The Insect Limestone is a discrete bed of fine-grained, hard, muddy, freshwater to hypersaline limestone near the base of the Bembridge Marls Member of the Bouldnor Formation (Munt Reference Munt2014; Ross & Self Reference Ross and Self2014). Its age is late Priabonian, thus latest Eocene (Hooker et al. Reference Hooker, Grimes, Mattey, Collinson and Sheldon2009). Insect and plant remains are relatively common, whilst vertebrate remains are exceptionally rare and are limited to fragmentary skeletal elements of fish, lizard, bird and mammal, bird feathers and pieces of shed lizard skin. They come from four sites on the Isle of Wight: the vicinity of Gurnard Point (including Gurnard Bay and Sticeletts Ledge); Saltmead Ledge; Thorness Bay; and St Helens. However, old collections made by A'Court Smith, which have subsequently passed through the hands of two other collectors, Brodie and Hooley, and are labelled Gurnet or Gurnard Bay, are likely to also include material from Thorness Bay and Saltmead Ledge (Ross & Self Reference Ross and Self2014). The vertebrate assemblage is unusual in its anatomical and preservational composition. The bird feathers and two pieces of lizard skin are from 19th-Century collections, whereas the rest are from recent collecting by Andy Yule. The material is housed in either the Dinosaur Isle Museum, Sandown (IWCMS), or the Natural History Museum, London (NHMUK).
1. Osteichthyes
Osteichthyes are restricted to indeterminate and largely fragmentary bones that do not warrant description here. One specimen (IWCMS.1998.7) from Thorness Bay is ‘in the round' and apparently partially articulated, but largely embedded in matrix. One vertebra is exposed, showing it to be a teleost. Another specimen (NHMUK.PV.P74981) from Saltmead Ledge shows the posterior part of the trunk, with the rest embedded in the block. Preparation or computed tomography scanning could reveal a complete fish, but this is beyond the scope of the present project.
2. Squamata
Western European lizard faunas were at their most diverse in the globally warm early Eocene, with records of pleurodont and acrodont iguanians, gekkotans, lacertiforms, scincoids and a variety of anguimorphs (reviewed in Augé Reference Augé2003a, Reference Augé, Wing, Gingerich, Schmitz and Thomasb, Reference Augé2005). This changed as the climate cooled, culminating in a major drop in diversity just after the Eocene–Oligocene boundary (e.g., Rage Reference Rage, Pomerol and Premoli-Silva1986, Reference Rage2006, Reference Rage2012; Augé Reference Augé2000, Reference Augé2005; Augé & Smith Reference Augé and Smith2009). Much of the published data on Eocene lizards come from deposits in the Franco–Belgium Basin and Germany (reviewed in Augé Reference Augé, Wing, Gingerich, Schmitz and Thomas2003b, Reference Augé2005), and recently Spain (Bolet & Evans Reference Bolet and Evans2013), but Eocene lizard faunas have also been recovered from a series of horizons in southern England (e.g., Milner et al. Reference Milner, Milner and Estes1982; Green Reference Green1998). The most diverse of these are from the late Eocene (Priabonian) of the Hampshire Basin (Green Reference Green1998), where Green recorded the presence of iguanians, gekkotans, scincoids, lacertids, necrosaurs, varanids, anguids and glyptosaurs. However, most of these groups had dropped out of the Hampshire Basin record by the level of the Bembridge Limestone deposits (Palaeotherium medium medium–Palaeotherium curtum curtum Zone, MP19), leaving only gekkotans, anguids and lacertids in horizons contemporaneous with the Insect Limestone.
2.1. Scincoidea
NHMUK.PV.R36683 is the mid-section of a left dentary, preserving ten tooth positions of which eight are filled (Fig. 1a–h). The specimen was exposed in labial view but it has been removed from the matrix to reveal details of the lingual surface. However, owing to the delicacy of the specimen, it was unsafe to remove the remaining matrix. As preserved, the dentary fragment is 4.7mm long and the whole bone was probably originally no more than 10mm in length (giving a maximal skull length of around 20mm). The jaw is shallow and its ventral edge is straight rather than convex.
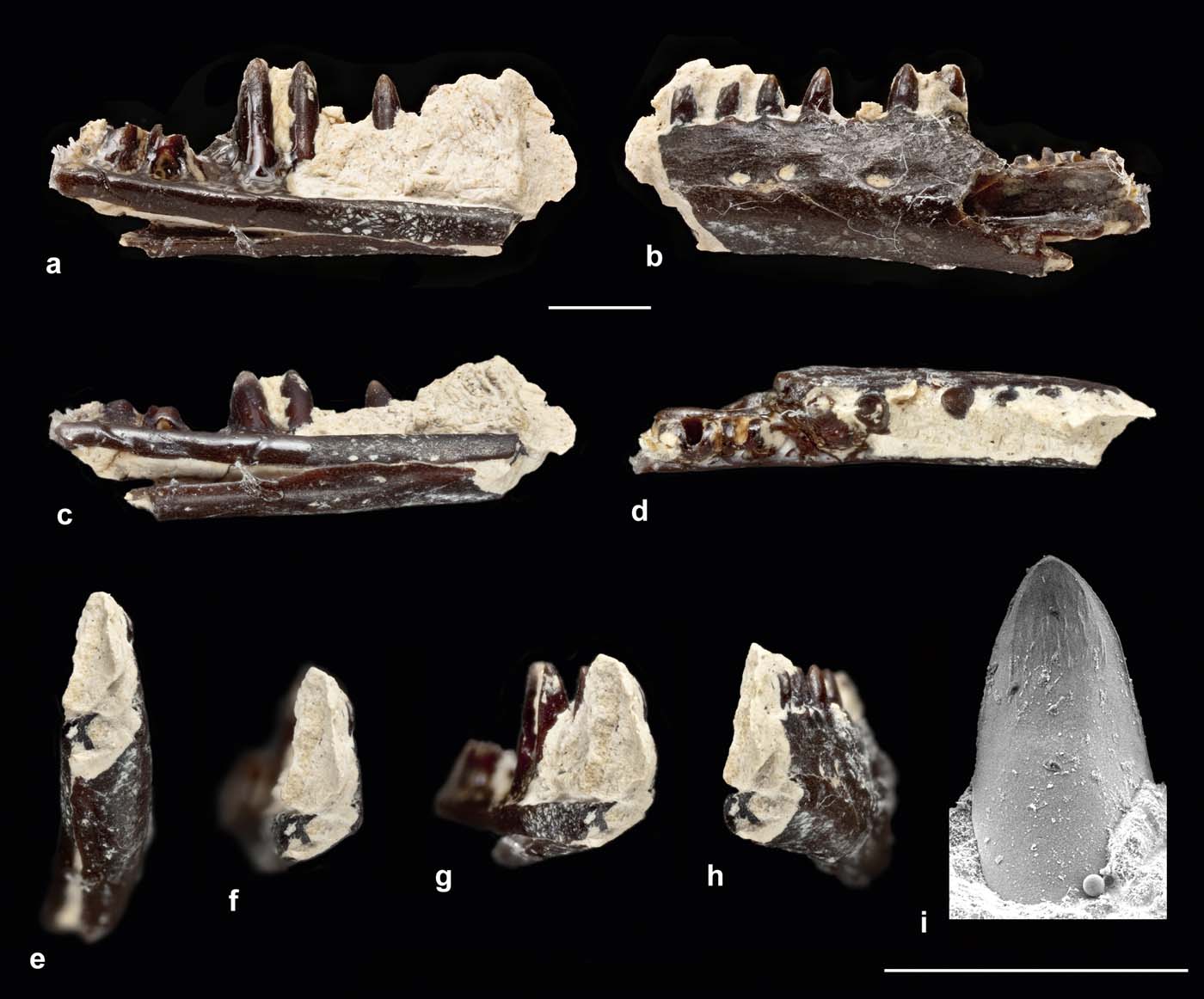
Figure 1 Left dentary of scincoid lizard, from Saltmead Ledge, NHMUK.PV.R36683. (a–h) Light photographs of entire dentary. (i) Scanning electron micrograph of tooth tip. Views are: (a, i) lingual; (b) labial; (c) ventrolingual; (d) occlusal; (e) anteroventral; (f) anterior; (g) anterolingual; (h) anterolabial. Scale bars=1mm (a–h); 0.5mm (i).
Seen in lingual view (Fig. 1a), the teeth are relatively large compared to the more ventral part of the bone and the implantation is fully pleurodont. There is a shallow subdental groove (Fig. 1a, c) and the dorsal margin of the subdental ridge is raised to form a low parapet lingual to the tooth bases. The subdental ridge is deeper anteriorly than posteriorly, but it narrows gradually so that the ventral margin is straight. Below it, the Meckelian fossa is small, shallow and mainly ventral in position (Fig. 1a, c, e–g). Up to the level of the sixth preserved tooth position, the fossa is closed by an expansion of the ventrolateral jaw margin, coupled with a distinct ventromedial angulation of the labial surface (Fig. 1f), which creates a slight ridge along this surface of the jaw (Fig. 1b). The ventral edge of the subdental ridge contacts the ventrolateral expansion to enclose the Meckelian fossa, but the two edges are sutured rather than fused (Fig. 1a, c). The dorsomedial and ventromedial borders separate further posteriorly but the Meckelian fossa remains shallow and ventral in position. The preserved portion of the subdental ridge bears no trace of a splenial facet and, if present, the splenial would have been small and posteriorly placed. The labial surface of the bone (Fig. 1b) is perforated by three neurovascular foramina and a slight groove at the anterior margin suggests at least one more foramen lay in front of this.
The teeth are cylindrical with a labiolingually compressed crown. This crown is smooth labially and slightly incurved. The teeth lack distinct distal and mesial angles and have a smooth, curved profile. Lingually, each has widely spaced striae dominantes (sensu Richter Reference Richter1994) and a short carina intercuspidalis, but the lingual and labial cusps are not prominent. Fine additional striae are visible on the lingual surface.
Fragmentary though the specimen is, attribution to Anguimorpha, Gekkota, Iguania or Lacertoidea can be excluded. Unlike anguimorph jaws, there is lingual tooth replacement (as opposed to posterior or posterolingual), a relatively deep subdental ridge (as opposed to a shallow shelf), little development of crown striations and a closed Meckelian fossa. The latter feature occurs in most living gekkotans, many iguanians and some scincoids. However, in gekkotans the upper and lower edges of the Meckelian fossa are usually fused with no trace of a suture. Gekkotan jaws typically also have a deep subdental gutter lingually, and the teeth have pronounced lingual and labial apical cusplets. None of these features are found in NHMUK.PV.R36683. Pleurodont iguanian tooth shafts are typically longer and more deeply pleurodont than in the Insect Limestone jaw, and the tooth crowns are generally tricuspid (Augé Reference Augé2005). Bi- or tricuspidy is also frequently found in lacertoid jaws, at least from the mid-section onwards. Moreover, the Meckelian fossa in lacertoids tends to be large and deep to accommodate an extension of the posterior adductor, and it is closed by a substantial splenial that generally reaches to or close to the level of the symphysis.
Taken together, the features found on NHMUK.PV.R36683 (shallow, straight ventral margin, closed Meckelian fossa, no anterior splenial facets, unicuspid teeth) are all compatible with attribution to the Scincoidea (Scincidae and Cordyliformes), and the specimen broadly resembles scincoid specimens described from the Paleocene and Eocene of Europe (e.g., Augé Reference Augé2003a, Reference Augé, Wing, Gingerich, Schmitz and Thomasb, Reference Augé2005; Folie et al. Reference Folie, Sigé and Smith2005; Augé & Smith Reference Augé and Smith2009). Scincid and cordylid jaws are difficult to distinguish (Augé Reference Augé, Wing, Gingerich, Schmitz and Thomas2003b). Both usually have unicuspid teeth, although scincids are less likely to have a prominent cuspis apicalis lingualis. A straight ventral margin is more typically associated with cordylids (Lang Reference Lang1991), but it can also occur in scincids, whereas a tendency toward closure of the Meckelian fossa is considered a scincid feature (Augé & Smith Reference Augé and Smith2009; Čerňanský Reference Čerňanský2012). On balance, the characters of NHMUK.PV.R36683 are probably more scincid than cordylid, but we attribute it to Scincoidea indet. pending the recovery of further material.
NHMUK.PV.R36683 does not provide a precise match for any known Paleogene scincoid. Scincoides haininensis (Folie et al. Reference Folie, Sigé and Smith2005), from the early Paleocene of Belgium, has more acuminate teeth and no anterior closure of the Meckelian fossa. Axonosaurus sabatieri (Augé Reference Augé2003a), a possible scincid from the early Eocene of Prémontré, France, has broadly similar features to the Insect Limestone jaw (straight lower margin, tooth bases increasing in size anteroposteriorly, unicuspid teeth), but differs in that the posterior part of the Meckelian fossa faces medially rather than ventrally and is deeper. Moreover, although the fossa is constricted anteriorly, due, in part, to an expansion of the ventral jaw margin as in NHMUK.PV.R36683, the closure is not complete. This is also the case in Berruva (late Paleocene, MP6, Cernay: Augé Reference Augé2005) and Orthoscincus (late Eocene, Malpérié: Augé Reference Augé2005), where the profile of the subdental ridge, the size and orientation of the posterior part of the Meckelian fossa and the shape of the ventral dentary margin also differ. Foliesaurus (Augé & Smith Reference Augé and Smith2009) from the earliest Oligocene of Boutersem, Belgium (MP21), has a shallow jaw with a straight lower margin like that from the Insect Limestone, and has tooth bases that increase in diameter posteriorly, but the teeth are more acuminate and the subdental ridge tapers abruptly in its posterior half rather than gradually. Furthermore, in both Foliesaurus and a second unnamed scincoid from the same locality, the Meckelian fossa is restricted by a ventral expansion of the subdental ridge rather than an expansion of the ventromedial edge of the jaw. Ayalasaurus tenuis (MP18-19, Quercy: Augé Reference Augé2005) shows the closest similarity to the Insect Limestone jaw. It is rather cordylid in appearance, as it is long and slender, and has a straight ventral margin. Like NHMUK.PV.R36683, the upper and lower margins of the Meckelian fossa meet to close the anterior part of the Meckelian fossa (a scincid rather than cordylid tendency; Augé Reference Augé2005). However, the profile of the subdental ridge in A. tenuis differs in that it varies abruptly in depth rather than narrows gradually, and it has a splenial fitting into a wider, medially open, Meckelian fossa posterior to the closure. The lizard represented by NHMUK.PV.R36683 may, therefore, be related to that from Quercy, but is probably generically distinct.
NHMUK.PV.R36683 also resembles a partial dentary (Yule collection) from the Osborne Member, Thorness Bay, Isle of Wight, UK, a somewhat older (ca.500ky) horizon than the Insect Limestone. The Osborne Member jaw was described and figured as a possible scincid in a PhD thesis (Green Reference Green1998), but has not been formally published. The two jaws resemble one another in all major features, including the mode and length of the Meckelian fossa closure and the general shape and striations of the tooth crowns, although the striae are coarser in the Osborne Member specimen.
2.2. Squamata, family indet
In addition to the partial dentary, lizards are represented in the Insect Limestone by nine matrix blocks (including part and counterpart in some cases) bearing pieces of shed skin (NHMUK.PI.In17345, In24564, Brodie and Hooley Collections, respectively, probably ex A'Court Smith Collection, Gurnard Bay) and Yule collection (IWCMS.2014.7, 2014.8 from Sticeletts Ledge; IWCMS.2014.9, 2014.11–2014.14 from Saltmead Ledge; and IWCMS.2014.10 with no locality, but likely Sticeletts or Saltmead). The largest of these is NHMUK.PI.In24564, a small block (Fig. 2a) bearing insect remains but also scale impressions. These scale impressions are hexagonal and grade in size, with the largest at the lower left of the impression and the smallest to the right. The size gradation is most marked on the left one-quarter to one-third of the impression where the scales range from ∼0.7mm in diameter to 0.2mm. On the rest of the block, however, they are smaller and of more uniform size (∼0.13mm in diameter). Depending on the lizard species, a change in scale size of this degree can be found between proximal and distal parts of the limbs or sometimes between the ventral and lateral parts of the torso. Smaller patches of scales are preserved on the other blocks, and are of similar shape and appearance, although mostly at the lower end of the size range on NHMUK.PI.In24564. The exception to this is IWCMS.2014.9, where the scales are roughly an order of magnitude smaller and may represent a hatchling or juvenile individual (Fig. 2b).

Figure 2 Fragments of shed skin of indeterminate squamates. (a) NHMUK.PI.In24564 from Gurnard Bay. (b) IWCMS.2014.9 from Saltmead Ledge. Scale bars=5mm.
As preserved, the scales are hexagonal rather than cycloid, rhomboid or rectangular, and are neither spiny nor, apparently, imbricate. They are, therefore, unlikely to have come from a scincid or cordylid, like the jaw, or from either a lacertid or anguid. No detailed comparative study of squamate scalation characters has been made, but a short survey of available specimens (S. E. Evans, pers. obs.) suggests the Insect Limestone scales most closely resemble the relatively simple, soft body scales of gekkotans and some iguanians, with scale size matching a living lizard of around 80mm snout to vent length. Both groups are known to have been present in the Eocene of western Europe and have been reported from the Priabonian of the Hampshire Basin (Green Reference Green1998).
2.3. Discussion
Analyses of the squamate fauna of western Europe agree that there was a major drop in diversity shortly after the Eocene–Oligocene boundary, at the time of the Grande Coupure (e.g., Rage Reference Rage, Pomerol and Premoli-Silva1986, Reference Rage2006, Reference Rage2012; Augé Reference Augé2000; Augé & Smith Reference Augé and Smith2009). Four groups disappeared from western Europe – pleurodont iguanians, gekkotans, glyptosaurines and helodermatids – although gekkotans and helodermatids returned later in the Oligocene. At species level, however, some 66–80% of squamate species are estimated to have been lost from western Europe (Rage & Augé Reference Rage and Augé1993). Scincoids (scincids, cordylids and possible stem-scincoids) were reportedly present both before and after the Grande Coupure, although relatively rare. The scincoid specimen described by Green (Reference Green1998) from the Osborne Member was, until now, one of the youngest records from the British Eocene, as she found no scincoids in the later Priabonian horizons (Bembridge Limestone Formation, Bembridge Marls) (Green Reference Green1998). The Insect Limestone jaw, fragmentary though it is, therefore extends the record of the group closer to the Eocene–Oligocene boundary.
3. Aves
3.1. Bones
Only one bird bone has so far been found (NHMUK.PV.A.9052). It is a long bone and is both fragmented and still largely embedded in matrix. It requires specialist preparation before it can be described.
3.2. Feathers
The feather fauna is dominated by body contour feathers (Lucas & Stettenheim Reference Lucas and Stettenheim1972). The feathers are well preserved with a high resolution of anatomical detail visible. The first record of feathers being discovered on the Isle of Wight is by Brodie (Reference Brodie1878), who noted that feathers were found from the Eocene deposits at Gurnet Bay, Isle of Wight. The Gurnet Bay that Brodie referred to is an old name for Gurnard Bay, near Cowes, but may in any case be inaccurate (see introductory paragraph). In the collection of the NHMUK there are 15 specimens of feathers from the Insect Limestone, all collected in the 19th Century. Two of these feathers (both bearing the number NHMUK.PV.A504) have documentation with them that states: ‘Feather of bird, Eocene, Gurnet Bay, Isle of Wight. The Rev P. B. Brodie M.A., FGS, Vicarage, Rowington, Warwick'. A third (NHMUK.PV.A9053, ex PI.In59734), also Brodie Collection, but donated by Mrs P. Hugh-Jones, May 1960, has the following written on the block: ‘Feather of a bird, Tertiary, Gurnet Bay, Bembridge Series, I of Wight'. According to Cleevely (Reference Cleevely1983), Peter Bellinger Brodie (1815–1 November 1897) was a rector of various parishes in southern and central England. He collected fossils wherever his ecclesiastical appointments took him. He pioneered the study of fossil insects and is best known for his 1845 publication ‘A history of the fossil insects in the secondary rocks of England'. His extensive collection was sold in 1895. The British Museum (Natural History) (BMNH; now NHMUK) purchased large numbers of specimens, including types at this sale. It also made a further purchase of Brodie specimens from his executors in 1898. As is shown in the following, the feather specimens cannot be definitely said to come from either of these purchases. Moreover, whereas one of the specimens is preserved in typical Insect Limestone lithology, the other is in a piece of brown rock, probably sideritic. In fact, Reid & Chandler (Reference Reid and Chandler1926, p. 3) noted that in the Bembridge Marls ‘there are many plants … preserved in dark, or light, clay-ironstone nodules'. These represent a more likely provenance for this other feather (see also Hayes & Collinson Reference Hayes and Collinson2014).
One of the feather specimens (NHMUK.PV.A1630) was purchased from Alfred Bell (28 June 1835–28 June 1925 – a noted collector of European Pliocene fossils) on 13 January 1874. The location for this specimen is given as Insect Limestone, lower part of the Bembridge Marls, Gurnard Bay, Cowes, Isle of Wight. However, like one of the Brodie specimens, the rock is brown and apparently sideritic, so the feather is not from the Insect Limestone and may likewise have come from a different horizon in the Bembridge Marls.
Ten specimens (NHMUK.PV.A1621–1629, A1632) are labelled as R. W. Hooley collection, 1924, mid-Oligocene (now latest Eocene) Insect Limestone, lower part of the Bembridge Marls, Gurnard Bay, Cowes, Isle of Wight. Reginald Walter Hooley (5 September 1865–5 May 1923) was the honorary curator of Winchester Museum and best known for his collection of Cretaceous vertebrates from the Isle of Wight. His fossil collection was purchased by the BMNH in 1923 for £500 (curiously, the labels record a 1924 date – possibly the date the specimens were curated or registered). To further complicate the matter, Hooley purchased the Bembridge Marls insects, mammals and plants of the James Edwin A'Court-Smith collection from a Southampton auction house in 1899. According to Cleevely (Reference Cleevely1983), James Edwin Ely A'Court-Smith (1814–1900) was also known to have exchanged material with P. B. Brodie. The above information seems to indicate that the actual origin of all of Brodie's feather specimens is likely to have been A'Court-Smith, who exchanged material with Brodie (in the late 1800s) and whose collection was then incorporated with that of Hooley's in 1899 (see also Reid & Chandler Reference Reid and Chandler1926 for biographical details of A'Court Smith). The 15th specimen (NHMUK.A9054, ex PI.In17194; Fig. 3a) was purchased directly from A'Court Smith in 1883 and the provenance is recorded as Gurnard Bay. It was figured by Jarzembowski (Reference Jarzembowski1980, fig. 61).

Figure 3 (a) Avian body contour feather, from Gurnard Bay, NHMUK.PV.A9054. (b) Left lower incisor of the theridomyid rodent Isoptychus sp., from Saltmead Ledge, NHMUK.PV.M45566, in mesial view. Scale bar=5mm.
After this time, there are no records of feathers being collected until recent decades. One such find was made by M. Barker on 8 October 1972 on a Geologists' Association field trip to the Isle of Wight when visiting the southern side of Gurnard Ledge (SZ 464945) to examine the Bembridge Marls succession (Daley & Edwards Reference Daley and Edwards1974, p. 290). The specimen is in the University of Portsmouth, UK, collections. More recently, numerous specimens have been collected by Andy Yule and these are housed in IWCMS (Martin New, pers. comm. 2014). Most are from Thorness Bay, but one (IWCMS.2014.37) is from the St Helens foreshore, N of St Helens tower.
4. Mammalia
The first record of a mammal from the Insect Limestone is a left lower incisor of the rodent Isoptychus (NHMUK.PV.M45566) (Fig. 3b). It was found recently by Andy Yule at Saltmead Ledge, Thorness Bay. Its discovery involved the oblique breakage of the specimen into two, the best exposed part being the mesial face together with the cross section, as illustrated. The identification is based on size and the cross-sectional shape, by comparison with other Bembridge Marls rodents. The shape is subtriangular, with the greatest width near the labial margin. Labio-lingual length is 1.70mm, whilst maximum mesiodistal width is 1.18mm. Isoptychus is a member of the extinct endemic European family Theridomyidae, subfamily Theridomyinae, which is well represented throughout the Solent Group. Isoptychus is the commonest theridomyid in the Bembridge Marls, where specimens have frequently been referred to the species Isoptychus pseudosiderolithicus de Bonis, Reference de Bonis1964 (e.g., Bosma Reference Bosma1974). Subtle differences from the type assemblage from earlier in the Priabonian of southern France suggest that Isoptychus sp. is the safer determination until the material from multiple levels is fully revised. The genus Isoptychus is often synonymised with the genus Theridomys, on the basis that it includes simply primitive species of the latter. However, Isoptychus does appear to have some derived character states, supporting its validity (Hooker Reference Hooker, Whitaker and Hart2010). From knowledge of postcranial remains, theridomyids were semiterrestrial mammals that, according to their dentition, fed on fruits and leaves (Collinson & Hooker Reference Collinson, Hooker, Friis, Chaloner and Crane1987), including those of marginal aquatics (Grimes et al. Reference Grimes, Collinson, Hooker, Mattey, Grassineau and Lowry2004). The Insect Limestone is a very low-energy subaqueous deposit in which remains of land mammals are relatively unexpected. The incisor shows no digestion-related etching of its enamel (Vasileiadou et al. Reference Vasileiadou, Hooker and Collinson2007), so is unlikely to have been dropped into the sediment by a bird of prey. In the limestone block in which it is embedded, it lies at the base of a slightly coarser unit consisting largely of 100–200μ-scale plant debris, which has a sharp contact on the fine-grained limestone beneath. The orientation of the block is confirmed by observations of a sample obtained in situ. Therefore, it is likely that the Isoptychus tooth was transported into the depositional environment by hydraulic flow of slightly higher energy than is usual for the bed.
5. Conclusions
Probably the most interesting aspects of this meagre vertebrate fauna are its taphonomic peculiarities. Reid & Chandler (Reference Reid and Chandler1926, pp. 10–13), studying the Insect Limestone plants, noted that a significant number of the remains of land plants were either specially adapted for wind dispersal or were capable of such transport. This aspect is treated in greater depth by Hayes & Collinson (Reference Hayes and Collinson2014). Apart from the fish, which were presumably living in the water body beneath which the Insect Limestone sediments accumulated, the remaining vertebrates, the lizards, birds and the mammal were essentially land-based. From their high surface to volume ratio and lightness, the feathers and shed skin fragments would clearly lend themselves to wind transport. The near absence of tetrapod remains likely to have been transported by water currents supports the contention that wind transport was the major factor in their accumulation in the Insect Limestone. Whereas the rodent incisor can be accounted for by its occurrence at the coarser base of a fining upwards cycle and, therefore, could have been transported by water, the lizard jaw is small enough to have been windblown, like the feathers and skin.
6. Acknowledgements
We thank Andy Yule for his dedicated collecting and generosity in making his specimens available for study, Peta Hayes (NHMUK, Earth Sciences Department) for field help, Mark Graham (NHMUK, Conservation Centre) for preparing the lizard jaw, Harry Taylor (NHMUK, Image Resources) for the light photographs, Alex Ball and Tomasz Goral (NHMUK, EMMA Unit) for SEM facility support and Martin New (IWCMS) for help in locating specimens and for a loan.





