INTRODUCTION
Increasing attention has been focused on the importance of antimicrobial resistance in nursing-home (NH) residents. Antimicrobial resistance in this rapidly growing segment of the population is important because these people often have multiple comorbidities and functional impairments that increase susceptibility to infection [Reference Smith and Rusnak1, Reference Yoshikawa2]. Furthermore, NH residents are frequently treated empirically with broad-spectrum antibiotics, which increase selection pressure for resistance. The crowded living environment, shared physical therapy activities, shared bathing equipment, and group dining facilities have the potential to foster unintentional and unrecognized person-to-person transmission of antibiotic-resistant organisms [Reference Fisch3, Reference O'Fallon, Gautam and D'Agata4]. Moreover, prevalence of colonization with such organisms is higher in older adults in NHs than in community-dwelling older adults [Reference Van der Donk5]. Inappropriate use of antibiotics and low adherence to hand hygiene by healthcare workers worsen the situation. Extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E) is currently one of the most important and common antibiotic-resistant organism isolated from NH residents [Reference Van der Donk5, Reference Loeb6]. Faecal flora represent a large potential repository for the evolution of such organisms and a site where resistance genes transfer from commensal flora to virulent microorganisms [Reference Donskey7–Reference Salyers and Amabile-Cuevas9].
NHs have been implicated as significant reservoirs of antibiotic-resistant organisms and the wider importance of NHs in the epidemiology has been examined by other groups over the past decade, especially in North America [Reference Bonomo10, Reference Juthani-Mehta11], but few comprehensive microbiological screening studies have been performed within these settings in China or with regard to the epidemiology of antimicrobial resistance [Reference Chan12]. Moreover, few studies have addressed the issue of colonization with Enterobacteriaceae in faecal flora or have attempted to determine risk factors of ESBL-E colonization at the facility level. Understanding the impact of these characteristics on resistance patterns would provide important insights to inform strategies to curb future emergence of resistance.
We conducted a cross-sectional study to assess bacterial colonization, antimicrobial resistance, molecular epidemiology of ESBL-E and to determine risk factors for faecal colonization of ESBL-E.
METHODS
Setting and participants
Putuo District is the largest district in the west of Shanghai and the number of adults aged ⩾65 years is about 1 56 000, accounting for 12·12% of the total population [13]. There were 31 registered NHs in this area which were officially authorized by the Putuo government to care for old people according to the law [14], seven of these NHs were randomly selected and asked to participate. This study was approved and reviewed by the Medical Ethics Committee and Review Board of Ruijin Hospital (Shanghai Jiao Tong University School of Medicine). All psychogeriatric and somatic residents of these NHs were eligible for participation. However, only those residents who supplied a consent form signed by themselves or their legal representative (someone who has been legally appointed to take charge of all the affairs of the resident) to allow access to their NH records and to culture a single faecal sample before the start of the study were included in the study (see Supplementary material). In each NH, the samples were collected during 1 day in the period between March 2014 and May 2014. NH records at the individual-resident level (level 1) were examined for details of age, gender, comorbidities, previous hospitalization, recent antibiotic use (last 3 months) and other potential risk factors from August 2013 onwards. Data recorded for each NH (level 2) included the following: number of resident beds, average monthly expenditure per resident (AMEPR); staffing levels (staff per 10 beds); and frequency of bathing. All data were collected using a standardized questionnaire.
Bacterial isolates
Rectal swabs were streaked across blood plate (non-selective medium) and two selective media (MacConkey agar with cefotaxime at 2 µg/ml for ESBL producers and meropenem at 2 µg/ml for carbapenemases producers) [Reference Lo15]. Colonies with morphology suggestive of Enterobacteriaceae were further tested. For each agar plate, at least one, and up to five colonies were investigated. If there were more than three colonies with morphology suggestive of Enterobacteriaceae growing on the selective media, we selected the colonies to perform antimicrobial susceptibility testing, screening test and confirmatory test for ESBL and carbapenemase production. Bacteria were identified by the VITEK 2 compact system and VITEK 2 Gram-negative identification card (bioMérieux, France).
Antimicrobial susceptibility testing
Susceptibility testing was done by disk diffusion according to Clinical and Laboratory Standard Institute (CLSI) recommendations [16] for ampicillin (AMP), gentamicin (GM), amikacin (AK), piperacillin (PRL), ampicillin-sulbactam (SAM), piperacillin-tazobactam (TZP), cefepime (CEF), ceftriaxone (CRO), cefuroxime (CXM), cefotaxime (CTX), ceftazidime (CAZ), aztreonam (ATM), ertapenem (ETP), imipenem (IPM), meropenem (MEM), doripenem (DOR), ciprofloxacin (CIP), levofloxacin (LEV), trimethoprim-sulfamethoxazole (SXT), fosfomycin (FOS), chloramphenicol (CL), tigecycline (TGC) and tetracycline (TE). Susceptibility testing and interpretation of tigecycline (TGC) was according to Food and Drug Administration (FDA) recommendations [Reference Jones17]. Escherichia coli ATCC 35218 was used as control strain for β-lactam/β-lactamase inhibitor combinations and E. coli ATCC 25922 was used as control strain for other antibiotics in antimicrobial susceptibility testing. The double-disk synergy test was used as a confirmatory test for ESBL producers according to CLSI recommendations [16]. Klebsiella pneumoniae ATCC 7 00 603 was used as positive control for ESBL production.
Molecular characterization
Template DNA was prepared from two fresh colonies resuspended in 100 ml distilled water and the cells were lysed by heating at 100 °C for 15 min. Cellular debris was removed by centrifugation at 21756 g for 10 min, the supernatant was diluted 1:10 in distilled water and utilized as a source of template DNA for amplification. Polymerase chain reaction (PCR) and sequencing of PCR products were performed as described previously to determine the bla SHV, bla CTX-M (-1, -2, -8, -9, -25 groups), bla OXA (-1, -2, -10 groups), bla VEB, bla GES, bla PER, bla VIM, bla IPM, bla KPC, bla OXA-48 and bla NDM-1 genes encoding for ESBLs and carbapenemases [Reference Woodford, Fagan and Ellington18–Reference Pang21].
Statistical analysis
The prevalence of intestinal colonization by ESBL-E was calculated as the percentage of carriers in the total number of residents included. Colonized and non-colonized subjects were compared using univariate analysis with the χ 2 test for categorical variables and Student's t test or the Mann–Whitney U test, as appropriate, for continuous variables. Multilevel regression analysis was performed using SAS software v. 9.3 (SAS Institute Inc., USA) [Reference Ni, Lu and Zhang22], and the multivariate logistic regression analysis was performed with the forward likelihood ratio logistic-regression model of SPSS software package v. 19 (IBM SPSS Statistics, USA) to determine variables independently related to intestinal colonization. All tests performed were two-sided, and a P value <0·05 was considered significant.
RESULTS
Overview of included residents and NHs
Seven homes were asked to participate, including residents from diverse socioeconomic backgrounds with a range of physical and cognitive functioning, but 73 of these residents either refused to provide faecal samples for analysis because of impaired mobility and serious illness or supplied incomplete resident data and were therefore excluded. Ultimately, 390 residents of the seven NHs (A–G) provided rectal swabs and 457 Enterobacteriaceae isolates were cultured from these specimens. There were more specimens from females (273, 70%) than from males (117, 30%). The age of residents ranged from 47 to 101 years (median age 84 years, interquartile range 79–88 years). Of these residents, 183 (46·92%) were colonized by ESBL-E. The AMEPR ranged from 2550 to 3700 yuan and number of staff per 10 beds ranged from 1·92 to 5·00 (Table 1). The length of stay at a NH ranged from 0 to 5918 days (median length 335 days, interquartile range 79·75–367·25 days). Other baseline characteristics of all enrolled subjects are given in Table 2. There were no significant age or gender differences between those positive and negative for faecal carriage of ESBL-E; however, carriers had significantly more episodes of cardio-cerebrovascular disease [odds ratio (OR) 3·155, 95% confidence interval (CI) 1·238–8·038, P = 0·012] and urinary tract infection (UTI) (OR 2·215, 95% CI 1·139–4·310, P = 0·017) than non-carriers and were more likely to treated by invasive procedures (OR 3·049, 95% CI 1·723–5·396, P < 0·001) and to have received any antibiotic (OR 1·752, 95% CI 1·171–2·621, P = 0·006). More specifically, residents found to carry ESBL-E were more likely to have been prescribed narrow-/broad-spectrum cephalosporins and quinolones; for other groups of antibiotics, no significant difference was found. Moreover, frequency of bathing per week was a notable variable associated with ESBL-E faecal carriage.
Table 1. Overview of included residents and nursing homes

NH, Nursing home; ESBL-E, extended-spectrum β-lactamase-producing Enterobacteriaceae; AMEPR, average monthly expenditure per resident.
* 9·63 yuan = 1 British pound.
† Bathing is available whenever the resident wants and it may be once, twice, or more per week.
Table 2. Baseline characteristics of the study population and nursing homes according to ESBL-E faecal carriage
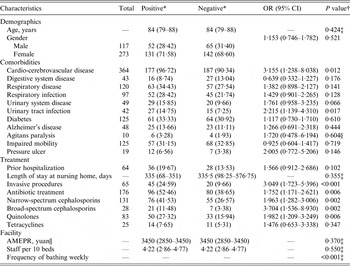
ESBL-E, Extended-spectrum β-lactamase-producing Enterobacteriaceae; OR, odds ratio; CI, confidence interval; AMEPR, average monthly expenditure per resident.
* Categorical variables: number (percentage); continuous variables: median (interquartile range).
† P value: χ 2 test, unless stated otherwise.
‡ Mann–Whitney U test.
§ Continuity correction χ 2 test.
|| 9·63 yuan = 1 British pound.
Distribution of Enterobacteriaceae isolates and characteristics of resistance genes
In total, 457 Enterobacteriaceae isolates were collected. Escherichia coli (365, 79·87%) predominated, followed by Proteus mirabilis (40, 8·75%), Klebsiella pneumoniae (24, 5·25%), Proteus vulgaris (6, 1·31%) and Enterobacter cloacae (5, 1·09%). The number of genus isolated <5 were: Citrobacter freundii (4), Enterobacter aerogenes (2), Klebsiella oxytoca (2), Citrobacter koseri (2), Citrobacter fameri (1), Morganella morganii (1), Providencia stuartii (1), Shigella sonnei (1), Yersinia enterocolitica (1), Serratia liquefaciens (1), Raoultella planticola (1). Two hundred isolates were ESBL producers, the distribution of ESBL is given in Table 3. CTX-M enzymes (198, 99%) predominated, with CTX-M-14 (84, 42%), CTX-M-15 (74, 37%), and CTX-M-3 (10, 5%) the most common types. Two E. coli isolates and seven K. pneumoniae isolates produced β-lactamase type SHV. Two carbapenemase producers were both isolated from NH F, harbouring the same genotype (KPC-2). No bla CTX-M-2, bla CTX-M-8, bla CTX-M-25, bla OXA-1, bla OXA-2, bla OXA-10, bla VEB, bla GES, bla PER, bla VIM, bla IPM, bla KPC, bla OXA-48 and bla NDM-1 genes were found.
Table 3. Distribution of resistance genotypes
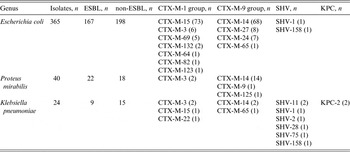
ESBL, Extended-spectrum β-lactamase.
Antimicrobial resistance profiles
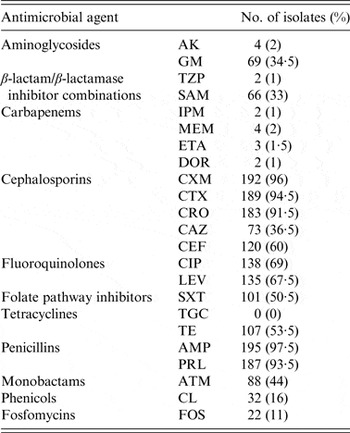
The resistance profiles of ESBL-E are presented in Table 4. No isolates were resistant to TGC. The highest rates of resistance were to penicillins (93·5–97·5%), CXM (96%), CTX (94·5%), CRO (91·5%), fluoroquinolones (67·5–69%) and CEF (60%). By contrast, resistance rates were low to carbapenems (1–2%), TZP (1%), AK (2%), FOS (11%) and CL (16%).
Table 4. Rates of antimicrobial resistance in 200 ESBL-E isolates
Risk factor analysis
In multilevel logistic regression analysis, the result of the empty model showed that the intra-class correlation coefficient was 0·05483 (P = 0·2359), so we just performed the multivariate logistic regression analysis. History of invasive procedures (OR 2·384, 95% CI 1·318–4·310, P = 0·004), narrow-spectrum cephalosporins (OR 1·635, 95% CI 1·045–2·558, P = 0·031) and broad-spectrum cephalosporins (OR 3·276, 95% CI 1·278–8·398, P = 0·014) were the only variables independently associated with carriage. The equation of the final model was:
 $$\eqalign{{\rm logit}\,P & = - 0\! \cdot \!285 + 0 \!\cdot \! 869 \hbox{invasive procedures} \cr & \quad +\! 0 \! \cdot \!492\;{\rm narrow} \cr & \quad - \!\hbox{spectrum cephalosporins} \cr & \quad + \! 1 \! \cdot \!187\;{\rm broad} - \hbox{spectrum cephalosporins}\comma} $$
$$\eqalign{{\rm logit}\,P & = - 0\! \cdot \!285 + 0 \!\cdot \! 869 \hbox{invasive procedures} \cr & \quad +\! 0 \! \cdot \!492\;{\rm narrow} \cr & \quad - \!\hbox{spectrum cephalosporins} \cr & \quad + \! 1 \! \cdot \!187\;{\rm broad} - \hbox{spectrum cephalosporins}\comma} $$
the –2 log likelihood was 531·5.
DISCUSSION
Over the past few years, there has been an increasing number of studies focused on antimicrobial resistance in NH residents which is closely associated with high mortality [Reference Allen23, Reference Kelley24]. Although some authors have commented that higher mortality in older adults in NHs is not because of NH residence but is related to their frailty, worse functional status, and greater number of comorbidities [Reference Chan25]; researchers from Hong Kong reported that NH residence remained an independent predictor of all-cause and infection-related mortality in Chinese older adults after taking into account these confounding predictors [Reference Chan12]. Shanghai has the oldest population in China, possibly in the world, and 50000 older adults live in NHs [26]; therefore we conducted a multi-centre active surveillance to assess the epidemiology of, and risk factors for, faecal ESBL-E carriage in local NH residents.
Our study shows that the incidence of ESBL-E isolates was 46·92%, much higher than NHs in Europe and Japan [Reference Olofsson27, Reference Yamamoto28]. This finding suggests that NHs have been implicated as significant reservoirs of antimicrobial-resistant organisms in this region; moreover, this carriage rate may be an underestimate for several reasons. First, only one sample was examined per subject, so intermittent excretion may have been missed. Second, we only investigated one colony in each bacterial species isolated from each sample, perhaps other clones of one bacterial species were missed. Third, not all residents in these seven NHs participated in the study. Of the seven NHs, the top two highest carriage rates of ESBL-E occurred in the top two NHs with the largest bed capacity, followed by NH G with the smallest bed capacity (Table 1), these findings indicate that the colonization rate of ESBL-E has nothing to do with NH bed capacity.
The profile of microorganisms in our study showed that E. coli, K. pneumonia and P. vulgaris were the most common, this result is in general accordance with the findings of a community-acquired colonization-based study [Reference Leistner29]. The ESBL genotypes we found were mainly CTXM-14 and CTX-M-15, similar to the results of a Hong Kong study [Reference Lo15]. Interestingly, Overdevest et al. found mostly CTX-M-1 in human isolates from the community but CTX-M-14 in clinical blood culture isolates [Reference Overdevest30]. For NHs as a type of long-term care facility, colonization should be viewed as healthcare-acquired ESBL colonization. The difference between healthcare-acquired genotypes and genotypes circulating in the community might be associated with the pressure deriving from the antibiotics used in hospital or transmission through the food chain [Reference Nicolas-Chanoine31, Reference Kola32].
Most residents were aged between 81 and 90 years (221, 56·67%) and were more often female (273, 70%), which is in parallel with the longer life expectancy for women in Shanghai [Reference Chan25], but no significant differences were found between ESBL-E carriers and non-carriers. Exposure to antibiotics was high in residents, with carriers previously receiving narrow-/broad-spectrum cephalosporins and quinolones; therefore, resistance to these antimicrobial agents was high (Table 4). This may reflect their increased incidence of UTIs or that ESBL-E isolates are more likely to survive and colonize the gut of antibiotic recipients. Prior invasive procedures (urinary catheter, stomach tube, central venous catheter, etc.) and UTI were significantly associated with ESBL-E carriage in the univariate analysis, in keeping with findings of other studies [Reference Gaynes33, Reference Loeb34]. However, a resistance rate <10% was found only for TZP, AK, TGC, CL, FOS and carbapenems, which may be options for empirical use. Notably, two carbapenemase-producing K. pneumoniae isolates were found in one NH, both carrying bla KPC-2 genes and the phylogenicity of these warrants further investigation.
All the values of NH level variables (AMEPR, staff per 10 beds, and frequency of bathing per week) were determined by NH managers and the government. Therefore, NH-level variables cannot be considered to be an individual measure. Interestingly, we found cardio-cerebrovascular disease was associated with colonization in the univariate analysis, but not an independent risk factor. Prior hospitalization has previously been found to be a risk factor for colonization with ESBL producers [Reference Leistner29, Reference Bisson35], but this association was not supported in the present study. Many of these residents must have acquired the organism while resident in the homes, as they had no history of hospital admission and had been long-term NH residents. Contrary to expectation, the length of stay in the NH did not correlate with an increased likelihood of a positive sample. In fact, the median length of stay was a little longer in residents who were found not to carry such organisms. It could be argued that the days spent resident in a NH in which ESBL-E were not present are protective and it is likely that all days spent prior to the appearance of the organism in May 2014 were risk-free. Similar to clinical setting-based studies [Reference Kelley24, Reference Gaynes33], narrow- and broad-spectrum cephalosporins were independently associated with ESBL-E carriage.
This is the first analytical study to comprehensively investigate variables related to ESBL-E carriage at the facility and individual levels in multi-centres, which is also a major strength of this study. There are some limitations of this study other than those in sampling mentioned above. First, it was a cross-sectional study and it is impossible to conclude the causal relationship between the variables and final outcome. Second, it was performed in one district only instead of a random sampling from all NHs in this region, and characteristics of other NH residents may be different in different districts of Shanghai.
In conclusion, NH residents have a very high prevalence of faecal carriage of ESBL-E, indicating that the faecal flora in NH residents is a major reservoir for ESBL-E. Continuous surveillance to monitor this potential growing problem is important, as are prudent infection control measures and antibiotic use to prevent and control the spread of these antibiotic-resistant strains.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268815001879.
ACKNOWLEDGEMENTS
The authors thank Qian Yang (Shanghai Putuo Hospital), Long-di Gao (Shanghai Baiyu Community Health Service), Shou-Xi Lu (Shanghai Changshou Community Health Service), Hai-Min Yang (Shanghai Yichuan Community Health Service), Hui Chen (Shanghai Ganquan Community Health Service), Zhi Yang (Shanghai Tongpu Community Health Service) and He-Ping Zhang (Shanghai Shenxin Nursing Home) for their kind support and cooperation in collecting clinical data. The authors also thank Pak-Leung Ho and Wai-U Lo (Queen Mary Hospital, The University of Hong Kong) for their professional suggestions.
This work was supported by the Special Fund for Health – Scientific Research in the Public Interest of China Programme (grant no. 201002021) and the National Natural Science Foundation of China (grant no. 81472010).
DECLARATION OF INTEREST
None.







