Significant outcomes
Plasma neurofilament light chain (NfL), a marker of neuronal injury, was significantly elevated in Niemann Pick Type C (NPC), compared to healthy controls
Plasma glial fibrillary acidic protein (GFAP), a marker of astrocytosis, was not elevated in NPC compared to healthy controls
NfL distinguished NPC from controls, with high accuracy
Limitations
This retrospective study had a small sample size and lack of many serial samples, limiting interpretations of treatment effects and changes over time
Our cohort only included patients with several years of symptoms, therefore limiting interpretation of diagnostic utility at earliest stages of the disease
Introduction
Niemann-Pick Type C (NPC) is a rare, severe, genetic neurodegenerative lysosomal storage disorder, associated with highly variable age at onset and symptoms. NPC is commonly associated with psychiatric symptoms, high rates of misdiagnosis and delay until accurate diagnosis and treatment (Walterfang et al., Reference Walterfang, Fietz, Fahey, Sullivan, Leane, Lubman and Velakoulis2006; Rego et al., Reference Rego, Farrand, Goh, Eratne, Kelso, Mangelsdorf, Velakoulis and Walterfang2019; Berry-Kravis, Reference Berry-Kravis2021). While there are no specific blood or cerebrospinal fluid (CSF) biomarkers which assist in the diagnosis of NPC, biomarkers of neuronal injury (e.g., neurofilament light chain protein, NfL), and astrocytosis (e.g., glial fibrillary acidic protein, GFAP) may be useful as non-specific markers. Elevated NfL and GFAP levels have been identified in neurodegenerative conditions such as Alzheimer disease and frontotemporal dementia (Khalil et al., Reference Khalil, Teunissen, Otto, Piehl, Sormani, Gattringer, Barro, Kappos, Comabella, Fazekas, Petzold, Blennow, Zetterberg and Kuhle2018; Gaetani et al., Reference Gaetani, Blennow, Calabresi, Di Filippo, Parnetti and Zetterberg2019; Ashton et al., Reference Ashton, Janelidze, Al Khleifat, Leuzy, van der Ende, Karikari, Benedet, Pascoal, Lleó, Parnetti, Galimberti, Bonanni, Pilotto, Padovani, Lycke, Novakova, Axelsson, Velayudhan, Rabinovici, Miller, Pariante, Nikkheslat, Resnick, Thambisetty, Schöll, Fernández-Eulate, Gil-Bea, López de Munain, Al-Chalabi, Rosa-Neto, Strydom, Svenningsson, Stomrud, Santillo, Aarsland, van Swieten, Palmqvist, Zetterberg, Blennow, Hye and Hansson2021), and NfL has been shown to distinguish neurodegenerative from primary psychiatric and non-neurodegenerative conditions, and potentially reduce misdiagnosis (Ashton et al., Reference Ashton, Janelidze, Al Khleifat, Leuzy, van der Ende, Karikari, Benedet, Pascoal, Lleó, Parnetti, Galimberti, Bonanni, Pilotto, Padovani, Lycke, Novakova, Axelsson, Velayudhan, Rabinovici, Miller, Pariante, Nikkheslat, Resnick, Thambisetty, Schöll, Fernández-Eulate, Gil-Bea, López de Munain, Al-Chalabi, Rosa-Neto, Strydom, Svenningsson, Stomrud, Santillo, Aarsland, van Swieten, Palmqvist, Zetterberg, Blennow, Hye and Hansson2021; Eratne et al., Reference Eratne, Kang, Malpas, Simpson-Yap, Lewis, Dang, Grewal, Coe, Dobson, Keem, Chiu, Kalincik, Ooi, Darby, Brodtmann, Hansson, Janelidze, Blennow, Zetterberg, Walker, Dean, Berk, Wannan, Pantelis, Loi, Walterfang, Berkovic, Santillo and Velakoulis2023; Eratne, Keem, et al., Reference Eratne, Keem, Lewis, Kang, Walterfang, Farrand, Loi, Kelso, Cadwallader, Berkovic, Li, Masters, Collins, Santillo and Velakoulis2022; Eratne, Loi, et al., Reference Eratne, Loi, Li, Stehmann, Malpas, Santillo, Janelidze, Cadwallader, Walia, Ney, Lewis, Senesi, Fowler, McGlade, Varghese, Ravanfar, Kelso, Farrand, Keem, Kang, Goh, Dhiman, Gupta, Watson, Yassi, Kaylor‐Hughes, Kanaan, Perucca, Dobson, Vivash, Ali, O'Brien, Hansson, Zetterberg, Blennow, Walterfang, Masters, Berkovic, Collins and Velakoulis2022; Kang et al., Reference Kang, Eratne, Dobson, Malpas, Keem, Lewis, Grewal, Tsoukra, Dang, Mocellin, Kalincik, Santillo, Zetterberg, Blennow, Stehmann, Varghese, Li, Masters, Collins, Berkovic, Evans, Kelso, Farrand, Loi, Walterfang and Velakoulis2023).
Studies have found elevated CSF and plasma NfL levels in NPC compared to primary psychiatric conditions and controls (Eratne et al., Reference Eratne, Loi, Li, Varghese, McGlade, Collins, Masters, Velakoulis and Walterfang2020; Dardis et al., Reference Dardis, Pavan, Fabris, Da Riol, Sechi, Fiumara, Santoro, Ormazabal, Milanic, Zampieri, Biasizzo and Scarpa2021), and associations with severity and miglustat treatment (Agrawal et al., Reference Agrawal, Farhat, Sinaii, Do, Xiao, Berry-Kravis, Bianconi, Masvekar, Bielekova, Solomon and Porter2023). No studies have investigated GFAP in NPC, except one that included only two people with NPC as a comparison group, not finding elevated levels (Welford et al., Reference Welford, Farine, Steiner, Garzotti, Dobrenis, Sievers, Strasser, Amraoui, Groenen, Giugliani and Mengel2022).
This study aimed to compare NfL and GFAP levels in patients with NPC to age- and sex-matched controls, and to compare NfL levels to the reference ranges we recently developed (Eratne et al., Reference Eratne, Kang, Malpas, Simpson-Yap, Lewis, Dang, Grewal, Coe, Dobson, Keem, Chiu, Kalincik, Ooi, Darby, Brodtmann, Hansson, Janelidze, Blennow, Zetterberg, Walker, Dean, Berk, Wannan, Pantelis, Loi, Walterfang, Berkovic, Santillo and Velakoulis2023). Exploratory analyses investigated associations with clinical variables and explored biomarker levels in patients who had serial bloods.
Methods
Patients were recruited from a tertiary specialist NPC assessment and management service at Neuropsychiatry Centre, Royal Melbourne Hospital, Australia. Data was available from an age-matched healthy control group with no cognitive, neurological, or psychiatric symptoms or conditions, no known renal impairment or other severe medical conditions. Plasma aliquots were collected, processed, and stored at −80°C. Plasma NfL and GFAP levels were measured on Quanterix Simoa HD-X analysers.
Statistical analyses were performed using R v4.2.2 (2022-10-31). Generalised linear models (GLMs) were used to examine relationships with log10-transformed biomarker levels, diagnostic group, age and sex as covariates, with 95% confidence intervals (nonparametric bootstrapping, 1000 replicates). Receiver operator characteristic curves were computed to estimate diagnostic performance, area under the curve (AUC), sensitivity, and specificity. For more precise exploration of NfL levels, levels were compared to and percentiles derived from reference ranges developed using generalised additive models for location, scale, and shape, previously described (Eratne et al., Reference Eratne, Kang, Malpas, Simpson-Yap, Lewis, Dang, Grewal, Coe, Dobson, Keem, Chiu, Kalincik, Ooi, Darby, Brodtmann, Hansson, Janelidze, Blennow, Zetterberg, Walker, Dean, Berk, Wannan, Pantelis, Loi, Walterfang, Berkovic, Santillo and Velakoulis2023).
This study, part of The Markers in Neuropsychiatric Disorders Study (The MiND Study, https://themindstudy.org), was approved by the Melbourne Health Human Research Ethics Committee (MH/HREC2020.142).
Results
There were 11 patients with NPC, all of whom had neurological symptoms, and 25 controls. There were no differences in age (mean age 33.9 years vs. 40.7, p = 0.098; median 42.9 (range 25–49) vs. 41.3 (17–48)) or sex (63.6% vs. 80%, p = 0.409). Four out of 11 (36%) patients had a juvenile onset; 7/11 (64%) were on treatment (details in Table 1). The mean duration of illness was 12.2 years (median 10, range 4–23) with no differences between juvenile and adult onset, or between younger age (<30 years) or older (>30 years).
Table 1. Study demographics and plasma biomarker levels

Data is mean (SD) or n (%). GFAP, glial fibrillary acidic protein; NfL, neurofilament light chain protein.
Plasma NfL and GFAP in Niemann-Pick Type C compared to controls
NfL levels were significantly higher in patients with NPC compared to controls (mean 17.1 vs. 7.4 pg/ml, GLM for logNfL: β = 1.81, 95% confidence interval: [1.50, 2.22], p < 0.001), Table 1 and Fig. 1. As demonstrated in Fig. 2, 100% of patients with Niemann-Pick Type C had significantly elevated NfL levels for their age: all were >98th percentile (z-score >2); 7/11 (64%) were >99th percentile (z-score >2.5).

Figure 1. Plasma neurofilament light chain protein (left) and glial fibrillary acidic protein (right) levels in patients with Niemann-Pick Type C and controls. + = mean level.

Figure 2. All patients with Niemann-Pick Type C had significantly elevated neurofilament light chain protein (NfL) levels for their age (all >98–99th percentile), using our previously developed interactive plasma NfL reference range app (themindstudy.org/apps).
By comparison, GFAP levels were not elevated in NPC compared to controls (66.6 vs. 75.1 pg/ml, β = −0.09 [−0.83, 0.63], p = 0.790).
Plasma NfL had high diagnostic accuracy to distinguish NPC from controls (AUC 0.96 [0.91, 1.00]). An optimal cut-off of 9.35 pg/mL was associated with 84% specificity, 100% sensitivity. A cut-off of 10.47 pg/mL resulted in better specificity (92%), but slightly reduced sensitivity (91%). GFAP did not have diagnostic utility (AUC 0.57 [0.38, 0.76]).
Exploratory analyses
Exploratory analyses investigated biomarker levels differences between treated versus untreated patients, different types of treatment, and juvenile versus adult onset. No differences were seen.
Three patients had serial bloods, as illustrated in Fig. 3. Over a two-year period, NfL levels in patient A (on miglustat and IV hydroxypropyl-beta-cyclodextrin (Cyclo Therapeutics, “cyclodextrin”) increased only slightly, by 11% (from 9.6 to 10.7 pg/ml), while GFAP increased by 36% (from 88.8 to 121 pg/ml). On the other hand, patient B (only on miglustat and acetylleucine), exhibited a 50% increase in NfL over 2 years (10.6 to 16 pg/ml), and a 76% increase in GFAP (65.3 to 114.8 pg/ml). Patient C (on acetylleucine only), had a significant neurological deterioration requiring hospitalisation at the time of their second blood sample. Their second NfL level (58 pg/ml) was markedly higher than a year prior (20 pg/ml), and GFAP increased by 28% (51.4 to 66 pg/ml).
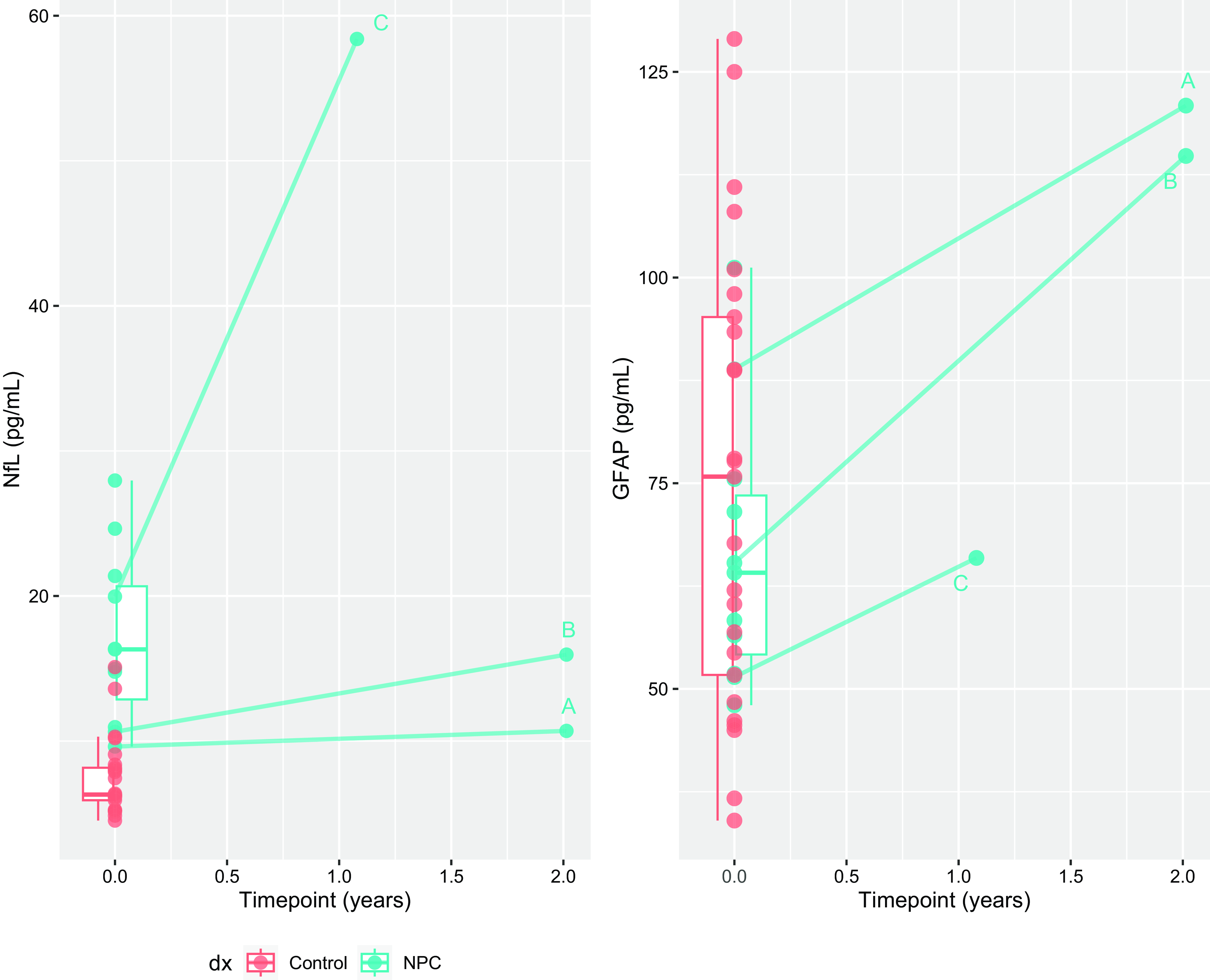
Figure 3. Longitudinal changes in neurofilament light chain protein and glial fibrillary acidic protein in three patients.
Discussion
This study found significantly elevated plasma NfL levels in NPC compared to controls, and NfL distinguished NPC from controls with high accuracy. These findings add important evidence on the potential diagnostic utility of plasma NfL in this devastating condition that is not uncommonly misdiagnosed as a primary psychiatric condition. In addition, this study provides an important negative finding of plasma GFAP in the largest number of NPC patients to date.
The differing profile of NfL and GFAP adds weight to the pathology in NPC primarily involving neuronal, and particularly axonal, degeneration (Walterfang et al., Reference Walterfang, Fahey, Desmond, Wood, Seal, Steward, Adamson, Kokkinos, Fietz and Velakoulis2010). It is likely that varying profiles of these biomarkers will be seen in different disorders, providing important information on the underlying pathophysiological processes in various conditions and within subgroups of syndromes, and combination biomarkers could have diagnostic and wider clinical utility in various conditions and differential diagnoses.
Secondary, purely exploratory analyses did not find any differences in NfL and GFAP levels between untreated and treated patients, however the main limitation of this study is the small sample size and lack of serial levels, which limit any confident interpretations of treatment effects and changes over time. Three patients with serial levels had large increases in GFAP levels over 1–2 years. One of these two patients was on IV cyclodextrin and did not have much increase in NfL, whereas the other had a more marked increase. This may reflect a treatment effect of IV cyclodextrin on neuronal injury, however, this was not seen for intrathecal cyclodextrin in a larger study (Agrawal et al., Reference Agrawal, Farhat, Sinaii, Do, Xiao, Berry-Kravis, Bianconi, Masvekar, Bielekova, Solomon and Porter2023). The increase in GFAP over time is notable, possibly pointing to differing timelines of NfL and GFAP changes in the disease course, with NfL changing earlier, and GFAP changing later, perhaps after neuronal loss has occurred. However, the serial GFAP levels, although increased compared to baseline, were still within the control range. Patient C’s dramatic increase in serial NfL levels corresponded with severe neurological deterioration. This adds weight to NfL reflecting severe/acute deterioration and severity/prognosis/rate of deterioration, on top of being diagnostic. Our cohort included patients with several years of symptoms, therefore we cannot conclude the diagnostic utility at the earliest stages of the disease. It would be important to investigate serial biomarkers and associations with other variables in larger, well characterised cohorts.
To conclude, this study found significantly higher plasma NfL levels in NPC and demonstrated strong diagnostic utility of plasma NfL to distinguish NPC from controls, while not finding elevated GFAP levels. Other plasma biomarkers can complement the investigation in case of an elevated NfL level, as more specific testing for NPC, before genetic testing, for example: N-palmitoyl-O-phosphocholineserine, cholestane-3β,5 α,6β-triol (C-triol), 7-ketocholesterol. (Jiang and Ory, Reference Jiang and Ory2021) This extends the literature on NfL to identify neurological/neurodegenerative causes of neurological and neuropsychiatric symptoms, especially in younger people where diagnostic challenges and misdiagnosis can be higher, and adds to our understanding of the pathophysiology and utility of biomarkers in NPC for diagnosis and potentially wider clinical utility. Further studies are underway in a larger cohort, to investigate NfL, GFAP, and other biomarkers and associations with clinical and treatment variables.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/neu.2024.14.
Acknowledgements
We are grateful for funding that supported this work: NorthWestern Mental Health Research Seed Grants, the CJDSGN Memorial Award in memory of Michael Luscombe, and MACH MRFF RART 2.2 and NHMRC (1185180) funding. The role of these funding sources was to support participant recruitment, research study staff, and biosample analyses.
Finally, the authors would like to thank all the patients and their families for their participation.
The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
A.F.S is primarily funded by the Swedish federal government under the ALF agreement (ALF 2022 YF 0017) and The Åke Wiberg Foundation.
Author contribution
Dhamidhu Eratne — Study design and coordination, data collection, participant recruitment, data analysis and interpretation, literature search, statistical analysis, writing manuscript.
Courtney Lewis — Study coordination, data collection, participant recruitment, writing reviewing manuscript.
Wendy Kelso — Data interpretation, participant recruitment, writing and reviewing manuscript.
Samantha Loi — Data interpretation, writing and reviewing manuscript.
Wei-Hsuan Michelle Chiu — Data collection, reviewing the manuscript.
Kaj Blennow — Data interpretation, reviewing the manuscript.
Henrik Zetterberg — Data interpretation, reviewing the manuscript.
Alexander F Santillo — Data interpretation, writing and reviewing manuscript.
Dennis Velakoulis — Data interpretation, writing and reviewing manuscript.
Mark Walterfang — Study design and coordination, participant recruitment, data interpretation, writing and reviewing manuscript.
Financial support
HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2023-00356; #2022-01018 and #2019-02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation, USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI-1003).
Competing interests
HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, which is a part of the GU Ventures Incubator Program (outside submitted work).
None of the other authors have anything to disclose.
Statistical analysis conducted by: Dr Dhamidhu Eratne.







