INTRODUCTION
The loss of cognitive capacity is not a function of aging itself, but rather a marker of accumulated neuropathological changes over time.Reference Meier, Gu and Guzaman 1 First described by Charcot and BouchardReference Bouchard 2 , Reference Fagge 3 as “miliary” aneurysms, cerebral microbleeds (MBs) are neurobiological markers of interest. The accumulation of MBs has been associated with decreased cognition in hypertensive patients,Reference Huijts, Duits, van Oostenbrugge, Kroon, de Leeuw and Staals 4 and the presence of lobar MBs has been associated with Alzheimer’s disease (AD)Reference van Rooden, Goos and van Opstal 5 resulting in prognostic significance.Reference Goos, van der Flier and Knol 6 - Reference Hommet, Mondon and Constans 9 Additionally, MBs are indirectly affecting cognitive functioning as a result of antithrombotic therapy in the cases of atrial fibrillation and strokesReference Vernooij, Haag and van der Lugt 10 or cerebral amyloid angiopathy.Reference Greenberg, Al-Shahi Salman and Biessels 11 , Reference Ringman, Sachs, Zhou, Monsell, Saver and Vinters 12 Likewise, MBs are considered to be an amyloid related imaging abnormality and are one of the exclusion criteria for entry into experimental amyloid-lowering therapies.Reference Sperling, Jack and Black 13 Also, an increasing number of MBs is a strong predictor of mortality in AD.Reference Henneman, Sluimer and Cordonnier 14
MBs in AD are thought to contribute to the pathophysiology of the illnessReference Goos, van der Flier and Knol 6 and are identified in all cortical regions, infratentorially, and within the basal ganglia. Although their presence is associated with localized tissue damage, disruption of white matter structural integrity,Reference Akoudad, Portegies and Koudstaal 15 and reduced cerebral blood flow,Reference Gregg, Kim and Gurol 16 they have been thought as clinically silent in AD because the nature of their contributions to global cognition in AD is thought to be negligible,Reference Cordonnier and van der Flier 8 or at most unclear.Reference van der Flier 7 - Reference Hommet, Mondon and Constans 9 The lack of a significant effect has been speculated to be due to small sample size,Reference Pettersen, Sathiyamoorthy and Gao 17 low MB counts, or severity of AD masking the subtle effect of MBs on cognition.Reference Hommet, Mondon and Constans 9 Additionally, heterogeneous classifications and lack of validation of the definition of presence and prevalence of MBs have obscured the potential relationships between neurocognitive functioning and MBs in AD. Furthermore, insensitivity of the assessment scales used to detect subtle cognitive deficits may have masked this effect. Many studies assessing global cognition in AD used the Mini Mental State Examination scale (MMSE), which is impervious to small subtle cognitive alterations.Reference Clark, Sheppard and Fillenbaum 18 , Reference Feher, Mahurin, Doody, Cooke, Sims and Pirozzolo 19 However, literature in the Mild Cognitive Impairment (MCI) and aging literature suggest that MBs tend to impact cognitive functions,Reference Martinez-Ramirez, Greenberg and Viswanathan 20 including processing speed, motor speed,Reference Poels, Ikram and van der Lugt 21 attention-related impairment,Reference van Norden, van den Berg, de Laat, Gons, van Dijk and de Leeuw 22 memory,Reference Staekenborg, Koedam and Henneman 23 executive processing,Reference Meier, Gu and Guzaman 1 and visuoconstructional function,Reference Hilal, Saini and Tan 24 and that MCI in the presence of MBs is potentially a predictor of progression to either AD or dementia.Reference Kirsch, McAuley and Holshouser 25 , Reference Ayaz, Boikov, Haacke, Kido and Kirsch 26
Because neuropsychological assessment plays a role in differential diagnosis and MBs potentially in cognitive impairment,Reference Martinez-Ramirez, Greenberg and Viswanathan 27 better understanding of MBs on neuropsychological functioning in AD is a critical next step. The prevalence of MBs in AD in light of the neuroimaging, demographic, and clinical moderating factors was recently appraised.Reference Sepehry, Lang, Hsiung and Rauscher 28 This meta-analysis showed that MB prevalence varies as a function of AD diagnosis, in that MB prevalence is higher for probable AD than for possible AD. Between studies, the strongest modifier of MB prevalence was neuroimaging modality, with susceptibility-weighted imagingReference Reichenbach, Venkatesan, Schillinger, Kido and Haacke 29 being twice as sensitive as conventional gradient echo magnetic resonance imaging (MRI).
Currently, little is known about the neuropsychological impact of MB in AD. To date, most cross-sectional studies examining the impact of MBs on cognition in AD have has a small sample size. The difference between global cognitive scores in AD with and without MB is unclear. In this review and meta-analysis, we summarize findings and make sense of the dispersed knowledge about the neuropsychological impact of MBs in AD. We sought to evaluate potential diagnostic and demographic moderating variables on the impact of MBs on global cognitive function.Reference Akoudad, Portegies and Koudstaal 15 , Reference Heringa, Reijmer and Leemans 30
MATERIALS AND METHODS
Search Strategies
Search of Medline and EMBASE (on Ovid platform) was carried out on May 15, 2015, using a priori determined key terms. The following search strategy was used to identify study abstracts: [((Microbleed* or microhemorrhage or “petechial haemorrhage” or hemosiderin or “Cerebral amyloid angiopathy” or Cerebral Hemorrhage).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier)) AND ((Alzheimer’s disease or Dementia or Alzheimer*).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier) AND (Cognitive or cognition or neuropsychology or neuropsychological).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]. Additionally, we examined the bibliographic sections of review papers relevant to the topic, and we referred to a meta-analysis conducted by our team on the prevalence of MBs in AD to substantiate our search of the literature.Reference Sepehry, Lang, Hsiung and Rauscher 28
Data Analysis Methods
Effect size [standardized mean difference, also known as Hedges’ (adjusted) g, ES] was generated using mean, standard deviation, and sample size to examine the magnitude of group differences between AD with and without MBs, AD with MBs and healthy controls with MBs on neurocognitive functions. Between-study heterogeneity was examined using the standard method of Q-value with p value and I2 to determine the existence and extent of variability. Based on current research theories, the extent of heterogeneity was examined. Publication bias was assessed by funnel plot and via quantitative analysis. The Begg and Mazumdar rank correlation testReference Begg and Mazumdar 31 and Egger’s test of the intercept testsReference Egger, Davey Smith, Schneider and Minder 32 were used to quantitatively appraise possible bias. A significant p value for both Begg and Mazumdar and Egger’s test is suggestive of bias. We have set to include cross-sectional studies examining cognition in AD with MBs versus AD without MBs or healthy control with MBs. We have excluded studies that included atypical AD such as frontal presentation or posterior cortical atrophy. Neuropsychiatric symptoms were examined if reported.
RESULTS
Study Selection Outcome
The search of Medline and EMBASE on Ovid platform revealed 118 possible studies after duplicates were removed. Four additional studies that reported on cognitive functioning in AD with MBs emerged from reading of review papers and screening of the bibliographic section of the included studies. From a total of 122 studies, six met selection criteria and were included in the meta-analysis (supplementary Figure 1).
Descriptive Statistics
Six studiesReference Pettersen, Sathiyamoorthy and Gao 33 - Reference van der Vlies, Goos, Barkhof, Scheltens and van der Flier 38 compared a total of 194 AD patients with MB (AD+MB) to 601 AD patients without (AD-MB) on global cognitive functioning as assessed via MMSE. Among them, two studiesReference Pettersen, Sathiyamoorthy and Gao 33 , Reference Goos, Kester and Barkhof 34 compared AD+MB to AD-MB on multiple cognitive functions. The neuropsychological tests included the Dementia rating scale, the Boston naming test, semantic fluency, phonemic fluency, the Wisconsin card sort test, the California verbal learning test, the Wechsler memory scale-revised for logical memory and delayed recall, the Boston judgment of line orientation, trail making test A and B, the western Aphasia battery with the apraxia subset, the Visual Association Task for object naming, and digit span forward and backward. These studies combined included 44 AD+MB and 99 AD-MB.
One studyReference Pettersen, Sathiyamoorthy and Gao 33 compared AD+MB (n =23) with healthy controls (n =25) without cognitive impairment or vascular risk factors on a battery of neuropsychological tests assessing executive, global, language, memory, and attention functioning; and AD-MB (n =57).
Globally, 59% to 100% of the MBs were reported to be lobar in location, and one study reported patients with mixed MB locations without reporting the percentage.Reference Nakata-Kudo, Mizuno and Yamada 36 In terms of MB criteria, with the exception of one study,Reference Goos, Kester and Barkhof 34 most included patients with 1 MB or more. All but one studyReference Nakata-Kudo, Mizuno and Yamada 36 included patients with a diagnosis of probable AD. These studies reported susceptibility weighted imaging (SWI), Gradient echo (GE), and gradient echo Echo Planar Imaging (EPI) for detection of MB. The year of publication for the included studies ranged from 2006 to 2013 (Tables 1 and 2).
Table 1 Studies comparing AD with and without MB on neuropsychological functioning (N =6)
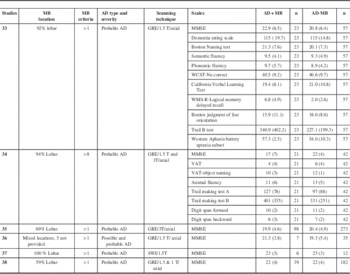
AD = Alzheimer’s disease; CVLT = California Verbal Learning Test; GE = gradient echo; GRE = gradient echo EPI; SWI = susceptibility weighted imaging; MB: Microbleed; MMSE: Mini Mental State Examination; VAT = visual association task; WCST = Wisconsin Card Sort Test; WMS = Wechsler Memory Scale. No study reported neuropsychiatric evaluation.
Table 2 Comparing mean cognitive scales scores between AD with MB and healthy control

Note: CVLT = California Verbal Learning Test; MB = microbleeds; WCST = Wisconsin Card Sort Test; MMSE = Mini Mental State Examination; WMS = Wechsler Memory Scale. With the exception to aphasia rating, the different between-mean scores on the neurocognitive scales are nearly from 1 to 3.2 standard deviation unite. Cut of 1 standard difference in mean was used to separate degree of differences, where below 1 was considered mild, between 1 and 2 was considered moderate, and 2 and higher was considered severe cognitive difference.
Neuropsychological Findings: Global Cognition
The aggregate standard difference in means between AD+MB and AD-MB for global cognitive functioning as measured by the MMSE was nonsignificant and heterogeneous (Q-value =12.744; degrees of freedom =5; p =0.026; I2 =60.766); Figure 1. Heterogeneity, publication bias, and moderating variables are discussed in detail later.
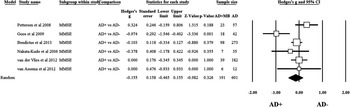
Figure 1 Forest plot showing standardized mean difference on MMSE scores for AD+MB and AD-MB.
For other cognitive domains, no aggregate can be generated given the low number of studies available for meta-analysis. However, these studies are descriptively examined (Figure 2).
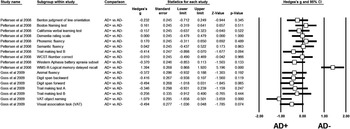
Figure 2 Presentation of the standard difference in means per scales and studies. AD = Alzheimer’s disease; AD+ = AD with MBs; AD- = AD without MBs; CVLT = California Verbal Learning Test: MB = microbleed; MMSE = Mini Mental State Examination; N: number of studies, VAT = visual association task; WCST = Wisconsin Card Sort Test; WMS = Wechsler Memory Scale.
Neuropsychological Findings: Individual Cognitive Function
Goos and colleagues reported that in 21 patients with and 42 without MBs, MBs were associated with cognitive dysfunction.Reference Goos, Kester and Barkhof 34 They found that the MB group performed worse on test of language functioning including Visual Association Task-object naming and animal fluency. There was a difference in the neuropsychological tests results between unadjusted and adjusted analyses for age, sex, medial temporal lobe atrophy, and white matter hyperintensities. After adjustment, patients with multiple MBs performed worse on language functioning tasks and tests of working memory including digit span (forward and backward) than the group without MBs (p < 0.05). Additionally, no significant associations between age and sex, with any of the neuropsychological measures, were reported.Reference Goos, Kester and Barkhof 34 This suggests that not the demographic factors, but potentially other factors contribute to the presence or absence of cognitive impairment resulting from MBs in AD including medial temporal lobe atrophy and white matter hyperintensities. No significant group difference was observed on psychomotor speed (trail A) or executive functioning (trail B) in this study. The results of this study are striking given that this study included AD patients with eight or more MBs, which is suggestive of more severe angiopathy in line with vascular dementia or possible AD, rather than probable AD.
Pettersen and colleagues (33) reported on patients with AD+MB (n=23) in comparison to healthy controls (n=25) matched (age, sex, education). They reported on an AD sample with MBs consisting of lobar predominance in 92% of AD patients, with 57% of MBs being localized to occipital regions. Among controls, the occipital lobe was also the most common location for MBs. Nonetheless, because of sample size and test sensitivity, the authors suggested that they were unable to demonstrate an association between MBs and cognitive performance on individual domains. For cognitive comparison between AD+MB and healthy adults as reported by Pettersen and colleagues, impairment was evident in the AD+MB group on various functions and the deficits ranged from mild to severe, as examined by standard mean difference between two groups.
Publication Bias, Heterogeneity, and Moderating Variables
The results of the quantitative analyses were nonsignificant at alpha 0.05, suggesting the absence of bias. There was between-study heterogeneity in the results as observed by the I2 value (I2 > 50%); one study seemed to act as an outlier given the use of more stringent criteria for MBs (e.g. >8). After exclusion of this study from the global analysis, the global effect-size estimate was no longer heterogeneous (I2=0.00).
Neither MRI scanning modality nor AD diagnosis significantly affected the effect-size estimate for global cognition. For scanning modality, no analysis was done for SWI (N=1), but for gradient echo EPI (N=5) a small effect-size estimate was obtained (Hedges’ g=−0.174; 95% confidence interval: −0.517 to 0.169; p value: 0.320; N=5; Q-value: 12.689; p-value: 0.013; I2: 68.476). For AD diagnosis, a lower effect-size estimate was obtained for the groups reporting on probable AD (N=5) than for probable and possible AD (ES=−0.132 and −0.378, respectively).
Mixed-effect univariate meta-regression was nonsignificant for percent lobar distribution, the year of publication, and imaging parameter (the field strength) on the global cognition as assessed by MMSE (p=0.73, 0.66, and 0.862, respectively).
Of note, other variables such as subject inclusion, including AD+MB patients with variable number of bleeds (anywhere from one to many MBs), would have affected the differences in global cognitive functioning between groups. In the studies included here, individuals with variable numbers of bleeds were enrolled in the AD+MB groups. Given the low number of studies available for analysis, further investigation of this factor was not performed because of the low probability of a reliable finding.
DISCUSSION
We have observed diversity across studies regarding differences between AD patients with and without MBs with respect to global cognition as assessed by MMSE. This variation in these differences appears to be the result of criteria of inclusion of MBs.
The identification of only one or more potential MBs is likely too low a threshold for inclusion given the concerns regarding accurate MB identification associated with imaging limitations. A better quantification approach will be to account both number and severity (size in diameter and location) of MBs instead of binary cutoff. Because the identification of MBs was based solely on visual ratings across these studies, image quality is a significant concern in the accurate identification of MBs. Although automated approaches exist at this time, this may not improve accurate identification of MBs because clinical judgement is required to differentiate true MBs from other susceptibility or flow effects in MRI. Accurate MB identification is strongly affected by imaging techniques. In our previously published work,Reference Sepehry, Lang, Hsiung and Rauscher 28 , Reference Denk and Rauscher 39 SWI was determined to be the optimal imaging approach for MB identification. Here, only one of six of the currently reviewed studies employed SWI.
We found that none of the studies controlled for the effect of neuropsychiatric symptoms such as depression, which are likely to obfuscate the very subtle effects of MBs on cognitive impairment assessed by MMSE.Reference Feng, Fang, Xu, Hua and Liu 40 Previously published data indicated that depressive symptoms are linked to cerebral small vessel disease such as MBs,Reference van Sloten, Sigurdsson and van Buchem 41 even in the presence of silent brain infarct.Reference Wu, Feng, Xu, Hua, Liu and Fang 42 This may suggest that the lack of difference between the homogeneous studies reporting on more than one MB is due to multiple reasons, and assessment of depression or other neuropsychiatric symptoms in AD when examining for MBs is recommended.
The major limitation of our study is the small number of included studies, especially those looking at MBs in multiple cognitive domains. We acknowledge that the research of neurocognitive functioning in relation to MBs in AD is relatively at an embryonic stage and it is typical to see few studies. How accurately the effects of MBs on individual cognitive domains can be interpreted remains challenging. Additionally, it is possible that MBs are just a by-product of amyloidosis present in AD and do not significantly affect cognitive functions. It is also possible that cognitive deficit in AD is overwhelmingly driven by AD pathology, and the emergence of MBs has comparatively minimal effects (compared with a vascular dementia case without underlying AD). These limitations warrant future studies. Furthermore, the number of subjects enrolled in these studies was limited, and that has potentially hindered further examination of the association between localized microbleeds (e.g. occipital lobes)Reference Pettersen, Sathiyamoorthy and Gao 17 and cognitive performance in specific domain (e.g. visuospatial function). Although, as seen via meta-regression that we have found no positive effect regarding distribution of MBs across the included studies specific to AD patients, given the ecological evidence supporting the effect of localized MBs (lobar vs basal ganglia) and their count,Reference Park, Seo and Kim 43 , Reference Yakushiji, Noguchi and Charidimou 44 the use of standardized neuroimaging technique for MB detection with larger sample size in future studies is warranted.
CONCLUSION
The role of MB on cognition in AD remains unclear because of limited number of neuropsychological studies. Future studies on MBs in AD should 1) use standardized imaging techniques with high sensitivity for MBs (i.e. SWI at 3T), 2) employ a common standard for MB definition, 3) use neuropsychological tests with high sensitivity, 4) compare pure AD patients to other dementia such as vascular dementia, and 5) screen for neuropsychiatric symptoms as possible confounders. Additionally, both severity (size and location) and number of MB should be recorded and reported. Taking these factors into consideration, further research in the field should be of interest and fruitful.
ACKNOWLEDGEMENTS
AAS is funded by the Alzheimer Society of Canada and Canadian Consortium on Neurodegeneration in Aging. AR is supported by Canada Research Chairs.
Disclosures
AAS has received a fellowship and postdoctoral grant from the Alzheimer Society of Canada and Canadian Consortium on Neurodegeneration in Aging. AR, G-YH, and DL do not have anything to disclose.
Statement of Authorship
AAS wrote the various drafts of the manuscript, designed the meta-analysis, and carried out the various meta-analytic procedures and statistics. AR, G-YH, and DL provided expertise on neuroimaging, and clinical expertise on the study’s implication, and revised the manuscript for content.
SUPPLEMENTARY MATERIAL
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/cjn.2016.296






