Impact statement
Plastics have become central to modern human life and have led to the new environmental problem of microplastics. Humans are unavoidably exposed to these microplastics through air, food and water and therefore microplastics have been detected in many different compartments of the body. This exposure could have implications for the function of cells and organs in our bodies, including how they develop and repair themselves when damaged. However, there is currently no comprehensive overview of the effects of microplastics on organ development and processes related to organ repair. Therefore, this review aims to provide an extensive overview of available evidence present in the public domain describing how microplastics and additives leaching from plastics can affect developing and repairing organs. The available studies suggest that we do not have the luxury to be complacent about microplastic pollution anymore, clear effects on development and repair have been found. It is therefore imperative that action is taken to reduce plastic use and prevent further contamination of the environment and ourselves with microplastics.
Introduction
The global production of plastic has surged dramatically from 1.5 million tons in 1950 to over 390 million tons in 2021 (Plastics Europe, 2021). The most widely used polymers include polyethylene, polypropylene, polystyrene, polyvinylchloride and polyamide (Peñalver et al., Reference Peñalver, Arroyo-Manzanares, López-García and Hernández-Córdoba2020). Their popularity arises from their versatility, durability, ease of use and cost-effectiveness (Wijesekara et al., Reference Wijesekara, Bolan, Bradney, Obadamudalige, Seshadri, Kunhikrishnan, Dharmarajan, Ok, Rinklebe, Kirkham and Vithanage2018). Nevertheless, their lack of biodegradability results in them persisting in the environment, causing considerable pollution (Bahl et al., Reference Bahl, Dolma, Jyot Singh and Sehgal2021).
Over time, environmental factors cause plastics to break down into smaller fragments (Gijsman and Dozeman, Reference Gijsman and Dozeman1996; Andrady, Reference Andrady2011; Min et al., Reference Min, Cuiffi and Mathers2020; Rodriguez et al., Reference Rodriguez, Mansoor, Ayoub, Colin and Benzerga2020). When these fragments are reduced to sizes smaller than 5 mm, they are classified as either microplastics (5 mm–1 μm) or nanoplastics (<1 μm) (Hartmann et al., Reference Hartmann, Hüffer, Thompson, Hassellöv, Verschoor, Daugaard, Rist, Karlsson, Brennholt, Cole, Herrling, Hess, Ivleva, Lusher and Wagner2019). These micro- and nanoplastics (MNP) are further categorized, based on their mode of environmental release, into primary MNP specifically produced to be small and secondary MNP originating from degradation of larger plastic waste (Figure 1) (Gijsman and Dozeman, Reference Gijsman and Dozeman1996; Andrady, Reference Andrady2011; Min et al., Reference Min, Cuiffi and Mathers2020; Rodriguez et al., Reference Rodriguez, Mansoor, Ayoub, Colin and Benzerga2020; Allen et al., Reference Allen, Allen, Abbasi, Baker, Bergmann, Brahney, Butler, Duce, Eckhardt, Evangeliou, Jickells, Kanakidou, Kershaw, Laj, Levermore, Li, Liss, Liu, Mahowald, Masque, Materić, Mayes, McGinnity, Osvath, Prather, Prospero, Revell, Sander, Shim, Slade, Stein, Tarasova and Wright2022). As a result, MNP are highly heterogeneous, varying in size, shape and polymer composition (Koelmans et al., Reference Koelmans, Redondo-Hasselerharm, Nor, De Ruijter, Mintenig and Kooi2022).
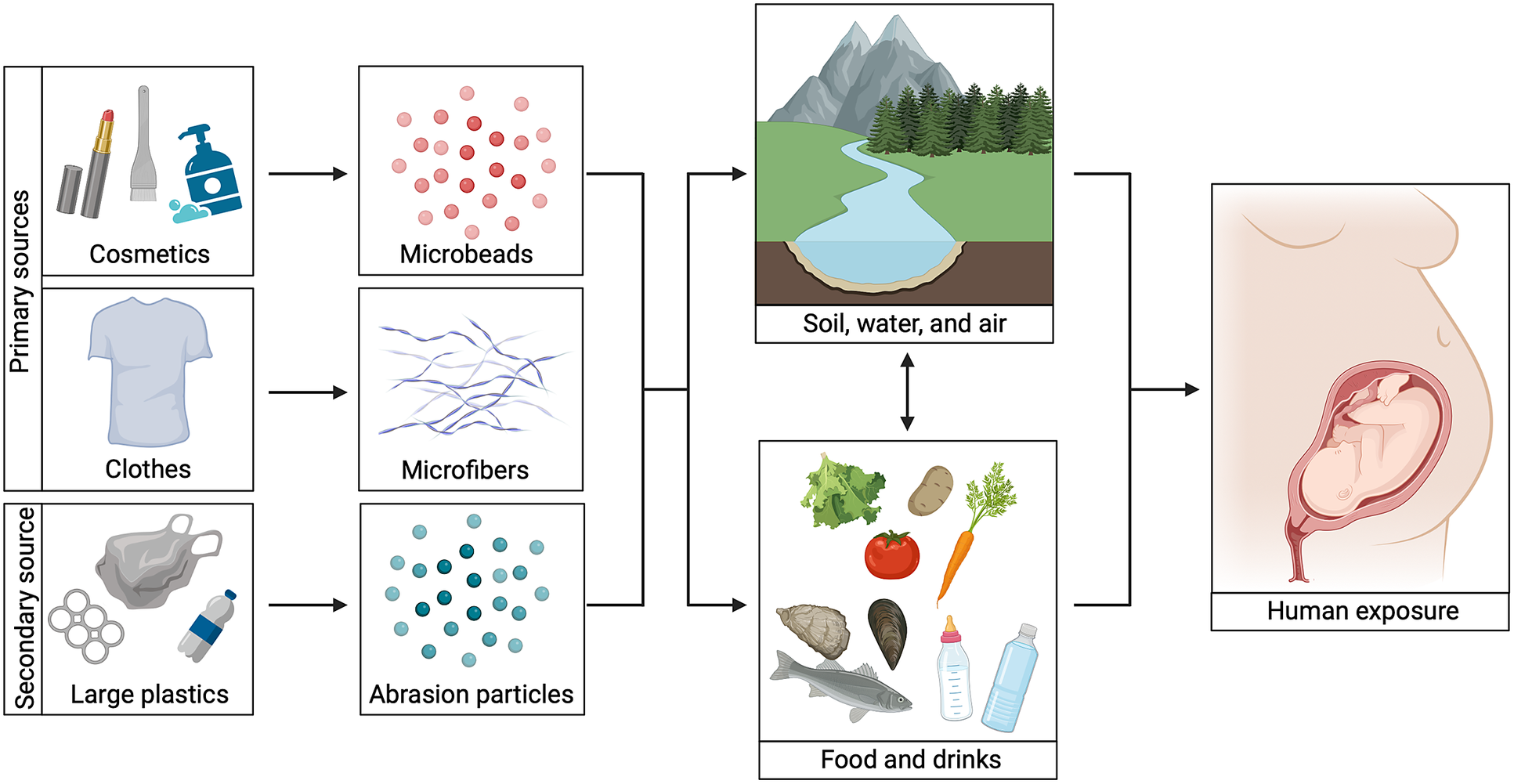
Figure 1. Microplastics exposure routes. An overview of different microplastic exposure routes. Primary sources include clothes and cosmetics, whereas secondary sources include larger pieces of plastic. Microbeads from cosmetics, microfibers from clothes and smaller plastic particles derived from plastic degradation can enter humans directly via food and/or drinks or via the natural environment. When pregnant women are exposed, a developing fetus can be exposed too. Image created with BioRender.com.
High amounts of MNP have been isolated from all environmental compartments ranging from water to soil and air (Figure 1) (Gasperi et al., Reference Gasperi, Wright, Dris, Collard, Mandin, Guerrouache, Langlois, Kelly and Tassin2018; Li et al., Reference Li, Liu and Paul Chen2018; Zhou et al., Reference Zhou, Wang, Zou, Jia, Zhou and Li2020). In the latter case, MNP have been found both in indoor and outdoor air (Evangeliou et al., Reference Evangeliou, Grythe, Klimont, Heyes, Eckhardt, Lopez-Aparicio and Stohl2020; Zhang et al., Reference Zhang, Wang and Kannan2020; Jenner et al., Reference Jenner, Sadofsky, Danopoulos and Rotchell2021). The levels of MNP indoors are higher compared to outdoors and this is worrying because we spend 90% of our time indoors (Gaston et al., Reference Gaston, Woo, Steele, Sukumaran and Anderson2020; Amato-Lourenço et al., Reference Amato-Lourenço, dos Santos Galvão, Wiebeck, Carvalho-Oliveira and Mauad2022). In addition, MNP are added on purpose to cosmetics and have been identified in our drinking water and food (Guerranti et al., Reference Guerranti, Martellini, Perra, Scopetani and Cincinelli2019; Koelmans et al., Reference Koelmans, Mohamed Nor, Hermsen, Kooi, Mintenig and De France2019; Jin et al., Reference Jin, Wang, Ren, Wang and Shan2021; Dronjak et al., Reference Dronjak, Exposito, Rovira, Florencio, Emiliano, Corzo, Schuhmacher, Valero and Sierra2022; Shi et al., Reference Shi, Dong, Shi, Yin, He, An, Tang, Hou, Chong, Chen, Qin and Lin2022). Consequently, humans are unavoidably exposed to MNP, that can enter the body via ingestion, inhalation and possibly dermal contact. Indeed, MNP have been detected in different compartments of the human body such as the blood, colon, liver, testes and lungs (Amato-Lourenço et al., Reference Amato-Lourenço, Carvalho-Oliveira, Júnior, dos Santos Galvão, Ando and Mauad2021; Ibrahim et al., Reference Ibrahim, Tuan Anuar, Azmi, Wan Mohd Khalik, Lehata, Hamzah, Ismail, Ma, Dzulkarnaen, Zakaria, Mustaffa, Tuan Sharif and Lee2021; Horvatits et al., Reference Horvatits, Tamminga, Liu, Sebode, Carambia, Fischer, Püschel, Huber and Fischer2022; Jenner et al., Reference Jenner, Rotchell, Bennett, Cowen, Tentzeris and Sadofsky2022; Leslie et al., Reference Leslie, van Velzen, Brandsma, Vethaak, Garcia-Vallejo and Lamoree2022; Zhao et al., Reference Zhao, Zhu, Weng, Jin, Cao, Jiang and Zhang2023). Notably, MNP were found in human placental tissue too and this may especially be of concern for a fetus (Ragusa et al., Reference Ragusa, Svelato, Santacroce, Catalano, Notarstefano, Carnevali, Papa, Rongioletti, Baiocco, Draghi, D’Amore, Rinaldo, Matta and Giorgini2021; Amereh et al., Reference Amereh, Amjadi, Mohseni-Bandpei, Isazadeh, Mehrabi, Eslami, Naeiji and Rafiee2022; Zhu et al., Reference Zhu, Zhu, Zuo, Xu, Qian and An2023). However, to date, there is no overview of effects of MNP on organ development and processes related to development such as regeneration and repair. Therefore, we here provide a comprehensive overview of current state-of-the-art knowledge regarding effects of MNP on developmental processes, including embryonic to childhood development and reactivation of developmental processes during regeneration in humans and rodent models.
Plastic additives
Not only the plastic particles themselves, but also plastic additives can influence biological processes. Properties of plastic polymers can be modified to achieve a desired material performance by the addition of chemical additives such as plasticizers, flame retardants, photo- and heat stabilizers, antioxidants and pigments (Fauser et al., Reference Fauser, Vorkamp and Strand2022). Notably, endocrine disruptors bisphenol A and phthalates are commonly used as additives in plastics and can therefore also leach from MNP (Liu et al., Reference Liu, Shi, Xie, Dionysiou and Zhao2019; Cao et al., Reference Cao, Lin, Zhang, Xu, Yan, Leung and Lam2022). In addition, MNP can still contain residual monomers that failed to polymerize, as well as unintentional byproducts of reactions during the manufacturing process (Lewandowski et al., Reference Lewandowski, Hayes and Beck2005; Klaeger et al., Reference Klaeger, Tagg, Otto, Bienmüller, Sartorius and Labrenz2019). MNP can also retain toxic organic compounds, like polycyclic aromatic hydrocarbons, polychlorinated biphenyls, pesticides and inorganic compounds such as heavy metals from the environment (Wu et al., Reference Wu, Cai, Jin and Tang2019; Mei et al., Reference Mei, Chen, Bao, Song, Li and Luo2020; Lu et al., Reference Lu, Zeng, Wei, Gao, Abdurahman, Wang and Liang2022). Most of these can leach from MNP into the surrounding environment, including inside the human body and may consequently cause damage or affect development.
Bisphenol A and phthalates are the most studied plastic additives and have been found in human tissues and blood. Both can also cross the placental barrier and reach a developing fetus (Tang et al., Reference Tang, Xu, Deng, Z-X and Yu2020; Mok et al., Reference Mok, Jeong, Park, Kim, Lee, Park, Kim, Choi and Moon2021; Warner et al., Reference Warner, Dettogni, Bagchi, Flaws and Graceli2021). Bisphenol A is thought to be the first synthetic estrogen produced and the majority is metabolized in the liver by UDP-glucuronosyltransferase enzymes (Hanioka et al., Reference Hanioka, Naito and Narimatsu2008). Bisphenol A and phthalates exhibit endocrine effects by modulating androgen receptor and estrogen receptors alpha and beta, thereby disturbing normal signaling (Rubin, Reference Rubin2011; Engel et al., Reference Engel, Buhrke, Imber, Jessel, Seidel, Völkel and Lampen2017). Notably, developing fetuses do not or only lowly express UDP-glucuronosyltransferase enzymes and are consequently exposed to higher bisphenol A concentrations (Hines, Reference Hines2008). However, the contribution of MNP to bisphenol A and phthalate exposure may be limited as humans are already exposed to substantial amounts of these chemicals by consuming food and beverages that are contaminated with leachate from reusable plastic bottles or food packages (Liu et al., Reference Liu, Shi, Xie, Dionysiou and Zhao2019; da Silva Costa et al., Reference da Silva Costa, Sainara Maia Fernandes, de Sousa Almeida, Tomé Oliveira, Carvalho Guedes, Julião Zocolo, Wagner de Sousa and do Nascimento2021; Sessa et al., Reference Sessa, Polito, Monda, Scarinci, Salerno, Carotenuto, Cibelli, Valenzano, Campanozzi, Mollica, Monda and Messina2021). Interestingly though, animals exposed to MNP showed higher levels of these endocrine disrupting chemicals compared to animals without or with less microplastic exposure (Fossi et al., Reference Fossi, Panti, Guerranti, Coppola, Giannetti, Marsili and Minutoli2012; Chen et al., Reference Chen, Yin, Jia, Schiwy, Legradi, Yang and Hollert2017; Barboza et al., Reference Barboza, Cunha, Monteiro, Fernandes and Guilhermino2020; Lu et al., Reference Lu, Chao, Mansor, Peng, Hsu, Yu, Chang and Fu2021). Recently, López-Vázquez et al. (Reference López-Vázquez, Rodil, Trujillo-Rodríguez, Quintana, Cela and Miró2022) showed that over 65% of bisphenol A or phthalates in MNP can become bioaccessible when exposed to physiologically relevant human digestive conditions. While precise data on the quantities of additives leaching from MNP remain unavailable, existing studies suggest that it does occur. Hence, the potential contribution of MNP to exposure levels of bisphenol A and phthalates warrants consideration. Relevant data concerning the impact of bisphenol A and phthalates on developmental and repair mechanisms will therefore also be addressed.
Microplastic exposure on various organs and tissues
Placental and fetal development
Human gestation starts when a sperm and egg cell fuse during fertilization to form a one-celled diploid totipotent zygote (Figure 2) (Clift and Schuh, Reference Clift and Schuh2013). This initiates a highly sensitive phase of development with the zygote maturing into a blastocyst containing an outer layer of trophoblasts and enclosing an inner cell mass, the precursors of the future fetus (Rossant and Tam, Reference Rossant and Tam2022). Trophoblasts will differentiate into components of the placenta, establishing a critical link between maternal and fetal tissues (Cindrova-Davies and Sferruzzi-Perri, Reference Cindrova-Davies and Sferruzzi-Perri2022). This stage of development is particularly vulnerable to environmental disturbances as it is susceptible to gene expression modifications in both the fetus and the placenta (Assou et al., Reference Assou, Boumela, Haouzi, Anahory, Dechaud, De Vos and Hamamah2011). Perturbations in gene expression can have deleterious consequences for the fetus, either directly or indirectly by impacting placental development, and such risks persist even after removal of the stressors (Nesan et al., Reference Nesan, Sewell and Kurrasch2018; Maitre et al., Reference Maitre, Bustamante, Hernández-Ferrer, Thiel, Lau, Siskos, Vives-Usano, Ruiz-Arenas, Pelegrí-Sisó, Robinson, Mason, Wright, Cadiou, Slama, Heude, Casas, Sunyer, Papadopoulou, Gutzkow, Andrusaityte, Grazuleviciene, Vafeiadi, Chatzi, Sakhi, Thomsen, Tamayo, Nieuwenhuijsen, Urquiza, Borràs, Sabidó, Quintela, Carracedo, Estivill, Coen, González, Keun and Vrijheid2022).

Figure 2. Early human development. An overview of human development at different time points. First, a sperm cell fuses with an egg cell during fertilization to form a zygote and this time point is referred to as gestational day 0. The zygote develops further into a blastocyte, consisting of an inner cell mass (purple cells) and trophoblasts (pink cells) on day 5. The inner cell mass further differentiates into ectoderm (blue cells), mesoderm (red cells) and endoderm (yellow cells) on day 15 and is called a gastrula. The embryo will then further develop and is called a fetus after week 8. Image created with BioRender.com.
Research investigating the effects of MNP on fetal development has produced important insights. Amereh et al. (Reference Amereh, Amjadi, Mohseni-Bandpei, Isazadeh, Mehrabi, Eslami, Naeiji and Rafiee2022) delineated a potential dose–response relationship in humans between placental plastics and reduced fetal growth, suggesting possible interference in nutrient exchange in the placenta. This is supported by several in vitro studies showing that microplastic exposure is toxic for a variety of human placental cell lines (Lee et al., Reference Lee, Amarakoon, C-I, Choi, Smolensky and Lee2021; Dusza et al., Reference Dusza, Katrukha, Nijmeijer, Akhmanova, Vethaak, Walker and Legler2022; Shen et al., Reference Shen, Li, Guo and Chen2022; Ragusa et al., Reference Ragusa, Matta, Cristiano, Matassa, Battaglione, Svelato, De Luca, D’Avino, Gulotta, Rongioletti, Catalano, Santacroce, Notarstefano, Carnevali, Giorgini, Vizza, Familiari and Nottola2022a; Dusza et al., Reference Dusza, van Boxel, van Duursen, Forsberg, Legler and Vähäkangas2023). In pregnant mice exposed to polystyrene microparticles, similar outcomes were found, demonstrating disruptions in placental metabolism (Chen et al., Reference Chen, Xiong, Jing, van Gestel, van Straalen, Roelofs, Sun and Qiu2022; Aghaei et al., Reference Aghaei, Mercer, Schneider, Sled, Macgowan, Baschat, Kingdom, Helm, Simpson, Simpson, Jobst and Cahill2022a; Aghaei et al., Reference Aghaei, Sled, Kingdom, Baschat, Helm, Jobst and Cahill2022b). Moreover, pregnant rats or mice exposed to polystyrene/polyethylene MNP showed detectable spread of particles throughout mothers and pups within 24 h, alongside a variety of other effects like lower fetal body weights, less vascularization of the placenta and higher expression of genes involved in cholesterol/lipid metabolism, the complement system and the coagulation cascade (Fournier et al., Reference Fournier, D’Errico, Adler, Kollontzi, Goedken, Fabris, Yurkow and Stapleton2020; Park et al., Reference Park, Han, Park, Seong, Lee, Kim, Son, H-Y and Lee2020; Huang et al., Reference Huang, Zhang, Lin, Liu, Sun, Liu, Yuan, Xiang, Kuang, Yang and Zhang2022; Aghaei et al., Reference Aghaei, Mercer, Schneider, Sled, Macgowan, Baschat, Kingdom, Helm, Simpson, Simpson, Jobst and Cahill2022a, Reference Aghaei, Sled, Kingdom, Baschat, Helm, Jobst and Cahillb; Chen et al., Reference Chen, Xiong, Jing, van Gestel, van Straalen, Roelofs, Sun and Qiu2023). These findings suggest that MNP exposure may disrupt both placental function and fetal development (Figure 3), thereby potentially leading to detrimental consequences for embryonic development.
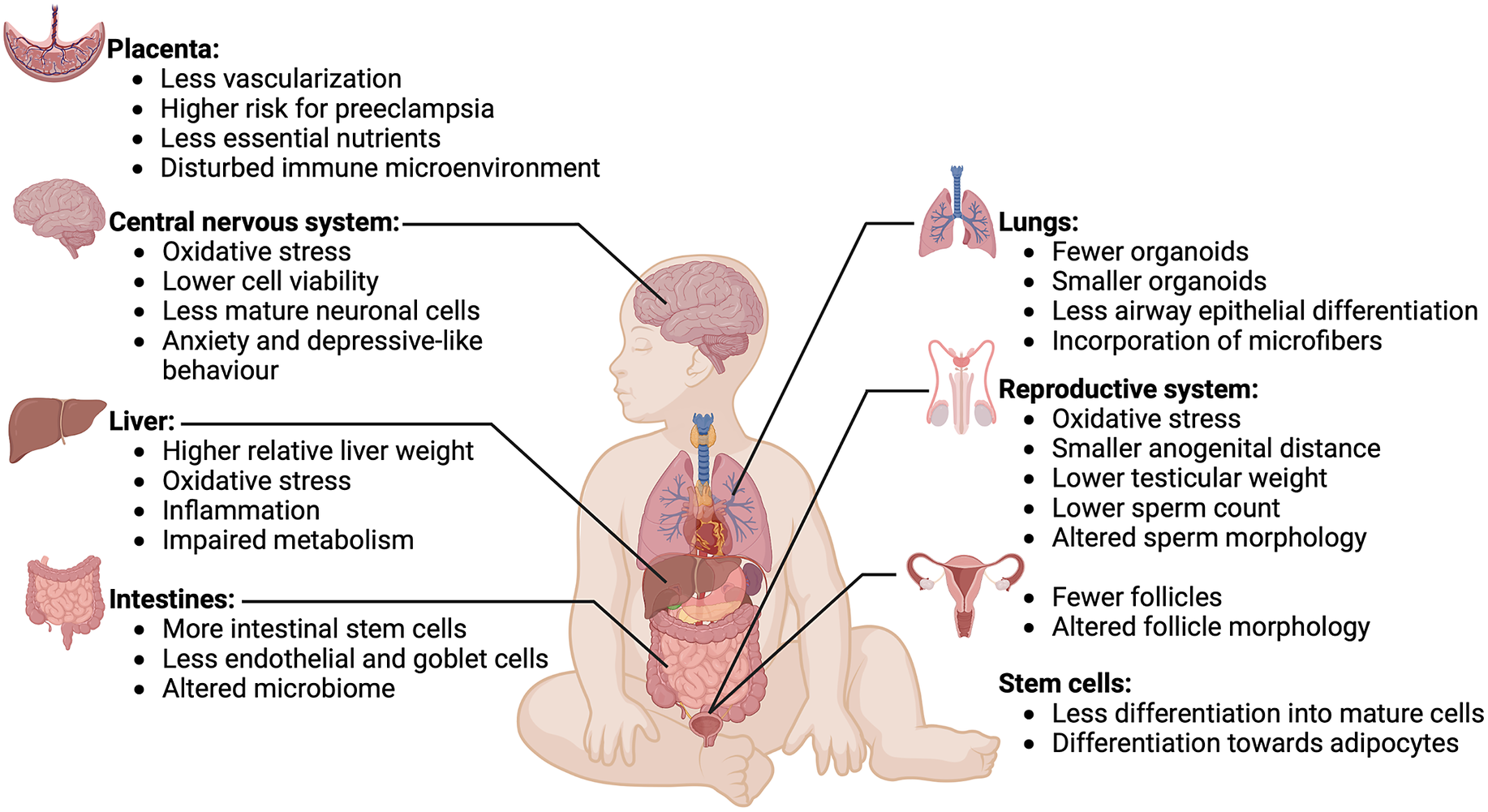
Figure 3. Effects of microplastics on various organs and tissues. Overview of effects of microplastics exposure on various organs and tissues of a developing fetus. Microplastics have detrimental effects on development of the placenta, central nervous system, liver, intestines, lungs, reproductive system and stem cells. Image created with BioRender.com.
With respect to developmental aberrations and endocrine disrupting chemicals like bisphenol A and phthalates, many more studies have been published. These have been elegantly reviewed by Rolfo et al. (Reference Rolfo, Nuzzo, De Amicis, Moretti, Bertoli and Leone2020) among others. In short, numerous studies, including in vitro studies, animal models and population-based studies, compellingly suggest that endocrine disrupting chemicals can adversely affect fetal and placental health. These disruptors potentially interfere with the developing embryonic epigenome, thereby predisposing individuals to disease in adulthood. Furthermore, endocrine-disrupting chemicals may trigger or contribute to serious pregnancy-related conditions such as preeclampsia, fetal growth restriction and gestational diabetes. Therefore, studies into effects of MNP should always consider additives contributing or being responsible for any effect that is found.
Reproductive system development
Reproductive structures already begin to form in the embryonic stage (Figure 2) (Pask, Reference Pask, Wilhelm and Bernard2016). The urogenital system is bipotential and undifferentiated until week 6 of gestation and can still develop into female and male primary sexual organs (Makiyan, Reference Makiyan2016; Garcia-Alonso et al., Reference Garcia-Alonso, Lorenzi, Mazzeo, Alves-Lopes, Roberts, Sancho-Serra, Engelbert, Marečková, Gruhn, Botting, Li, Crespo, Van Dongen, Kiselev, Prigmore, Herbert, Moffett, Chédotal, Bayraktar, Surani, Haniffa and Vento-Tormo2022). The presence of the sex-determining region Y gene on the Y chromosome is the main determining factor in development of the testes (Koopman et al., Reference Koopman, Gubbay, Vivian, Goodfellow and Lovell-Badge1991). The testes subsequently secrete anti-Müllerian hormone and the androgen testosterone, which induce differentiation toward male sex organs (Josso et al., Reference Josso, Lamarre, Picard, Berta, Davies, Morichon, Peschanski and Jeny1993; Nassar and Leslie, Reference Nassar and Leslie2023). In contrast, when an embryo lacks the Y chromosome, and thus the sex-determining region Y, there is no formation of the testes. Therefore, anti-Müllerian hormone and testosterone are not produced and this eventually leads toward development of female sex organs (Healey, Reference Healey, Mann, Blair and Garden2012; Cunha et al., Reference Cunha, Robboy, Kurita, Isaacson, Shen, Cao and Baskin2018).
Effects on reproductive system development were studied for two types of MNP, polystyrene and polyethylene. Maternal exposure to polystyrene nanoplastics resulted in lower testicular weights and altered morphology with lower sperm count in male offspring, while polyethylene microplastics reduced oocyte maturation and fertility in female offspring (Huang et al., Reference Huang, Zhang, Lin, Liu, Sun, Liu, Yuan, Xiang, Kuang, Yang and Zhang2022; Zhang et al., Reference Zhang, Wang, Zhao, Zhao, Yu, Yao, Zhao, Yu, Liu and Su2023). In both studies, oxidative stress was shown to be associated with the effects found. Further information on the effects of MNP on development of the male or female reproductive system is unfortunately limited.
An abundance of data exists concerning the impact of various plastic additives. For instance, bisphenol A is implicated in causing developmental abnormalities, as evidenced in animal models. These adverse effects seem to be particularly pronounced in the female reproductive organs. Different rodent studies found that fetal bisphenol A exposure resulted in abnormal follicle development (Susiarjo et al., Reference Susiarjo, Hassold, Freeman and Hunt2007; Rodríguez et al., Reference Rodríguez, Santambrosio, Santamaría, Muñoz-de-Toro and Luque2010; Karavan and Pepling, Reference Karavan and Pepling2012). Human oogenesis only takes place during embryonic development of the ovaries, meaning that the number of oocytes is established at birth and abnormalities during development will therefore impact fertility later in life (Feher, Reference Feher and Feher2012). Furthermore, Hunt et al. (Reference Hunt, Lawson, Gieske, Murdoch, Smith, Marre, Hassold and Vandevoort2012) treated pregnant rhesus monkeys with bisphenol A and found that second trimester fetuses had more oocytes with an abnormal number of chromosomes and abnormal morphology. Male offspring of pregnant rats exposed to bisphenol A during pregnancy and beyond had lower sperm counts and motility and less expression of steroid receptors in the testes. Worryingly this resulted in less fertility in these animals and their offspring up to the F3 generation (Salian et al., Reference Salian, Doshi and Vanage2009). Various other studies have confirmed that prenatal bisphenol A exposure can lead to sperm with abnormal morphology and to a lower sperm count and function (Vilela et al., Reference Vilela, Hartmann, Silva, Cardoso, Corcini, Varela-Junior, Martinez and Colares2014; Hass et al., Reference Hass, Christiansen, Boberg, Rasmussen, Mandrup and Axelstad2016; Rahman et al., Reference Rahman, Kwon, Karmakar, Yoon, Ryu and Pang2017). Moreover, bisphenol A exposure was also associated with a lower anogenital distance in humans (Mammadov et al., Reference Mammadov, Uncu and Dalkan2018; Sun et al., Reference Sun, Li, Liang, Miao, Song, Wang, Zhou and Yuan2018). This anogenital distance is a biomarker of fetal androgen exposure. A short distance in males is associated with genital malformations and reproductive disorders later in life (Schwartz et al., Reference Schwartz, Christiansen, Vinggaard, Axelstad, Hass and Svingen2019). Moreover, the anogenital distance is linked to adult testicular function, which is defined by sperm and testosterone production (Foresta et al., Reference Foresta, Valente, Di Nisio, Cacco, Magagna, Cosci, Presciutti and Garolla2018; Priskorn et al., Reference Priskorn, Petersen, Jørgensen, Kyhl, Andersen, Main, Andersson, Skakkebaek and Jensen2018, Reference Priskorn, Bang, Nordkap, Krause, Mendiola, Jensen, Juul, Skakkebaek, Swan and Jørgensen2019). Notably, prenatal exposure to phthalates has also been linked to a smaller anogenital distance in human infants (Swan et al., Reference Swan, Main, Liu, Stewart, Kruse, Calafat, Mao, Redmon, Ternand, Sullivan and Teague2005; Bornehag et al., Reference Bornehag, Carlstedt, Jönsson, Lindh, Jensen, Bodin, Jonsson, Janson and Swan2015). Various animal studies confirmed these human findings in offspring of rats or mice who were treated with different concentrations of phthalates during pregnancy (Ma et al., Reference Ma, Yin, Han, Ding, Zhang, Han and Li2017; Hsu et al., Reference Hsu, Jhong, Huang, Lee, Chen and Guo2021). The offspring of phthalate-exposed animals also showed signs of histological damage in the testes and apoptosis of cells in the seminiferous tubule, which is responsible for sperm production (Ma et al., Reference Ma, Yin, Han, Ding, Zhang, Han and Li2017). The female reproductive system was also affected by phthalate exposure. Mice that were prenatally exposed to phthalates had fewer healthy follicles and this effect was found up to the F3 generation also having lower total follicle numbers (Brehm et al., Reference Brehm, Rattan, Gao and Flaws2018; Repouskou et al., Reference Repouskou, Panagiotidou, Panagopoulou, Bisting, Tuck, Sjödin, Lindberg, Bozas, Rüegg, Gennings, Bornehag, Damdimopoulou, Stamatakis and Kitraki2019).
In summary, though the evidence from animal models is limited, it suggests that MNP could negatively affect the development of the reproductive system in fetuses, potentially leading to infertility or other reproductive issues (Figure 3). There is more robust and compelling evidence for the adverse effects of additives, indicating that MNP can harm developing reproductive organs through these substances. Therefore, studies investigating how much of the additive presence in humans is derived from microplastics exposure will be invaluable.
Central nervous system development
One of the first steps in the development of the nervous system begins in the third week of gestation and is the differentiation of ectoderm into neuroectoderm (Pleasure et al., Reference Pleasure, Pleasure, Pleasure, Polin, Abman, Rowitch, Benitz and Fox2017). Neuroectoderm cells are neural stem cells that are capable of self-renewal and can differentiate into neurons (Sansom et al., Reference Sansom, Griffiths, Faedo, Kleinjan, Ruan, Smith, Van Heyningen, Rubenstein and Livesey2009; Zhang et al., Reference Zhang, Huang, Chen, Pankratz, Xi, Li, Yang, Lavaute, Li, Ayala, Bondarenko, Du, Jin, Golos and Zhang2010; Thakurela et al., Reference Thakurela, Tiwari, Schick, Garding, Ivanek, Berninger and Tiwari2016). These processes can be modeled in vitro with embryonic or pluripotent stem cells and can then be used to study effects of MNP. Neurospheres generated from human embryonic stem cells were exposed to polyethylene nanoplastics and particles were found to penetrate deep into these neurospheres, causing oxidative stress with higher levels of malondialdehyde. Importantly, the expression of genes that play crucial roles in embryonic neural development was lower indicating that polyethylene nanoplastics can impair neural development (Hoelting et al., Reference Hoelting, Scheinhardt, Bondarenko, Schildknecht, Kapitza, Tanavde, Tan, Lee, Mecking, Leist and Kadereit2013). Another study used human-induced pluripotent stem cells and differentiated them into human forebrain cortical spheroids. Exposing these cortical spheroids to polystyrene MNP resulted in lower cell viability and expression of mature neuronal markers indicating that polystyrene MNP can also affect neural differentiation (Hua et al., Reference Hua, Kiran, Li and Sang2022).
Others have investigated the effects of microplastic exposure in mouse models. Yang et al. treated pregnant mice with polystyrene MNP. Polystyrene nanoplastics were observed throughout the fetus including the brain, especially in the thalamus. Excessive production of reactive oxygen species, more apoptosis, less proliferation, less gamma-aminobutyric acid synthesis and less expression of mature neuronal genes were found compared to unexposed fetuses. Moreover, mice showed more anxiety-like behavior in several tests after nanoplastic treatment. Together these findings indicate that polystyrene nanoparticle exposure during gestation can inhibit fetal brain development, which may result in anxiety (Yang et al., Reference Yang, Zhu, Zhou, Pan, Nan, Yin, Lei, Ma, Zhu, Chen, Han, Ding and Ding2022).
In addition to in utero exposure, maternally ingested polystyrene nanoplastics were also found to reach the brain via breast milk (Jeong et al., Reference Jeong, Baek, Koo, Park, Ryu, Kim, Zhang, Chung, Dogan, Choi, Um, Kim, Lee, Jeong, Shin, Lee, N-S and Lee2022). Milk-exposed pups showed less proliferation and lower expression of mature neuronal genes in the hippocampus, which indicates impairment of neuronal development. This reduced brain development resulted in neuronal dysfunction and cognitive deficit, which was dependent on estrogen receptor alpha and was more severe in exposed females.
Finally, bisphenol A and phthalates also have detrimental effects on neural development. Various studies found that these endocrine disrupting chemicals can diminish neural differentiation, impair neurotransmission pathways and diminish myelination (Zhou et al., Reference Zhou, Chen, Feng, Zhou, Li and Chen2015; Grohs et al., Reference Grohs, Reynolds, Liu, Martin, Pollock, Lebel and Dewey2019; Tiwari et al., Reference Tiwari, Agarwal, Chauhan, Mishra and Chaturvedi2019; Lucaccioni et al., Reference Lucaccioni, Trevisani, Passini, Righi, Plessi, Predieri and Iughetti2021). Moreover, exposure to bisphenol A and phthalates during gestation is linked to behavioral problems (particularly in girls), lower nonverbal IQ scores, anxiety and depression-like behavior (Zhou et al., Reference Zhou, Chen, Feng, Zhou, Li and Chen2015; Ejaredar et al., Reference Ejaredar, Lee, Roberts, Sauve and Dewey2017; Daniel et al., Reference Daniel, Balalian, Insel, Liu, Whyatt, Calafat, Rauh, Perera, Hoepner, Herbstman and Factor-Litvak2020; Van Den Dries et al., Reference Van Den Dries, Guxens, Spaan, Ferguson, Philips, Santos, Jaddoe, Ghassabian, Trasande, Tiemeier and Pronk2020; Guilbert et al., Reference Guilbert, Rolland, Pin, Thomsen, Sakhi, Sabaredzovic, Slama, Guichardet and Philippat2021; Rolland et al., Reference Rolland, Lyon-Caen, Thomsen, Sakhi, Sabaredzovic, Bayat, Slama, Méary and Philippat2023).
Together these studies indicate that MNP and/or their additives can directly or indirectly impair neural development. Whether this is happening in humans will depend on the number of particles that can reach the human brain and this information is sadly lacking (Figure 3). Moving forward in this field, large longitudinal studies following brain development in children in combination with analysis of MNP exposure will be necessary. Having the possibility to image MNP presence in brain tissue would be an enormous step forward, but for now is still science fiction.
Intestinal development and regeneration
Intestines develop from endoderm forming a hollow cylinder surrounded by cells of the mesoderm resulting in a primitive intestinal tube, whereas the ectoderm forms the enteric nervous system during week three of gestation (Spence et al., Reference Spence, Lauf and Shroyer2011). Then the foregut develops into esophagus, lung, stomach, liver and pancreas and the midgut and hindgut into the small and large intestines (Sheaffer and Kaestner, Reference Sheaffer and Kaestner2012; Chin et al., Reference Chin, Hill, Aurora and Spence2017; McCracken and Wells, Reference McCracken and Wells2017; Zhang et al., Reference Zhang, Jiang, Kim, Lin, Liu, Lan and Que2017). Signaling pathways controlling intestinal stem cell self-renewal include Wnt and Notch (Van Camp et al., Reference Van Camp, Beckers, Zegers and Van Hul2014; Demitrack and Samuelson, Reference Demitrack and Samuelson2016).
Unfortunately, no studies investigated effects of microplastics or additives on intestinal development, but some did use organoids. Intestinal organoids are an excellent way to study intestinal repair mechanisms and reactivated developmental pathways. Intestinal mouse organoids exposed to polystyrene MNP had higher expression of Notch pathway genes, other intestinal stem cell markers and proliferation markers compared to unexposed organoids. In contrast, the expression of endothelial and goblet cell markers was lower, indicating that polystyrene MNP can stimulate stemness but impair cell differentiation by overstimulation of Notch signaling (Xie et al., Reference Xie, Zhang, Li, Liu, Chen and Yu2023).
Effects of MNP on microbiome development were also investigated and this is of relevance because the microbiome can influence the function of the gut. Environmental stimuli in their turn can influence development of the microbiome, especially up until the age of 5 (Stiemsma and Turvey, Reference Stiemsma and Turvey2017; Roswall et al., Reference Roswall, Olsson, Kovatcheva-Datchary, Nilsson, Tremaroli, Simon, Kiilerich, Akrami, Krämer, Uhlén, Gummesson, Kristiansen, Dahlgren and Bäckhed2021; Wernroth et al., Reference Wernroth, Peura, Hedman, Hetty, Vicenzi, Kennedy, Fall, Svennblad, Andolf, Pershagen, Theorell-Haglöw, Nguyen, Sayols-Baixeras, Dekkers, Bertilsson, Almqvist, Dicksved and Fall2022). Infants can be exposed to high levels of MNP through bottle feeding, since MNP are found in various milk products and can be released from feeding bottles (Li et al., Reference Li, Shi, Yang, Xiao, Kehoe, Gun’Ko, Boland and Wang2020; Da Costa Filho et al., Reference Da Costa Filho, Andrey, Eriksen, Peixoto, Carreres, Ambühl, Descarrega, Dubascoux, Zbinden, Panchaud and Poitevin2021). In addition, infants can ingest MNP through breastfeeding, as MNP have been found in human breastmilk (Ragusa et al., Reference Ragusa, Notarstefano, Svelato, Belloni, Gioacchini, Blondeel, Zucchelli, De Luca, D’Avino, Gulotta, Carnevali and Giorgini2022b; Liu et al., Reference Liu, Guo, Liu, Yang, Wang, Sun, Chen and Dong2023). Notably, MNP were found in both infant formula and in the feces of infants consuming these types of milk (Zhang et al., Reference Zhang, Wang, Trasande and Kannan2021; Liu et al., Reference Liu, Guo, Liu, Yang, Wang, Sun, Chen and Dong2023). Fournier et al. processed stool of infants in a novel fermentation system that simulates physicochemical and microbial conditions in the intestines of a toddler. Exposing the microbiome of infants to polyethylene MNP resulted in lower numbers of healthy microbes and more opportunistic pathogens. Consequently, an altered microbial metabolic activity was found including a changed volatile organic compounds profile with less butyrate production (Fournier et al., Reference Fournier, Ratel, Denis, Leveque, Ruiz, Mazal, Amiard, Edely, Bezirard, Gaultier, Lamas, Houdeau, Engel, Lagarde, Etienne-Mesmin, Mercier-Bonin and Blanquet-Diot2023). This is of interest because higher butyrate production is associated with protection against allergies and asthma (Roduit et al., Reference Roduit, Frei, Ferstl, Loeliger, Westermann, Rhyner, Schiavi, Barcik, Rodriguez-Perez, Wawrzyniak, Chassard, Lacroix, Schmausser-Hechfellner, Depner, von Mutius, Braun-Fahrländer, Karvonen, Kirjavainen, Pekkanen, Dalphin, Riedler, Akdis, Lauener and O’Mahony2019; Depner et al., Reference Depner, Taft, Kirjavainen, Kalanetra, Karvonen, Peschel, Schmausser-Hechfellner, Roduit, Frei, Lauener, Divaret-Chauveau, Dalphin, Riedler, Roponen, Kabesch, Renz, Pekkanen, Farquharson, Louis, Mills, von Mutius, Genuneit, Hyvärinen, Illi, Laurent, Pfefferle, Schaub, von Mutius and Ege2020). These findings indicate that polyethylene MNP could cause significant disturbances in the microbiota of infants (Fournier et al., Reference Fournier, Ratel, Denis, Leveque, Ruiz, Mazal, Amiard, Edely, Bezirard, Gaultier, Lamas, Houdeau, Engel, Lagarde, Etienne-Mesmin, Mercier-Bonin and Blanquet-Diot2023).
Despite the intestinal system’s pivotal role in MNP entry into the body, knowledge regarding their effects on intestinal development and repair remains surprisingly sparse. A limited number of existing studies suggest that the ingestion of MNP may hinder the differentiation of intestinal epithelial cells and cause microbiota dysbiosis (Figure 3). Given the vital nature of the intestinal system as a primary entry point for MNP, it is imperative that this area receives a significantly greater level of research focus than it currently does.
Liver development
While the intestines originate from the midgut and hindgut, the liver develops from the more distal end of the foregut in the third week of gestation (Sheaffer and Kaestner, Reference Sheaffer and Kaestner2012; Chin et al., Reference Chin, Hill, Aurora and Spence2017). Endoderm-derived cells develop further in hepatoblasts, that differentiate with hepatocyte growth factor and Wnt signaling into hepatocytes or cholangiocytes (Shin and Monga, Reference Shin and Monga2013; Giancotti et al., Reference Giancotti, Monti, Nevi, Safarikia, D’Ambrosio, Brunelli, Pajno, Corno, Di Donato, Musella, Chiappetta, Bosco, Benedetti Panici, Alvaro and Cardinale2019).
With respect to effects of MNP on liver development, offspring of pregnant mice exposed to polystyrene MNP had higher liver weights in comparison to control mice. Moreover, expression of hepatic genes involved in fatty acid metabolism was lower. However, the observed results were sex-specific and dependent on the size of the polystyrene MNP. Whereas anabolic pathways were slower in males, females maintained the synthesis of lipids at the costs of amino acids. Eventually, these changes induced by maternal polystyrene microplastic exposure resulted in a higher risk of hepatic lipid accumulation and the development of metabolic disorders in offspring (Luo et al., Reference Luo, Zhang, Wang, Wang, Zhou, Shen, Zhao, Fu and Jin2019a, Reference Luo, Wang, Pan, Jin, Fu and Jin2019b). Interestingly, no significant change in liver weight in offspring was found after maternal exposure to nanoplastic in a study of Huang et al. (Reference Huang, Zhang, Lin, Liu, Sun, Liu, Yuan, Xiang, Kuang, Yang and Zhang2022). Therefore, the development of a higher liver weight is probably size-dependent since it was only found after maternal exposure of polystyrene microplastic of 5 μm (Luo et al., Reference Luo, Wang, Pan, Jin, Fu and Jin2019b). Interestingly, exposure of pregnant mice to polystyrene nanoplastics did induce higher levels of malondialdehyde and pro-inflammatory cytokines in liver tissue of their offspring indicating oxidative stress induction. Moreover, metabolomics revealed that levels of metabolites involved in carbohydrate metabolism were lower. Therefore, these results show that maternal exposure to polystyrene nanoplastics can trigger hepatic oxidative stress and inflammation resulting in a disrupted carbohydrate metabolism in their offspring (Huang et al., Reference Huang, Zhang, Lin, Liu, Sun, Liu, Yuan, Xiang, Kuang, Yang and Zhang2022).
In addition to studies on the direct effects of MNP on liver development, some examined the effects of additives. Different studies showed that prenatal bisphenol A and phthalate exposure can impair liver development, resulting in metabolic disorders including changes to glucose and lipid metabolism (Maranghi et al., Reference Maranghi, Lorenzetti, Tassinari, Moracci, Tassinari, Marcoccia, Di Virgilio, Eusepi, Romeo, Magrelli, Salvatore, Tosto, Viganotti, Antoccia, Di Masi, Azzalin, Tanzarella, Macino, Taruscio and Mantovani2010; Strakovsky et al., Reference Strakovsky, Wang, Engeseth, Flaws, Helferich, Pan and Lezmi2015; DeBenedictis et al., Reference DeBenedictis, Guan and Yang2016; Sol et al., Reference Sol, Santos, Duijts, Asimakopoulos, Martinez-Moral, Kannan, Jaddoe and Trasande2020; Long et al., Reference Long, Fan, Wu, Liu, Wu, Liu, Chen, Su, Cheng, Xu, Su, Cao, Zhang, Hai and Wang2021).
Although the evidence is not abundant, the available studies suggest that maternal exposure to MNP and additives leaching from plastics can detrimentally affect liver development, resulting in metabolic disorders (Figure 3). Again, given the proximity of the liver to a main port of MNP entry, more focus on this organ is warranted.
Lung development and regeneration
Another organ that develops from the primitive foregut is the lung. First, two independent outpouchings of the more proximal end of the foregut arise and these two lung buds develop into a tree-like system of airways ending in respiratory units called alveoli (Warburton et al., Reference Warburton, Bellusci, De Langhe, Del Moral, Fleury, Mailleux, Tefft, Unbekandt, Wang and Shi2005; Schittny, Reference Schittny2017). Eventually, airways consist of pseudostratified epithelial cells with basal cells, ciliated cells and secretory cells such as goblet and club cells, while alveoli are lined with alveolar epithelial cell types I and II (Desai et al., Reference Desai, Brownfield and Krasnow2014; Li et al., Reference Li, He, Wei, Cho and Liu2015). Some of these epithelial cells have progenitor functions that reactivate developmental pathways when repair of damaged structures is needed (Levardon et al., Reference Levardon, Yonker, Hurley and Mou2018; Olajuyin et al., Reference Olajuyin, Zhang and Ji2019; Davis and Wypych, Reference Davis and Wypych2021). Like for the intestine, pathways important in regulation of lung development and repair include Notch and Wnt (Kiyokawa and Morimoto, Reference Kiyokawa and Morimoto2020; Aros et al., Reference Aros, Pantoja and Gomperts2021).
We recently generated human and mouse lung organoids that self-assemble from isolated primary lung epithelial cells. These developing mouse and human lung organoids were exposed to polyester or polyamide 6,6 microfibers for 14 days. Both polyester and polyamide 6,6 microfiber exposure resulted in lower numbers and sizes of mouse and human organoids with the effects of polyamide 6,6 being most profound. Interestingly, this effect of polyamide 6,6 was mediated by compounds leaching from the microfibers. The observed effects of the leachate did not affect stemness of epithelial progenitors, or fully developed epithelial cells, but specifically inhibited differentiation of progenitor cells into airway epithelial cell types. Therefore, these results indicate that exposure to plastic microfibers and compounds leaching from them may affect developing lungs by impairing epithelial differentiation (Dijk et al., Reference Dijk, Song, Eck, Wu, Bos, Boom, Kooter, Spierings, Wardenaar, Cole, Salvati, Gosens and Melgert2021).
Winkler et al. (Reference Winkler, Cherubini, Rusconi, Santo, Madaschi, Pistoni, Moschetti, Sarnicola, Crosti, Rosso, Tremolada, Lazzari and Bacchetta2022) examined the effects of polyester microfiber exposure on developing human airway organoids in more detail. In this study, organoid growth was not affected by exposure to polyester microfibers and exposure did not induce oxidative stress or cell activation. However, lower levels of club cell markers were found, suggesting that exposure to polyester microfibers can inhibit generation of this epithelial cell type from progenitors.
Collectively, these two studies demonstrate that MNP and compounds leaching from MNP can have significant effects on differentiation of lung epithelial cells (Figure 3).
Regeneration in other tissues
Fetal development is highly dependent on specific pathways that are subsequently downregulated after birth. Interestingly, these pathways are reactivated when tissue damage occurs and regeneration is required. This includes, for instance, regeneration of damaged skeletal muscles after exercise, which is mainly governed by normally quiescent satellite cells (Relaix and Zammit, Reference Relaix and Zammit2012). After reactivation, these stem cells proliferate and differentiate into myogenic cells to repair damaged myofibers (Yin et al., Reference Yin, Price and Rudnicki2013; Zhang et al., Reference Zhang, Zhang, Gu, Lan, Liu, Wang, Su, Ge, Wang, Yu, Liu, Li, Li, Zhao, Yu, Wang, Li and Meng2018). When mice were exposed to polystyrene MNP for 30 days and skeletal muscle injury was induced on day 25, polystyrene microplastic exposure resulted in lower relative muscle weights, less and smaller myofibers, lower gene and protein expression of myogenic differentiation markers, more lipid deposition and more expression of adipogenic markers after injury compared to control mice (Shengchen et al., Reference Shengchen, Jing, Yujie, Yue and Shiwen2021). In another study, pregnant mice were exposed to polystyrene nanoplastics, which resulted in an abnormal morphology of skeletal muscles and in lower expression of genes involved in muscle development in their offspring (Chen et al., Reference Chen, Xiong, Jing, van Gestel, van Straalen, Roelofs, Sun and Qiu2023).
Other cells capable of regeneration are mesenchymal stromal cells (Pittenger et al., Reference Pittenger, Discher, Péault, Phinney, Hare and Caplan2019). These multipotent cells can differentiate into osteocytes, chondrocytes and adipocytes (Uccelli et al., Reference Uccelli, Moretta and Pistoia2008). Najahi et al. exposed human bone marrow and adipose mesenchymal stromal cells to polyethylene terephthalate MNP. Less proliferation and self-renewal and more senescence, reactive oxygen species and DNA damage was found in both types of mesenchymal stromal cells after exposure. Polyethylene terephthalate exposure also induced higher expression of early adipose markers in adipose mesenchymal stromal cells and expression of early chondrocyte markers in bone marrow mesenchymal stromal cells compared to untreated mesenchymal stromal cells. These findings indicate that polyethylene terephthalate MNP can induce stress and impair differentiation of mesenchymal stromal cells into more mature cells (Najahi et al., Reference Najahi, Alessio, Squillaro, Conti, Ferrante, Di Bernardo, Galderisi, Messaoudi, Minucci and Banni2022).
In a study conducted by Im et al., the exposure of human bone marrow mesenchymal stromal cells to polystyrene nanoplastics led to a decrease in reactive oxygen species levels and suppressed expression of markers for osteocytes, chondrocytes and neurons. Intriguingly, genes associated with adipogenesis were again markedly elevated, suggesting a shift in differentiation toward adipocytes (Im et al., Reference Im, Kim, Jo, Yoo, Kim, Park, Hyeon, G-R and Bhang2022). Therefore, MNP exposure appears to slow down muscle repair and promote adipogenic differentiation of mesenchymal stromal cells (Figure 3).
Perspectives and conclusion
The presence of MNP in our environment and our food and drinks is evident and exposure to significant levels appears unavoidable (Gasperi et al., Reference Gasperi, Wright, Dris, Collard, Mandin, Guerrouache, Langlois, Kelly and Tassin2018; Li et al., Reference Li, Liu and Paul Chen2018; Koelmans et al., Reference Koelmans, Mohamed Nor, Hermsen, Kooi, Mintenig and De France2019; Evangeliou et al., Reference Evangeliou, Grythe, Klimont, Heyes, Eckhardt, Lopez-Aparicio and Stohl2020; Zhang et al., Reference Zhang, Wang and Kannan2020; Zhou et al., Reference Zhou, Wang, Zou, Jia, Zhou and Li2020; Jenner et al., Reference Jenner, Sadofsky, Danopoulos and Rotchell2021; Jin et al., Reference Jin, Wang, Ren, Wang and Shan2021; Dronjak et al., Reference Dronjak, Exposito, Rovira, Florencio, Emiliano, Corzo, Schuhmacher, Valero and Sierra2022; Shi et al., Reference Shi, Dong, Shi, Yin, He, An, Tang, Hou, Chong, Chen, Qin and Lin2022). Consequently, MNP have been found throughout the entire human body including the placenta, which is of great concern for development (Ibrahim et al., Reference Ibrahim, Tuan Anuar, Azmi, Wan Mohd Khalik, Lehata, Hamzah, Ismail, Ma, Dzulkarnaen, Zakaria, Mustaffa, Tuan Sharif and Lee2021; Ragusa et al., Reference Ragusa, Svelato, Santacroce, Catalano, Notarstefano, Carnevali, Papa, Rongioletti, Baiocco, Draghi, D’Amore, Rinaldo, Matta and Giorgini2021; Horvatits et al., Reference Horvatits, Tamminga, Liu, Sebode, Carambia, Fischer, Püschel, Huber and Fischer2022; Jenner et al., Reference Jenner, Rotchell, Bennett, Cowen, Tentzeris and Sadofsky2022; Leslie et al., Reference Leslie, van Velzen, Brandsma, Vethaak, Garcia-Vallejo and Lamoree2022; Zhu et al., Reference Zhu, Zhu, Zuo, Xu, Qian and An2023). However, the effects of microplastic exposure on (human) development or repair mechanisms are not well known and the absence of evidence of risk cannot be translated into evidence for the absence of risk (Leslie and Depledge, Reference Leslie and Depledge2020; Gouin et al., Reference Gouin, Cunliffe, De France, Fawell, Jarvis, Koelmans, Marsden, Testai, Asami, Bevan, Carrier, Cotruvo, Eckhardt and Ong2021; Wardman et al., Reference Wardman, Koelmans, Whyte and Pahl2021).
Most studies have predominantly explored the impacts of MNP composed of polystyrene or polyethylene terephthalate, typically focusing on a singular size category or specific additive types such as bisphenol A or phthalates. Yet MNPs are diverse in their polymer composition, size and additives. As such, there is a pressing need for broader research into the impacts of environmentally relevant MNPs, incorporating varying types, sizes and their leachates.
The design and methodological approaches of these investigations are crucial. This includes the selection of the cell types and the experimental setup, which should closely emulate our constant exposure to probably low to moderate MNP levels. Regrettably, most current experimental models do not reflect these conditions, as they often focus on short-term, high-level exposure, resulting in immediate toxicological outcomes such as cell death, oxidative stress or cytokine release (Weis and Palmquist, Reference Weis and Palmquist2021). The study of continuous, low-dose exposure, relevant exposure routes and developmental or repair processes are rarely prioritized and neither are studies into mechanisms, even though they are highly pertinent to real-life exposure scenarios. The frequent use of cancer cell lines and culture conditions that do not represent in vivo conditions, such as serum-free cultures, further confounds the ability to predict the biological impact of MNP exposure accurately.
Organoids derived from primary cells could help overcome some of these limitations by mimicking complex organ systems. Nonetheless, they also have their own constraints, including atypical physiology, irrelevant exposure pathways and lack of interorgan communication. Thus, the use of animal models remains essential to study the complex human physiological interactions. Care should be taken to use these models in a way that represents common human exposure with credible exposure levels and modes and to focus on mechanisms behind any effects found in these systems.
Another significant factor to keep in mind when evaluating literature is the presence of a positive publication bias that also affects the field of microplastics research. This bias tends to favor studies demonstrating harmful effects of microplastics on biological systems, which can inadvertently skew the overall understanding of their impact. As a result, studies that find no significant effects often face a higher hurdle in terms of getting published, which can potentially silence an essential aspect of the conversation. This imbalance can limit the diversity of our knowledge, creating a distorted picture of microplastics’ overall influence. It is crucial to address this bias to ensure a more comprehensive, balanced and accurate understanding of microplastics and their effects, or lack thereof, on biological systems. Ensuring the publication of all research outcomes, irrespective of their direction, will help to avoid overestimating the effects of microplastics and support the development of effective and proportionate response strategies.
In conclusion, we have provided an extensive overview of studies investigating effects of MNP on developmental and regenerative processes. MNP and additives commonly leaching from them can impair differentiation and/or promote aberrant differentiation. Therefore, MNP could have detrimental effects on a developing fetus and/or on repair processes in adult tissues of humans. The scant available data indicate that for all discussed organs, there is reason for concern (Vethaak and Legler, Reference Vethaak and Legler2021). By addressing these knowledge gaps in years to come, we may be able to protect ourselves from potential health risks induced by MNP.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/plc.2023.19.
Data availability statement
Data sharing not applicable – no new data generated.
Author contribution
L.T.H. and B.N.M. conceived the outline of the manuscript. L.T.H. collected published studies to include in this overview and drafted a first version of the manuscript. All the authors critically reviewed and revised the manuscript and approved the final version for publication.
Financial support
The authors thank ZonMw for their financial support with the ZonMw/Health Holland consortium grant MOMENTUM (458001101) led by Prof Dr. J. Legler and Prof Dr. D. Vethaak and awarded to them and Melgert among others.
Competing interest
The authors declare no competing interests exist.






Comments
No accompanying comment.