexandr Bakaev (Faculty Member at Russian Academy of Science) for significantly improving the manuscript and sharing important literature. Also, we thank an anonymous reviewer for a useful critique which helped to generate better ideas for this manuscript. Moreover, we thank Vaida Kirkliauskaitė (Vilnius University) and Marijus Ravickas, who helped safely transport samples. Many thanks to Laurynas Šiliauskas (Nature Research Center, Lithuania) for the scanning electron microscope micrographs.
7. Financial support
This research was carried out by the Open Access to the research infrastructure of the Nature Research Centre (Vilnius) under the Lithuanian open access network initiative. The fieldwork to Poland was partly financed by Sepkoski Grant 2018 of Paleontological Society (PaISIRP). Professor Dr Sigitas Radzevičius (co-author) thanks the Polish National Agency for Academic Exchange (NAWA PPN/ULM/2020/1/00306). Besides which, this research was supported by the project S-MIP-21-9 ‘The role of spatial structuring in major transitions in microevolution’.
8. Competing interests
None.










