Flow batteries, with two fluid electrodes, are promising energy-storage devices especially for grid-scale electrical energy storage due to their good scalability and excellent durability. However, these batteries have not yet been widely implemented, partly due to their limited output voltage currently, which constrains their energy-storage capacity. William Chueh from Stanford University and co-workers now report boosting the output voltage of flow batteries by coupling a liquid sodium-potassium alloy anode with a potassium-containing alumina solid-state electrolyte. This breakthrough was published recently in Joule (doi:10.1016/j.joule.2018.04.008).
To address the low-voltage challenge of flow batteries, the researchers first focused on the anode material. Conventional anodes for flow batteries are aqueous solutions. They tend to decompose at potentials above 1.5 V when water is electrolyzed into hydrogen and oxygen gases. This value is only about one-third the voltage delivered by Li-ion batteries with solid electrodes. To increase the voltage, the researchers chose a Na-K alloy, a low-cost and liquid metal, as the anode. The (water-free) Na-K alloy pushed the output voltage over 3 V.
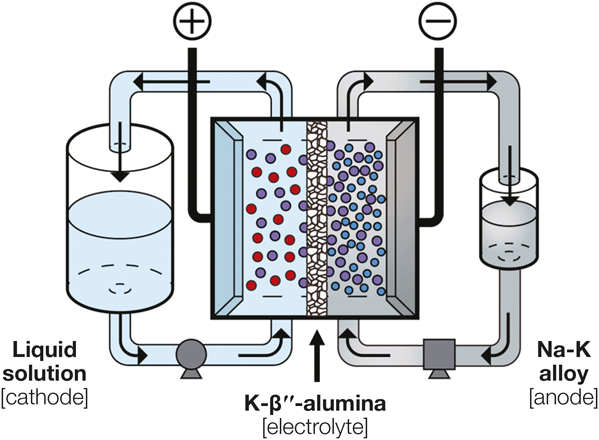
Schematic depicting the structure of the developed high-voltage flow battery. The cathodes include aqueous ferrocyanide, nonaqueous triiodide, and nonaqueous ferrocenium solutions. Credit: Joule.
The researchers also explored alternative electrolytes. Previously, no solid-state electrolytes were known that were compatible with the Na-K alloy. The typical solid-state electrolyte for other liquid-state Na-based electrodes, Na-β´´-alumina (Na+-containing β´´-phase alumina), unfortunately pulverizes when placed in contact with a Na-K alloy. This instability is caused by K+-Na+ ion exchange that constantly degrades the structural integrity. The researchers thus switched to K-β´´-alumina, which is synthesized by replacing Na+ with K+ in the lattice structure. Experiments verified that this K+-conducting electrolyte worked well with the Na-K anode: it maintained structural integrity across a broad range of K+ content (40–80 mol%) and pressures (100∼400 kPa) for at least 16 h.
The novel flow battery with a Na-K anode and a ∼300-µm-thick K-β´´-alumina electrolyte exhibited encouraging electrochemical performance. The maximal open-circuit voltage was 3.4 V, more than two times higher than those of conventional flow batteries. At 57°C, the flow battery achieved the highest power density of 115 mW cm–2 with an aqueous ferrocyanide cathode at a current density of ∼65 mA cm–2.
Dongliang Chao of Nanyang Technological University, Singapore, highlights the significance of this work from the angle of operating temperature: “The liquid sodium metal anode is also able to enhance the open-circuit voltage of flow batteries, but the enhancement is at the cost of high operating temperatures of 270–350°C. This study pairs Na-K alloy anode and K-β´´-alumina solid electrolyte, which realizes high-voltage flow batteries at room temperature.” Chao was not involved in this study.
The researchers are progressing toward commercial feasibility of the developed flow battery. “There are two main areas where materials-related improvements are needed: discovering a suitable liquid cathode, which should be low cost and compatible with the K-β´´-alumina solid electrolyte; and manufacturing a thin (∼100 μm) K-β´´-alumina membrane,” says Antonio Baclig of Stanford University, the first author of the article. “We are also refining our understanding of how pressure and wetting interact at the Na-K/K-β´´-alumina interface.”


