Diabetes mellitus is a widespread metabolic disease in developing and developed countries. In Iran, the prevalence rate of known diabetes and impaired fasting glucose has been reported to be 16·3 and 11·9 %, respectively. The prevalence of diabetes has been found to be higher in women (25·3 %) than in men (9·2 %)( Reference Lotfi, Saadati and Afzali 1 ). This disease is characterised by the presence of hyperglycaemia together with insulin resistance, oxidative stress as well as elevated production of cytokines, such as C-reactive protein, IL-6 and TNF-α( Reference Goldberg 2 ). In recent years, it has been documented that a change in the composition of gut microflora towards Gram-negative bacteria, particularly an elevated Firmicutes:Bacteroidetes ratio, plays an important role in the cascade of inflammation and in the development of systemic insulin resistance and other metabolic disorders in type 2 diabetic patients( Reference Zhang and Zhang 3 ). Lipopolysaccharide is a major component of the outer cell membrane in Gram-negative bacteria, and it is known to be an initiator of metabolic impairments such as chronic low-grade inflammation and insulin resistance in obese subjects and onset of type 2 diabetes( Reference Zhang and Zhang 3 ). Recently, prebiotics such as resistant dextrin have been proposed as a new therapeutic approach in the management of type 2 diabetes and its complications( Reference Kaczmarczyk, Miller and Freund 4 ). NUTRIOSE®06 is a purified resistant dextrin, a glucose polymer (rich in α-1,4 and α-1,6 linkages) derived from wheat (NUTRIOSE®FB06) or maize (NUTRIOSE®FM06). NUTRIOSE®06 is incompletely hydrolysed and absorbed in the small intestine. About 15 % of this resistant dextrin is digested in the small intestine while 75 % is fermented in the colon and about 10 % of it is excreted in the faeces( Reference Lefranc-Millot 5 ). Its available energy value ranges from 7·1 to 8·4 kJ/g (1·7 to 2·0 kcal/g). NUTRIOSE®06 is well tolerated by the human digestive system up to a dose of 45 g/d( Reference Lefranc-Millot 5 ). It has been shown that NUTRIOSE®06 can induce metabolic and health benefits via selective modulation of the human gut microflora towards Lactobacillus spp., and bacteroides and butyrogenic genera such as Clostridium cluster XIVa and Roseburia genus( Reference Hobden, Martin-Morales and Guérin-Deremaux 6 ). There is limited evidence on the effects of resistant dextrin on glycaemic status and inflammation. Some animal studies have reported the beneficial effects of resistant dextrin on inflammation( Reference Pouillart, Depeint and Abdelnour 7 ) and glycaemic status( Reference Knapp, Parsons and Bauer 8 ). For example, NUTRIOSE®06 has been shown to reduce postprandial glycaemic kinetics in dogs( Reference Knapp, Parsons and Bauer 8 ), whereas it has been shown to increase the number of intestinal butyrogenic bacterial strains and decrease inflammatory disorders in colitis piglets( Reference Li, Guérin-Deremaux and Pochat 9 ). Some human studies have evaluated the effects of NUTRIOSE®06 on glycaemic status and insulin resistance( Reference Li, Guérin-Deremaux and Pochat 9 ), energy intake, and body weight( Reference Guérin-Deremaux, Pochat and Reifer 10 ). To the best of our knowledge, no study has evaluated the effects of resistant dextrin on inflammatory biomarkers in human subjects. The review of the literature related to this area has shown a need for further research on the health effects of NUTRIOSE®06 especially on humans. Therefore, the present study tests the hypothesis that NUTRIOSE®06, as a resistant dextrin, can modulate insulin resistance, inflammation and metabolic endotoxaemia in women with type 2 diabetes.
Materials and methods
Patients
Female patients (n 75; aged 30–65 years) from the Iran Diabetes Society and Endocrinology and Metabolism Clinics at Tabriz University of Medical Sciences voluntarily participated in the present triple-blind randomised controlled study conducted from December 2011 to February 2012. Patients were included if they had type 2 diabetes for more than 6 months, used anti-diabetic drugs and maintained on them during the study period, and had a normal diet and BMI >25 kg/m2 in the last 3 months. Type 2 diabetes was defined as a fasting plasma glucose (FPG) level ≥ 7 mmol/l ( ≥ 126 mg/dl)( 11 ). Patients were excluded if they had a history of gastrointestinal, CVD, renal, thyroid, liver or pancreatic diseases; were pregnant, smokers or lactating; were consuming pre/probiotic products, antibiotics, antacids, alcohol, antidiarrhoeal, anti-inflammatory and lipid-lowering drugs, laxatives, or insulin; and, finally, had a typical fibre intake of >30 g. At the beginning of the trial, data including age, medication history and diabetes duration were collected using a questionnaire. The trial was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of Tabriz University of Medical Sciences and were registered on the Iranian Registry of Clinical Trials website (http://www.irct.ir/; IRCT201110293253N4). Written informed consent was obtained from each patient.
Experimental design
Patients were randomly divided into two groups: the intervention group (n 30) and the control group (n 25). Randomisation was based on the block randomisation procedure with a block size of 4. To match the groups, patients with the same categories of BMI and age were allocated to each block. The allocation sequence was generated randomly by using random allocation software. This software enables investigation to control different attributes of the random allocation sequence and produces qualified lists for parallel-group trials( Reference Saghaei 12 ). The intervention group received a supplement of 10 g/d of resistant dextrin (NUTRIOSE®06FM; Roquette) and the control group received a similar amount of maltodextrin as placebo (Jiujiang Huirong Trade Company Limited) for 8 weeks. Both NUTRIOSE®06 and maltodextrin were powdered and given to the patients in similar opaque packages. The supplements were divided into two packages of 5 g each to be taken during breakfast and dinner with a cup of water. These supplements were divided between patients in accordance with the allocation codes after randomisation. The patients received half of the packages at the beginning of the trial and the remaining packages in the middle of the trial. To maintain blinding, allocation was performed by an investigator with no clinical involvement in the study, and the investigators as well as the statistician remained blind until the end of the analysis. The supplements were distributed among the patients in accordance with the allocation codes after randomisation. To minimise the patients' withdrawal and to ensure their consumption of supplements, they received a phone call every week. Throughout the trial, the patients were asked to have their usual physical activity and diet. All the collected data were coded for analysis.
The sample size was determined based on the primary outcome of changes in TNF-α level, which was obtained from a pilot study on five patients. A minimum sample size of twenty-two was determined for each group according to Pocock's formula( Reference Pocock 13 ), with a 95 % CI and a power of 0·80. To account for an anticipated dropout of 25 %, the sample size was increased to twenty-seven in each group. The primary outcomes of the study were FPG, HbA1c, fasting insulin, homeostasis model assessment of insulin resistance (HOMA-IR), quantitative insulin sensitivity check index (QUICKI), high-sensitivity C-reactive protein (hs-CRP), TNF-α, IL-6, malondialdehyde (MDA) and endotoxin, while the secondary outcomes were changes in body weight and energy intake. Changes in energy intake and body weight for glycaemic parameters, MDA and inflammatory biomarkers were considered as covariates in the present study.
Body-weight and dietary intake assessment
Anthropometric indices including body weight and height were measured at baseline and at the end of the trial. BMI was calculated as weight (in kg) divided by squared height (in m2). Dietary intakes were evaluated using a 3 d food diary (2 weekdays and 1 weekend) at baseline and at the end of the trial. Dietary intakes were analysed using Nutritionist 4 software (First Databank, Inc., Hearst Corporation) using the database from tables of content and nutritional value of Iranian food products.
Biochemical measurements
At baseline and at the end of the trial, after an overnight fast, 10 ml of venous blood samples were collected and transferred into two Vacutainer tubes (Wuhan Desheng Chemical Technology Co., Ltd.), one of which contained EDTA for the measurement of HbA1c and the other was used for the measurements of FPG, insulin, MDA, inflammatory biomarkers including hs-CRP, TNF-α, IL-6 and serum endotoxin concentrations. The serum samples were separated from whole blood by centrifugation at 2500 rpm for 10 min (Beckman Avanti J-25; Beckman Coulter) at room temperature. Glycaemic indices were analysed on the day of sampling and the remaining serum was stored at − 70°C until assay time. FPG concentration was measured by an enzymatic method using an Abbott Model Alcyon 300, USA autoanalyser with a kit from Pars Azmoon Company. Serum insulin concentration was measured by a chemiluminescent immunoassay method (LIAISON Analyser 310360; Diasorin S.P.A.). HOMA-IR( Reference Matthews, Hosker and Rudenski 14 ) and QUICKI( Reference Katz, Nambi and Mather 15 ) were calculated according to the following formulae:
HbA1c level was determined in whole blood using an automated HPLC analyser with commercially available kits (Bio-Rad D-10; Bio-Rad Laboratories).
Serum hs-CRP concentration was determined using an immunoturbidimetric assay (Pars Azmoon Company). IL-6 and TNF-α levels were measured with an ELISA kit (eBioscience). The level of serum MDA, a thiobarbituric acid-reactive substance, was measured by the reaction with thiobarbituric acid to produce a pink-coloured complex. Its fluorescence intensity was measured at 547 nm with excitation at 525 nm by a spectrofluorimeter (model SFM 25A; Kontron)( Reference Del Rio, Pellegrini and Colombi 16 ). Serum endotoxin concentration was measured by a limulus amoebocyte lysate assay kit (LAL kit endpoint-QCL1000; Cambrex BioScience). Its fluorescence intensity was measured at 547 nm.
Statistical analyses
Data were analysed using SPSS software (version 13; SPSS Inc.). All statistical analyses were performed based on an intention-to-treat analysis. Results are presented as means and standard deviations. The normality of data distribution was evaluated by the one-sample Kolmogorov–Smirnov test. The following analyses were performed for both primary and secondary outcomes. Unpaired t tests (for baseline measurements) and ANCOVA were used to compare quantitative variables after the intervention. Quantitative data, collected at the beginning and end of the trial, were compared by the paired t test. hs-CRP analyses were performed after log transformation. The effects of medications used in the two groups were compared by the Mann–Whitney U test. ANCOVA adjusting for baseline measurements of the primary outcome and covariates (including changes in energy intake and body weight) was used to identify any differences between the two groups after the intervention. For calculating the percentage of mean changes in the biomarkers, at the beginning and end of the trial, mean changes in the biomarkers from baseline in each group were calculated by the following formula:
Mean changes in the biomarkers between the groups were calculated as follows:
P< 0·05 were considered as statistically significant.
Results
Of a total of seventy-five patients, fifty-five completed the trial (intervention group n 30 and control group n 25; Fig. 1). The patients did not report any adverse effects or symptoms with respect to resistant dextrin supplementation. Table 1 presents the baseline characteristics of the patients in the two groups. The initial characteristics were similar at baseline in both groups.

Fig. 1 Flow chart of the study design.
Table 1 Baseline characteristics of the study patients (Mean values and standard deviations or ranges; number of participants and percentages)

Effects of resistant dextrin supplementation on body weight and dietary intakes
At baseline, no significant differences were observed between the two groups in relation to body weight and BMI (Table 1). After 8 weeks of supplementation, body weight and BMI did not change significantly in the maltodextrin group, but decreased significantly in the resistant dextrin group (Table 2) . These changes were found to be significant in the resistant dextrin group compared with those at baseline (P< 0·05; paired Student's t test).
Table 2 Anthropometric indices and dietary intakes of the study patients at baseline and at the end of the trial (Mean values and standard deviations)

* Mean value was significantly different from that at baseline (P< 0·05; paired Student's t test).
† Mean value was significantly different from that of the maltodextrin group (P< 0·05; ANCOVA adjusted for baseline values).
Dietary intakes of macronutrients are given in Table 2. There were no significant differences in the intakes of energy and macronutrients between the two groups at baseline. Intakes of energy, carbohydrate and total fat were found to be significantly different between the two groups at the end of the trial (P< 0·05; ANCOVA adjusted for baseline values). Intakes of energy and total fat decreased significantly in the resistant dextrin group compared with those at baseline (P< 0·05; paired Student's t test), but did not differ significantly in the maltodextrin group.
Effects of resistant dextrin supplementation on glycaemic indices, inflammatory parameters and malondialdehyde
At baseline, no significant differences in glycaemic status between the resistant dextrin and maltodextrin groups were observed (Table 3). However, at the end of the trial, there was a significant decrease in fasting insulin concentration (20·1 pmol//l, 22·8 %), HOMA-IR (1·3, 24·9 %) and QUICKI (0·2, 7·2 %) in the resistant dextrin group compared with the maltodextrin group (P< 0·05; ANCOVA adjusted for energy intake, weight changes and baseline values). The reduction in FPG (0·05 mmol/l, 0·6 %) and HbA1c (0·5 %, 9·6 %) levels was not significant in the resistant dextrin group compared with the maltodextrin group (P>0·05; ANCOVA adjusted for energy intake, weight changes and baseline values). The two groups did not show any significant difference in baseline inflammatory biomarkers, i.e. MDA and endotoxin (Table 4). After 8 weeks of supplementation, significant decreases in the levels of IL-6 (1·4 pg/ml, 28·4 %), TNF-α (5·4 pg/ml, 18·8 %), MDA (1·2 nmol/ml, 25·6 %) and endotoxin (6·2 endotoxin units/ml, 17·8 %) were observed in the resistant dextrin group compared with the maltodextrin group (P< 0·05; ANCOVA adjusted for energy intake, weight changes and baseline values). The reduction in the levels of hs-CRP (2·7 ng/ml, 35·1 %; P>0·05) was not significant.
Table 3 Changes in the glycaemic status of the study patients at baseline and at the end of the trial (Mean values and standard deviations; mean differences (MD) and 95 % confidence intervals)
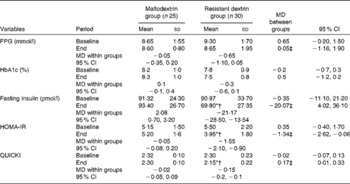
FPG, fasting plasma glucose; HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index.
* Mean value was significantly different from that at baseline (P< 0·05; paired Student's t test).
† Mean value was significantly different from that of the maltodextrin group (P< 0·05; ANCOVA adjusted for energy intake, weight changes and baseline values).
‡ Adjusted for changes in energy intake, body weight and baseline values using ANCOVA.
Table 4 Changes in lipopolysaccharide, malondialdehyde (MDA) and inflammatory biomarkers of the study patients at baseline and at the end of the trial (Mean values and standard deviations; mean differences (MD) and 95 % confidence intervals)
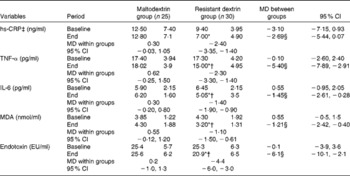
hs-CRP, high-sensitivity C-reactive protein; EU, endotoxin units.
* Mean value was significantly different from that at baseline (P< 0·05; paired Student's t test).
† Mean value was significantly different from that of the maltodextrin group (P< 0·05; ANCOVA adjusted for energy intake, weight changes and baselines values).
‡ hs-CRP analyses were performed after log transformation.
§ Adjusted for changes in energy intake, body weight and baseline values using ANCOVA.
Discussion
It has been hypothesised that prebiotics can modulate metabolic disorders such as blood glucose homeostasis, oxidative stress and inflammation by reducing metabolic endotoxaemia( Reference Pourghassem Gargari, Dehghan and Aliasgharzadeh 17 , Reference Dehghan, Pourghassem Gargari and Asghari Jafar-Abadi 18 ). The results of the present study demonstrated that supplementation with resistant dextrin for 8 weeks significantly decreased the levels of body weight, BMI, fasting insulin, HOMA-IR, QUICKI, IL-6, TNF-α, MDA and endotoxin in the intervention group compared with the control group. However, reductions in the levels of FPG, HbA1c and hs-CRP were not significant.
The results of the present study for the changes in body weight and BMI are similar to those reported by Guérin-Deremaux et al. ( Reference Guérin-Deremaux, Pochat and Reifer 10 , Reference Guérin-Deremaux, Li and Pochat 19 ), suggesting that supplementation with NUTRIOSE®06 at a dosage of 17 g/d for 12 weeks and dosages of 14, 18 and 24 g/d for 9 weeks decreased body weight and BMI in overweight men. In the present trial, energy intake of the intervention group significantly decreased (7414·0 (sd 1324·6)–6009·3 (sd 951·8) kJ). The exact mechanism(s) of weight reduction by resistant dextrin remains unclear. It has been hypothesised that increased the levels of leptin and some gut satiety hormones, including glucagon-like peptide (GLP)-1, peptide YY and ghrelin, as well as reduction in appetite, play an important role in weight reduction( Reference Guérin-Deremaux, Pochat and Reifer 10 , Reference Guérin-Deremaux, Li and Pochat 19 ). Furthermore, it has been reported that there is a positive relationship between increased endotoxaemia and obesity( Reference Amar, Burcelin and Ruidavets 20 ). Resistant dextrin may decrease body weight through the reduction in metabolic endotoxaemia. Only one study has reported the effects of NUTRIOSE®06 supplementation on glycaemic status in overweight men. It has shown that NUTRIOSE®06 reduces the levels of glucose (4 %), insulin (12 %) and HOMA-IR (18 %) in overweight men( Reference Li, Guérin-Deremaux and Pochat 9 ). Regarding insulin and HOMA-IR, the present results are consistent with the findings of that study. We observed non-significant reductions in the levels of FPG and HbA1c. The discrepancy in the results for FPG and HbA1c may be due to study duration and dosage of the supplement. The results obtained for the effects of other prebiotics on glycaemic status in diabetic patients are inconsistent. We have reported the positive effects of inulin-type fructans, as a prebiotic, on glycaemic status in diabetic patients( Reference Pourghassem Gargari, Dehghan and Aliasgharzadeh 17 , Reference Dehghan, Pourghassem Gargari and Asghari Jafar-Abadi 18 ). By contrast, another study has reported no effects of inulin-type fructans in diabetic patients( Reference Bonsu Nana and Johnson 21 ). This difference in results may be attributed to the basal levels of glycaemic indices, the dosage and type of supplementation, as well as the pathological state of patients. Prebiotics such as resistant dextrin may promote hypoglycaemic effects via several mechanisms discussed below.
Modification in gut hormone secretion
Supplementation with prebiotics has been reported to promote L-cell differentiation in the colon and increase the secretion of gut hormones including peptide YY, GLP-1 and gastric inhibitory polypeptide( Reference Cani, Lecourt and Dewulf 22 ). Prebiotics probably modulate these effects through an increase in the bacterial production of butyrate and propionate that activates G-protein-coupled receptors, free fatty acid receptor 2 and free fatty acid receptor 3( Reference Lin, Frassetto and Kowalik 23 ). These hormones contribute to the regulation of appetite and control of glucose metabolism and insulin resistance, respectively. By these mechanisms, resistant dextrin, as a prebiotic, may control glycaemic and insulinaemic responses.
Reduction in body weight
Excess body weight can affect the expression of inflammatory biomarkers such as TNF-α, which reinforce insulin resistance via suppressing insulin intracellular signals such as inhibitory phosphorylation of insulin receptor substrates (IRS-1 and IRS-2)( Reference Kueht, McFarlin and Lee 24 ). Resistant dextrin is likely to decrease hyperglycaemia by increasing anorexigenic hormone levels, decreasing body weight as well as BMI, and subsequently reducing inflammation( Reference Guérin-Deremaux, Li and Pochat 19 ).
Improvement in metabolic endotoxaemia
Endotoxin levels are higher (76 %) in individuals with type 2 diabetes than in healthy individuals( Reference Creely, McTernan and Kusminski 25 ). Increased endotoxin levels (metabolic endotoxaemia) cause disturbance in food intake and energy expenditure control, which may favour weight gain( Reference Amar, Burcelin and Ruidavets 20 ) and consequently develop insulin resistance. Moreover, metabolic endotoxaemia causes an increase in the expression of pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α( Reference Cani and Delzenne 26 ). The increase in the levels of these pro-inflammatory cytokines is related to the decrease in insulin action via molecular mechanisms such as inhibitory phosphorylation of IRS-1 and IRS-2 by activating c-Jun NH2-terminal kinase and IκB kinase, reducing the expression of IRS-1 and IRS-2 via p38 mitogen-activated protein kinase, and suppressing the expression of IRS-1 and IRS-2 via the extracellular signal-related kinase pathway( Reference Fujishiro, Gotoh and Katagiri 27 ). Resistant dextrin may contribute to the modulation of glycaemic status by reducing body weight and inflammatory biomarkers through decreased metabolic endotoxaemia.
Modulation of butyrate and NEFA levels
In diabetic patients, a reduction in insulin anti-lipolytic activity helps to increase NEFA levels, leading to cellular dysfunction of insulin in several tissues by decreasing IRS-1-associated phosphatidylinositol 3-kinase activity and consequently insulin resistance. Animal studies have indicated that butyrate, as a SCFA, reverses diet-induced insulin resistance, possibly by enhancing the expression of PPAR-γ that increases fatty acid oxidation in muscles( Reference Gao, Yin and Zhang 28 ). Butyrate reduces gut permeability by increasing the release of GLP-2, which, in turn, helps to reduce the level of serum endotoxin( Reference Cani, Possemiers and Van de Wiele 29 ) that is known to induce inflammation and subsequently resistant insulin( Reference Cani and Delzenne 26 , Reference Fujishiro, Gotoh and Katagiri 27 ). Moreover, GLP-2 improves glucose homeostasis and insulin sensitivity by activating phosphatidylinositol 3-kinase signalling( Reference Shi, Zhou and Li 30 ). NUTRIOSE®06, as a butyrogenic prebiotic, may modulate glycaemic status by the aforementioned mechanisms.
Another outcome of resistant dextrin supplementation was to improve inflammatory biomarkers, i.e. MDA and metabolic endotoxaemia. To the best of our knowledge, no previous study has evaluated the effects of resistant dextrin on the mentioned biomarkers in human subjects. In colitis piglets, supplementation with NUTRIOSE®06, as a resistant dextrin, decreased systemic concentrations of IL-1β, IL-12 as well as TNF-α and stimulated the T helper (Th)2-related immune pathway (IL-10 and secretory IgA)( Reference Pouillart, Depeint and Abdelnour 7 ). In ob/ob mice, supplementation with oligofructose led to decreases in the levels of cytokines, such as TNF-α, IL-1β, IL-1, IL-6 and interferon-γ, and the hepatic expression of inflammatory and oxidative stress markers( Reference Cani, Possemiers and Van de Wiele 29 ). Some human studies have reported the positive effects of other prebiotics on inflammatory biomarkers( Reference Malaguarnera, Vacante and Antic 31 – Reference Vulevic, Juric and Tzortzis 33 ). In patients with non-alcoholic steatohepatitis, it has been shown that supplementation with Bifidobacterium longum plus fructo-oligosaccharides significantly reduces the levels of TNF-α, C-reactive protein, HOMA-IR, serum endotoxins and steatosis( Reference Malaguarnera, Vacante and Antic 31 ). Research has shown that supplementation with inulin and xylo-oligosaccharide (3 g inulin+1 g xylo-oligosaccharide) for 4 weeks significantly decreases the expression of IL-1β and TNF-α and increases the expression of IL-13 and IL-10 in the blood of healthy volunteers( Reference Lecerf, Depeint and Clerc 32 ). It has been reported that supplementation of trans-galacto-oligosaccharide (5·5 g/d), as a prebiotic, for 12 weeks improves IgA, insulin, lipid and C-reactive protein status in overweight adults( Reference Vulevic, Juric and Tzortzis 33 ). Moreover, it has previously been reported that inulin-type fructans, as a prebiotic, improve glycaemic indices, oxidative stress( Reference Pourghassem Gargari, Dehghan and Aliasgharzadeh 17 ), some inflammatory biomarkers and metabolic endotoxaemia in women with type 2 diabetes( Reference Dehghan, Pourghassem Gargari and Asghari Jafar-Abadi 18 , Reference Dehghan, Gargari and Jafar-Abadi 34 ).
In contrast, Macfarlane et al. ( Reference Macfarlane, Cleary and Bahrami 35 ) reported that supplementation of older people with a synbiotic (B. longum+Synergy 1) for 4 weeks significantly decreases the level of TNF-α. They observed a non-significant reduction in the levels of interferon-γ, IL-4, IL-6, IL-8, monocyte chemoattractant protein-1 and IL-1 as well as a non-significant increase in the level of IL-10. Anderson et al. ( Reference Anderson, McNaught and Jain 36 ) found that supplementation with oligofructose plus probiotics (for 1–2 weeks in patients undergoing elective abdominal surgery) did not affect systemic inflammation. The diversity of these results may be due to the differences in ethnicity, genotype, study duration, dosage, type and time of supplementation, pathological state, as well as basal status of inflammatory/anti-inflammatory status of patients.
The underlying mechanism(s) of resistant dextrin affecting inflammation are not yet known. Some probable proposed mechanism(s) are presented below.
Shift in the gut ecosystem
NUTRIOSE®06, as a resistant dextrin, has been reported to shift the bacterial microbiota profile to butyrogenic genera such as Peptostreptococcus, Fusobacterium and Bifidobacterium ( Reference van den Heuvel, Wils and Pasman 37 ). Probiotics of the Bifidobacterium genus are well known for their anti-inflammatory activities( Reference Suzuki, Mitsuyama and Koga 38 ). Moreover, human studies have shown that NUTRIOSE®06 changes the bacterial microbiota profile towards Lactobacillus spp.( Reference Lefranc-Millot, Wils and Neut 39 ). It has been shown that the Lactobacillus genus down-regulates IL-12 and TNF-α, enhances IL-10 in dendritic cells, and controls the regulation of dendritic cell functions, resulting in their inability to induce CD4+T-cell activation( Reference Mohamadzadeh, Pfeiler and Brown 40 ).
SCFA from resistant dextrin fermentation in the colon
Clinical trials on healthy subjects have shown that resistant dextrin stimulates the growth of acid-resistant bacteria( Reference van den Heuvel, Wils and Pasman 37 ). These bacterial genera are well known to produce butyrate( Reference Lefranc-Millot, Wils and Henry 41 ). Butyrate controls inflammation by preventing inhibitor of κβ degradation and the production of NF-κβ( Reference Place, Noonan and Giardina 42 ) and by increasing the expression of cytokine signalling 3 suppressor( Reference Weber and Kerr 43 ). These changes shift lymphocyte differentiation into Th2 rather than Th1 cells, thereby increasing the secretion of IL-10 and decreasing the expression of pyrogenic factors, such as myeloperoxidase( Reference Knapp, Parsons and Bauer 8 ). Th2 inhibits the production of inflammatory cytokines by interfering with the Toll-like receptor 4-dependent signalling pathway by activating PPAR-γ( Reference Schwab, Reynders and Loitsch 44 ). PPAR-γ exerts its anti-inflammatory effect by directly binding to as well as modulating the expression and activity of the peptidoglycan recognition protein 3 (PGlyRP3) gene, which, in turn, activates inhibitor of κβ and inhibits NF-κβ translocation into the nucleus, resulting in the down-regulation of different pro-inflammatory cytokines( Reference Zenhom, Hyder and de Vrese 45 ).
Reduction of oxidative stress by increasing Lactobacillus and decreasing hyperglycaemia and NEFA levels
Increased oxidative stress results in an increase in intestinal permeability and the levels of endotoxin in the blood. Lipopolysaccharide is a major component of the outer cell wall in Gram-negative bacteria, and is known as an inflammatory agent in obesity and type 2 diabetes( Reference Cani, Possemiers and Van de Wiele 29 ). Lactic acid bacteria produce superoxide dismutase. An in vitro study has shown that antioxidant properties of lactic acid bacteria leads to the elimination of free radicals( Reference Rishi, Mavi and Bharrhan 46 ). Also, Lactobacillus spp. resident in gut lyses release their intracellular antioxidative constituents, which, in turn, help to decrease the level of MDA( Reference Rishi, Mavi and Bharrhan 46 ). Moreover, it has been reported that NUTRIOSE®06 can increase β-glucosidase activity that is known for its antioxidative effects( Reference van den Heuvel, Wils and Pasman 37 ). Hyperglycaemia and probably increased levels of NEFA induce high concentrations of reactive oxygen species( Reference King and Loeken 47 ). We have found that resistant dextrin improves the lipid profile (P Dehghan et al., unpublished results).
Reduction of serum endotoxin levels
Resistant dextrin, as a prebiotic, may reduce the tone of inflammation via the reduction of intestinal permeability due to increased GLP-2 and normalisation of the Gram-negative:Gram-positive ratio that leads to the reduction of endotoxin levels (endotoxaemia)( Reference Cani, Possemiers and Van de Wiele 29 ). The probable mechanisms are shown in Fig. 2.
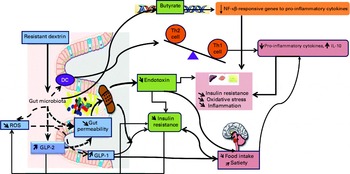
Fig. 2 Probable mechanisms of the effect of resistant dextrin on inflammation and insulin resistance. Th, T helper; DC, dendritic cells; ROS, reactive oxygen species; GLP, glucagon-like peptide. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
The present trial had some limitations, including its sample size, fairly short duration of its intervention, as well as no assessment of serum SCFA and NEFA levels among other inflammatory and anti-inflammatory biomarkers. Additionally, gut and faecal microbial compositions were not evaluated in the present study. Based on the results of the present trial, it can be hypothesised that resistant dextrin supplementation may improve insulin resistance and some of the oxidative stress and inflammatory biomarkers in type 2 diabetic patients. These findings suggest resistant dextrin to be a safe intervention for the management of type 2 diabetes and its complications. This dietary fibre can be considered as a supplement in the food industry, especially as a substitute for sugar and fat in foods for diabetic patients. Further investigations are needed to confirm the positive effects of resistant dextrin on insulin resistance and inflammatory/anti-inflammatory indices in type 2 diabetic patients.
Acknowledgements
The authors thank all the patients who participated in the study.
The present study was financially supported by the Nutrition Research Center of Tabriz University of Medical Sciences, Iran. The study received no specific grant from commercial or not-for-profit sectors. The Nutrition Research Center of Tabriz University of Medical Sciences had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: P. D., M. A. J.-A. and B. P. G. designed the study; P. D. and A. A. conducted the trial and collected the data; P. D. and M. A. J.-A. analysed the data; A. A., B. P. G. and P. D. wrote and revised the final manuscript.
The authors declare that there are no conflicts of interest.








