Current evidence suggests that approximately half of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections are due to transmission from symptom-free individuals. Reference Zou, Ruan and Huang1,Reference Bai, Yao and Wei2 Healthcare workers (HCWs) may have an increased risk of SARS-CoV-2 infection, although it is also possible that the risk of infection among HCWs might be similar to community risk, as was recently reported in New York. Reference Voytko3
Currently, data on the point prevalence of infection among symptom-free HCWs are limited. Given the evidence that patients are avoiding medically necessary healthcare visits for fear of becoming infected, prevalence estimates in HCWs can inform the potential risk that a patient might encounter an infected symptom-free HCW. Reference Wong, Hopkins, Langness, Murrell, Iris and Sammann4
To address this question, we screened symptom-free HCWs for SARS-CoV-2. Additionally, to preserve personal protective equipment (PPE), we implemented a protocol for self-collection of nasopharyngeal swabs (NPSs) and surveyed participants about their perceived quality of a self-collected versus provider-collected NPSs.
Methods
A convenience sample of individuals working in Minnesota healthcare facilities located in the Minneapolis–St Paul metropolitan area were enrolled. Participants were identified via social media advertisements and enrolled from April 20 to June 24, 2020. We applied the following eligibility criteria: (1) employed or volunteering in a healthcare facility; (2) free of fever, chills, anosmia, pharyngitis, recently developed persistent cough, nasal congestion suspected to be unrelated to season allergies; (3) aged 18–80 years; and (4) not pregnant. In total, 489 participants provided self-collected NPSs (Fig. 1). The study was approved by the University of Minnesota Institutional Review Board. All participants provided informed consent.
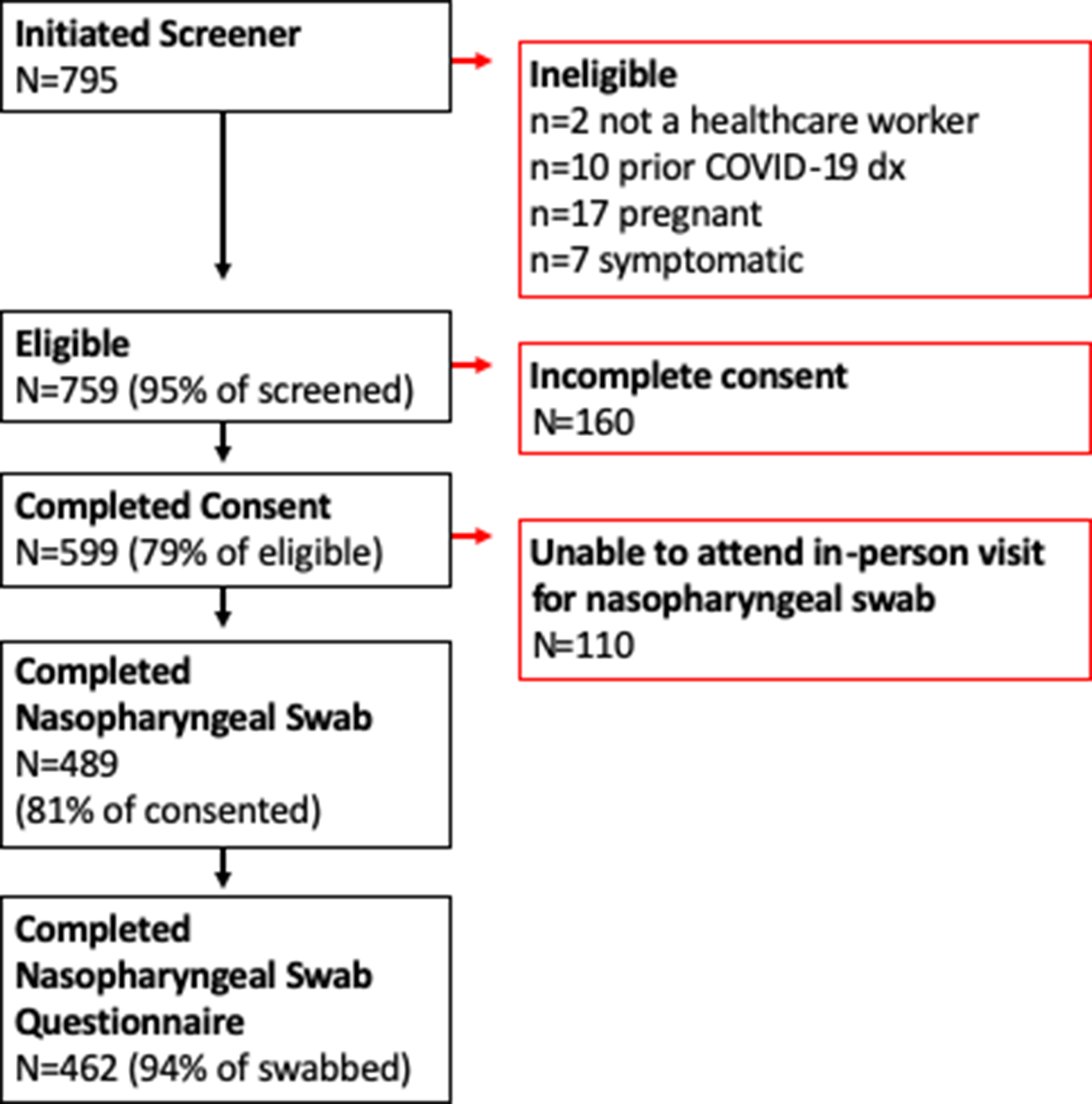
Fig. 1. Participant flow diagram.
Participants advanced a nylon flocked-tip NPS through the nasal passage (bilaterally) 7.6–10 cm (3–4 inches) into the nasopharynx and swirled the swab 360° for 5 seconds. The swab tip was preserved in 3 mL 95% ethanol, immediately placed on an ice bath, and transferred to a −80°C freezer. Samples were shipped overnight on dry ice to University of California–San Diego.
Samples were processed within 48 hours of receipt at UC San Diego. Total nucleic acid was extracted from the swab heads using the MagMAX Microbiome Ultra Nucleic Acid Isolation Kit (no. A42357, Thermo Fisher Scientific, Waltham, MA) and eluted in 100 µL nuclease-free H2O. SARS-CoV-2 screening was performed using the one-step Applied Biosystems TaqPath COVID-19 Combo Kit (no. A47814, Applied Biosystems, Waltham, MA) following the manufacture’s protocol with the following exceptions. The reaction volume was scaled down to 3 µL with proportional reagent scaling and replacement of ∼94% of the water with participant RNA. Additionally, the MS2-phage spike-in control was diluted 160-fold to improve sensitivity through reducing competition for reagent material within the multiplex RT-qPCR reaction. Samples were prepared in 384-well reaction plates using a mosquito HV Robotic Liquid Handler (SPT Labtech, Melbourn, UK) and a mosquito X1 (HV) Robotic Liquid Handler (SPT Labtech). The RT-qPCR was analyzed in a QuantStudio5 qPCR instrument (Thermo Fisher Scientific). Positive controls for each SARS-CoV-2 target amplified as expected, as well as all MS2 sample controls. None of the negative controls amplified.
Prior to swabbing, participants completed online surveys. After the NPS procedure, participants were queried about their perception of the swabbing procedure relative to NPS they have performed on patients. They reported their level of discomfort with the self-swab on a scale of 1 (no discomfort) to 10 (the most discomfort they have ever experienced), and their likelihood of repeating a self-collected NPS for clinical or research purposes.
Descriptive characteristics are reported as mean (±SD) for continuous variables and number (%) for categorical variables. We used bivariate analyses, t tests, and χ Reference Bai, Yao and Wei2 tests to assess statistical significance. We had >95% power to detect at least 1 positive test if the true underlying prevalence of SARS-CoV2 was ≥1%.
Results
Among 489 participants enrolled, the mean age was 41 (±11) years and 80% were female. All participants worked in facilities located in the 7-county Minneapolis–St Paul metropolitan area. The average number of people living with participants was 2 (SD±1.4) and 12% reported living alone. The average number of children living with participants was 0.9 (±1.1), and 50% reported having at least 1 child at home.
The average time between NPS collection and laboratory testing was 36 (±18) days (range, 2–68). SARS-CoV-2 was not detected in any sample.
In the 14 days prior to enrollment, 40% of participants reported a known COVID-19 exposure. This proportion varied by venue (P < .0001) and role (P < .01) (Table 1). PPE use was high with only 1.4% of participants reporting no PPE use and this occurred among individuals without patient contact.
Table 1. General Characteristics of 488 Minnesota Healthcare Workers a According to COVID-19 Exposures Within 14 Days Preceding Enrollment b
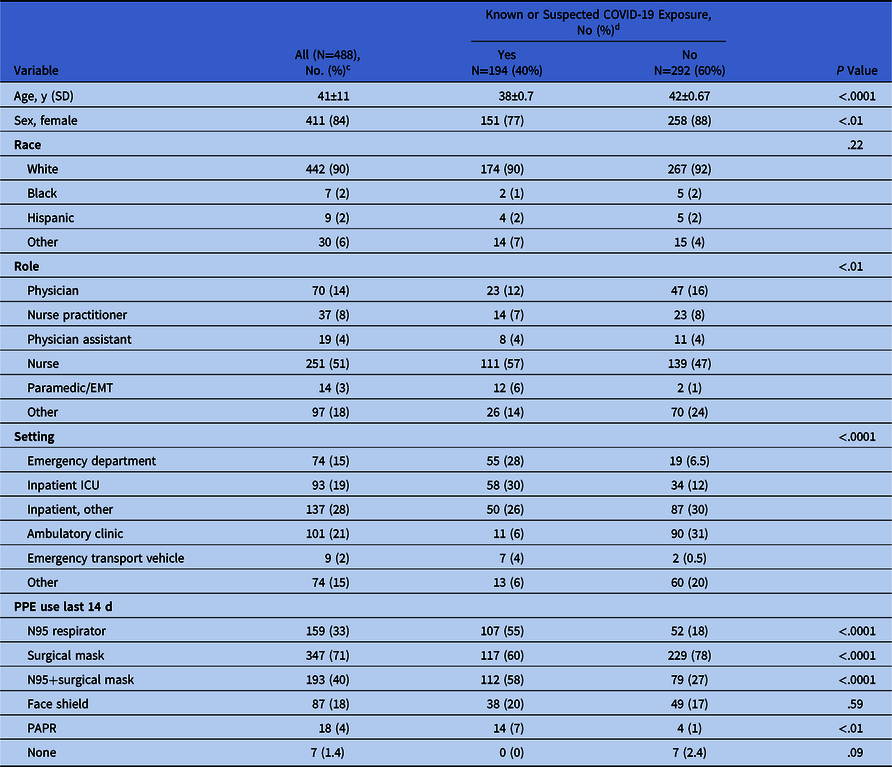
Note. SD, standard deviation; SE, standard error; EMT, emergency medical technician; ICU, intensive care unit; PPE, personal protective equipment; PAPR, powered air-purifying respirator.
a 489 received an NPS, but survey data were not available for 1 participant who did not complete the questionnaire and was excluded from the table.
b Enrollment occurred between April 20 and June 24, 2020.
c Data presented as mean±SD.
d Data presented as mean±SE.
The mean score for discomfort related to the self-collected NPS was 4.5 (±2.0; range, 1–10). Among the 287 participants (62%) who reported performing an NPS on a patient, 89% indicated that their self-swabbing depth was greater than or equal to the depth of prior patient swabs, and 95% reported that their self-swab was greater than or equal to the duration of previous patient swabs. More than 95% of participants reported a willingness to repeat a self-collected NPS for either clinical or research purposes; 24% preferred a provider-collected swab, 57% preferred self-collection, and 19% reported no preference.
Discussion
The major finding in this study was the lack of any SARS-CoV-2–positive individuals among a convenience sample of symptom-free HCWs. Based on our power calculations, this strongly suggests that the point prevalence of SARS-CoV-2 in our study sample of symptom-free HCWs was <1%. This finding is consistent with results in the US population and in Minnesota during the period these samples were collected. National seroprevalence estimates reported by the Centers for Disease Control ranged from 1% to 7%, and the estimate from Minnesota during the period from April 20 to May 12, 2020, was 2.2%. Reference Havers, Reed and Lim5 Low SARS-CoV-2 point prevalence in HCWs, despite increased relative risk for infection compared to the general population, Reference Nguyen, Drew and Graham6 is plausible because HCWs are prioritized to receive PPE and are trained in infection control. Our findings from the self-collected NPS survey suggest that the self-collection of NPS was acceptable to HCWs and that the collections were properly done.
The sensitivity of our screening tests might have been low due to the use of self-collected NPSs, although recent studies report self-collection protocols to have acceptable sensitivity. Reference Altamirano, Govindarajan and Blomkalns7,Reference Tu, Jennings and Hart8 Tests among symptom-free individuals could also have reduced sensitivity; however, prior studies in asymptomatic pregnant women Reference Sutton, Fuchs, D’Alton and Goffman9 and residents of long-term care facilities Reference Bigelow, Tang and Barshick10 have detected high SARS-CoV-2 prevalence. More research is necessary to validate PCR tests among symptom-free individuals, although preliminary findings from the SalivaDirect test, which has Federal Drug Administration emergency use authorization, are encouraging (https://www.fda.gov/media/141194/download). Because we used a convenience sample, our sample is not representative of all HCWs in Minnesota, nor is it representative of what future SARS-CoV-2 prevalence estimates might be among symptom-free HCWs in settings with high community prevalence. Our sample was largely comprised of individuals working in acute-care settings, and representation from long-term care facilities, where infection risk has been notably higher in Minnesota, was limited.
Our results suggest that even though the HCWs are very likely to be at increased relative risk for infection compared to the general population, Reference Nguyen, Drew and Graham6 the point prevalence of SARS-CoV-2 infection was low in symptom-free Minnesota HCWs. If true, the probability of encountering an infected symptom-free HCWs during a medically necessary healthcare visit is likely low when community point prevalence is low. Self-collected NP swabs are acceptable to participants. Ongoing monitoring of infection in HCWs will be important as the pandemic progresses and community transmission rises across the country.
Acknowledgments
We are profoundly grateful for the study participants who donated valuable time to advance our understanding about SARS-CoV-2 prevalence in HCWs. This study was also made possible by Prof. Jian Xu (Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Science), who generously donated nasopharyngeal swabs.
Financial support
This study was supported by funding from the University of Minnesota Office of the Vice President for Research, by the Minnesota Population Center (funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Population Research Infrastructure Program (grant no. P2C HD041023) and by the University of Minnesota’s National Institutes of Health (NIH) Clinical and Translational Science Award (grant no. UL1TR002494). Dr Ulrich was supported by a grant from the NIH (grant no. T32AI05543315).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.





