INTRODUCTION
Timings of wheat developmental stages such as the onset of reproductive development, meiosis, anthesis and grain filling are adaptive traits (Slafer et al. Reference Slafer, Kantolic, Appendino, Miralles, Savin, Sadras and Calderini2009). Genetic control of vernalization requirement with Vrn alleles, earliness per se (eps) and photoperiod sensitivity (Ppd) (Reynolds et al. Reference Reynolds, Foulkes, Furbank, Griffiths, King, Murchie, Parry and Slafer2012), all contribute to resource capture, dry matter partitioning and stress avoidance during critical developmental stages for grain yield formation in different environments (Barber et al. Reference Barber, Carney, Alghabari and Gooding2015). With regard to anthesis, early flowering can confer an advantage if excessive heat or drought stress is likely to develop during maturation such as in Southern Europe (Worland & Law Reference Worland and Law1986; Worland et al. Reference Worland, Korzun, Röder, Ganal and Law1998). In such conditions, early development can reduce the risk of negative effects on: gamete development prior to anthesis; photosynthate supply during floret development; pollen release and fertilization; and grain filling (Saini & Aspinall Reference Saini and Aspinall1982; Kato & Yokoyama Reference Kato and Yokoyama1992; González et al. Reference González, Miralles and Slafer2011). Early development in short days can be conferred by photoperiod insensitivity (PI). Ppd-D1a is the most potent known PI allele and the dominant source of PI in European (Snape et al. Reference Snape, Leckie, Parker, Nevo, Casely, Cussans and Atkin1991) and Asian cultivars (Yang et al. Reference Yang, Zhang, Xia, Laurie, Yang and He2009; Kiss et al. Reference Kiss, Balla, Veisz, Láng, Bedö, Griffiths, Isaac and Karsai2014). Two further Ppd-1 homeologous genes have been mapped to the short arm of group 2 chromosomes in wheat and alleles conferring PI have been identified: Ppd-A1a allele, as for Ppd-D1a, is associated with an upstream deletion within a pseudo-response element (Beales et al. Reference Beales, Turner, Griffiths, Snape and Laurie2007; Wilhelm et al. Reference Wilhelm, Turner and Laurie2009) and predominates in modern durum wheat (Bentley et al. Reference Bentley, Turner, Gosman, Leigh, Maccaferri, Dreisigacker, Greenland and Laurie2011); Ppd-B1a is a result of an increased gene copy number (Díaz et al. Reference Díaz, Zikhali, Turner, Isaac and Laurie2012; Kiss et al. Reference Kiss, Balla, Veisz, Láng, Bedö, Griffiths, Isaac and Karsai2014), carried by almost a quarter of wheat genotypes from Europe, Asia and America, and therefore the second most important Ppd-1a allele in global wheat germplasm (Kiss et al. Reference Kiss, Balla, Veisz, Láng, Bedö, Griffiths, Isaac and Karsai2014).
Effects of Ppd-1 alleles on mean flowering date are well-documented and in the field are likely to depend on daylength progression during different growth stages (GSs), and hence interact with latitude, sowing date, temperature and other genetic components of developmental rate such as Vrn and eps status (Snape et al. Reference Snape, Leckie, Parker, Nevo, Casely, Cussans and Atkin1991; Foulkes et al. Reference Foulkes, Sylvester-Bradley, Worland and Snape2004; González et al. Reference González, Slafer and Miralles2005; Kiss et al. Reference Kiss, Balla, Veisz, Láng, Bedö, Griffiths, Isaac and Karsai2014). As well as mean flowering date, however, an adaptive trait that has received little attention to date is the extent to which duration of flowering, i.e. the time during which pollination may occur within and/or between ears, might be related to yield stability. For instance, extending the period over which a crop flowered would mean that a damaging spike in temperature would disrupt the fertilization of a smaller proportion of florets (Lukac et al. Reference Lukac, Gooding, Griffiths and Jones2012). Modelling studies have demonstrated the importance of flowering duration for estimating the impact of brief periods of high temperature on crop yield (Challinor et al. Reference Challinor, Wheeler, Craufurd and Slingo2005). Conversely, Barber et al. (Reference Barber, Carney, Alghabari and Gooding2015) suggest that, variable GSs between stems of a poorly geographically adapted crop may be associated with poor grain set and increased susceptibility to stress.
Wheat varieties are known to vary for flowering duration (Hucl Reference Hucl1996; Matus-Cadiz et al. Reference Matus-Cadiz, Hucl, Horak and Blomquist2004), but little is known concerning the effects of Ppd alleles on the duration of flowering. The present study has particular relevance to increasing the knowledge of the potential adaptive traits of Ppd alleles beyond the known PI: the effect of Ppd-1 alleles in near isogenic lines (NILs) is compared in pot and field experiments with regard to flowering time and duration within and between stems, and a novel ‘overlap index’ to characterize the coincidence of flowering on different stems is presented.
MATERIALS & METHODS
Plant material
Near isogenic lines, carrying PI alleles Ppd-A1a, Ppd-B1a, Ppd-D1a from different germplasms in a Ppd ‘Paragon’ background, were used in the experiments. Single NILs or introgression lines carried either Ppd-A1a (‘GS-100’), Ppd-B1a (‘Sonora64’) or Ppd-D1a (‘Sonora64’) (Bentley et al. Reference Bentley, Turner, Gosman, Leigh, Maccaferri, Dreisigacker, Greenland and Laurie2011). Two triple introgression lines (Ppd-1a on all three genomes), developed from the single NILs (Shaw et al. Reference Shaw, Turner and Laurie2012) were also used. The first triple Ppd-1a introgression line (Triple1) carried the same alleles as the single introgression lines, but jointly in one line (Ppd-A1a + Ppd-B1a + Ppd-D1a = Ppd-1a). The second triple Ppd-1a introgression line (Triple2) was the same, but for the Ppd-B1a allele coming from ‘Chinese Spring’. ‘Paragon’, which had the photoperiod sensitive allele at all loci (Ppd-A1b + Ppd-B1b + Ppd-D1b = Ppd-1b), was also included.
Pot experiments
Pot-based experiments were carried out at the Plant Environment Laboratory facility, University of Reading, UK (51°24′N, 0°56′W, 43·980 m asl). Individual wheat plants were grown in 12·5 cm diameter pots filled with a 4 : 4 : 2 : 1 mixture of steam-sterilized 6 mm gravel, medium vermiculite, 3 mm sharp sand and peat-based potting compost. Osmocote Pro 3 to 4 months (Scotts, UK) was added to the mixture at the rate of 2 kg per cubic metre to provide plant nutrition throughout the experiment. Pots filled with the planting medium were soaked with water for 24 h prior to sowing with single seeds to a depth of 2·5 cm.
The first pot-based experiment, henceforth referred to as the ‘pot-based ambient (A) experiment’ was carried out with plants grown in outside conditions. Four replicate pots per line were randomly distributed in an outside enclosure in 2011 and 2012; the first batch sown on 25 February 2011 (2011 growing season) and the second sown on 9 December 2011 (2012 growing season). All pots were initially kept in an unheated polytunnel and regularly assessed for GS (Zadoks et al. Reference Zadoks, Chang and Konzak1974). Plants were transferred to an open area protected by bird netting at GS 31. Pots were raised on frogged bricks (i.e. bricks with an indentation on one or more sides) to allow for free drainage and irrigated daily through an automatic drip system to full water capacity. All plants were treated against powdery mildew as required with Flexity (300 g/l (25·2% w/w) metrafenone; BASF Plc, UK in 0·5 l/ha). Temperature data were recorded at half hour intervals (Table 1).
Table 1. Mean monthly temperature data (°C) for ambient pot-based experiment

The second pot-based experiment utilized controlled-environment growth cabinets to subject plants to two different temperature regimes throughout their life-cycle. Henceforth referred to as the ‘controlled environment (CE) experiment’, it was sown on 23 December 2012. The plants were initially kept in a polytunnel as outlined above and transferred to CE Saxcil growth cabinets (photon flux 650 µMol/m2/s; 70% relative humidity; 390 ppm atmospheric CO2) when plants reached the double ridge stage (Kirby & Appleyard Reference Kirby and Appleyard1981). Sixteen replicate pots per line were randomly distributed between and within six growth cabinets, eight in ambient (cool) and eight in warm (ambient + 5 °C) cabinets. The day and night temperatures in the cabinets followed ‘cool’ (ambient) and ‘warm’ (ambient + 5 °C) weather patterns as informed by the Waddington Meteorological station (53°10′N, 0°32′W) from 2012 (Met Office 2012, contains public sector information licenced under the Open Government Licence v2.0). Similarly, daylength adjustment took place at weekly intervals and was based on UK daylight variation (Table 2).
Table 2. Temperature for the cool regime (ambient) and warm regime (ambient + 5 °C) in the CE experiment with daylength change (hours). Temperatures and daylength change were carried out on a weekly basis; the day at which the change took place is indicated. Temperatures were extrapolated from the Waddington weather station. Contains public sector information licenced under the Open Government Licence v2.0 (Met Office 2012)

Flowering assessment in pots
All plants were allowed to develop four tillers in addition to the main stem (MS); any further tillers were removed. Tillers were labelled consecutively according to order of emergence, where Tiller 1 refers to the first ear coming into flower after the MS. The spikelets on each ear were numbered from the collar upwards, the lowest being ‘1’ and subsequent numbers alternating between sides such that one side of the ear is ‘odd’ and the other ‘even’. All ‘odd’ spikelets were assessed for flowering progress on each of the emergent tillers. Five florets within each spikelet were identified, following the scheme of Kirby and Appleyard (Kirby & Appleyard Reference Kirby and Appleyard1984). The first floret from the lower glume was labelled as ‘A’, with subsequent florets up the spikelet alternating between sides such that florets ‘B’ and ‘D’ were on the same side. Flowering assessments started when tillers reached GS 57 and continued until all flowering stopped on the last tiller.
Observations started at 10.00 h and were consistently completed before 15.00 h. Care was taken to vary the first set of plants to be assessed each day to avoid systemic bias due to any potential diurnal pattern of flowering activity. Scoring of a spikelet was initiated when the lower glume could be opened easily with a thumbnail and flowering assessment in the A and CE experiment followed as described by Lukac et al. (Reference Lukac, Gooding, Griffiths and Jones2012). Each floret that could be opened was scored using four developmental stages of both anthers and stamens. In the ‘A’ experiment in 2012, each spike was split into top, middle and bottom third and presence/absence of active flowers was assessed as defined in Lukac et al. (Reference Lukac, Gooding, Griffiths and Jones2012). For the purpose of the present analysis, only data relating to active flowering were used. Anthers were defined as ‘active’ when showing signs of pollen dehiscence, whereas stigmas were considered ‘active’ when receptive to pollen. Flowering was deemed to have started at the presence of the first dehiscent anther and/or receptive stigma, and finished when all anthers and stigmas for a given spike had passed the active stage. For the purposes of the present paper, ‘male’ flowering refers to anther activity and ‘female’ flowering refers to stigma activity.
Field experiment
Plots (minimum 2 × 5 m) were drilled (300 seeds/m2) into a free-draining sandy loam overlying coarse red–brown sand of the Sonning series (Jarvis Reference Jarvis1968), following a clover-rich grass ley at the Crop Research Unit, Sonning, University of Reading, UK (51°28′N, 0°54′W, 56·296 m asl). Paragon (Ppd-1b) was compared with NILs incorporating Ppd-A1a, Ppd-B1a and Ppd-D1a in four randomized blocks. The Triple 1 and 2 lines were not included due to a limited volume of seed. Plots were maintained with fertilizer, fungicide and herbicide applications as per local agricultural practice. Flowering was assessed in three randomly placed 0·1 m2 circular quadrats per plot from when the first plots started to extrude anthers till all anther extrusion had finished, indicated by an absence of yellow anthers on all tillers. The quadrat position avoided plot edges, and all tillers within the quadrat were assessed. Ten to 14 assessments were carried out per allele per plot over the duration of flowering.
Statistical analyses
Pot-based experiments
Analysis of the ‘A’ experiment used restricted maximum likelihood (REML) with a random model of year/replicate and a fixed model of line, and for the ‘CE’ experiment the REML analysis comprised a random model of Cabinet/Pot and a fixed model of environment × line. All analyses were carried out using Genstat (13th edn, VSN International Ltd.) and REML was used to account for a minimum amount of missing data for individual plants, where the full number of five tillers did not develop.
In addition to flowering duration, a simple metric – the flowering overlap index (FOI) – was defined to express the degree of overlap of flowering at the plant level (FOI = 0 if there was no overlap of flowering time between any pair of tillers considered and was ‘+1’ or ‘−1’ if all tillers considered flowered on exactly the same day). First, for each pair of ears (i, j), the FOI (i, j) is calculated using the start (Fs) and end (Fe) dates of flowering as follows:
 $$\matrix{{\rm if}\;{\rm MIN}({\rm Fe}({\rm i}), {\rm Fe}({\rm j})) \lt {\rm MAX}({\rm Fs}({\rm i}),{\rm Fs}({\rm j}))\hfill &{\rm FOI}( {\rm i,j}) = 0 \cr {\rm if}\;{\rm MIN}({\rm Fe}({\rm i}), {\rm Fe}({\rm j})) \ge {\rm MAX}({\rm Fs}({\rm i}),{\rm Fs}({\rm j}))\hfill &{\rm FOI}( {\rm i,j})= \displaystyle{{\rm MIN}({\rm Fe}({\rm i}), {\rm Fe}({\rm j})) - {\rm MAX}({\rm Fs}({\rm i}),{\rm Fs}({\rm j}))+ 1 \over {\rm MAX}({\rm Fe}({\rm i}), {\rm Fe}({\rm j})) - {\rm MIN}({\rm Fs}({\rm i}),{\rm Fs}({\rm j})) + 1}}$$
$$\matrix{{\rm if}\;{\rm MIN}({\rm Fe}({\rm i}), {\rm Fe}({\rm j})) \lt {\rm MAX}({\rm Fs}({\rm i}),{\rm Fs}({\rm j}))\hfill &{\rm FOI}( {\rm i,j}) = 0 \cr {\rm if}\;{\rm MIN}({\rm Fe}({\rm i}), {\rm Fe}({\rm j})) \ge {\rm MAX}({\rm Fs}({\rm i}),{\rm Fs}({\rm j}))\hfill &{\rm FOI}( {\rm i,j})= \displaystyle{{\rm MIN}({\rm Fe}({\rm i}), {\rm Fe}({\rm j})) - {\rm MAX}({\rm Fs}({\rm i}),{\rm Fs}({\rm j}))+ 1 \over {\rm MAX}({\rm Fe}({\rm i}), {\rm Fe}({\rm j})) - {\rm MIN}({\rm Fs}({\rm i}),{\rm Fs}({\rm j})) + 1}}$$
For example, if an ear on the MS flowers from days 1 to 4 and an ear on the first primary tiller (T1) flowers from days 3 to 5, then FOI (MS,T1) = 2/5 = 0·4. For a given plant, FOI is then calculated as the mean of the FOIs of all possible pairs of tillers considered, e.g. if only the first three tillers are considered:
A script was written in Fortran to calculate the FOI for the first three flowering tillers (FOI_3) and for all five tillers (FOI_5) using the algorithms as described above.
Field experiment 2013
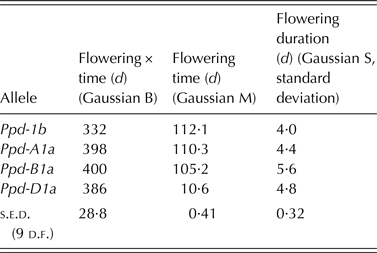
Percentage of ears in flower over time was fitted with a Gaussian curve for each separate field plot with constant omitted for each plot to provide estimates for duration of flowering (Gaussian S = standard deviation), time of peak flowering (Gaussian M = time scalar) and the area under the time × flowering curve (Gaussian B). The model parameters thus derived were then subject to analysis of variance for a balanced, randomized block experiment.
RESULTS
Controlled environment experiment
Male flowering
The delay in flowering over the different tillers from MS to T5 is evident in Figs 1(b)–(f). The earliest lines to flower were those, where the three Ppd-1a alleles had been combined. Where only one insensitivity allele was present, Ppd-D1a was the most potent, being significantly earlier to flowering than those carrying Ppd-B1a and Ppd-A1a. The Ppd-1b control was the last to flower. This pattern in timing of flowering was broadly evident on all tiller classes. The differences among the Ppd-insensitivity alleles tend to be less distinct in the warmer environment; an observation that contributed to significant (P < 0·05) environment × line interactions for the start or end of flowering on the MS (Fig. 1(b)) and T4 (Fig. 1(e)).
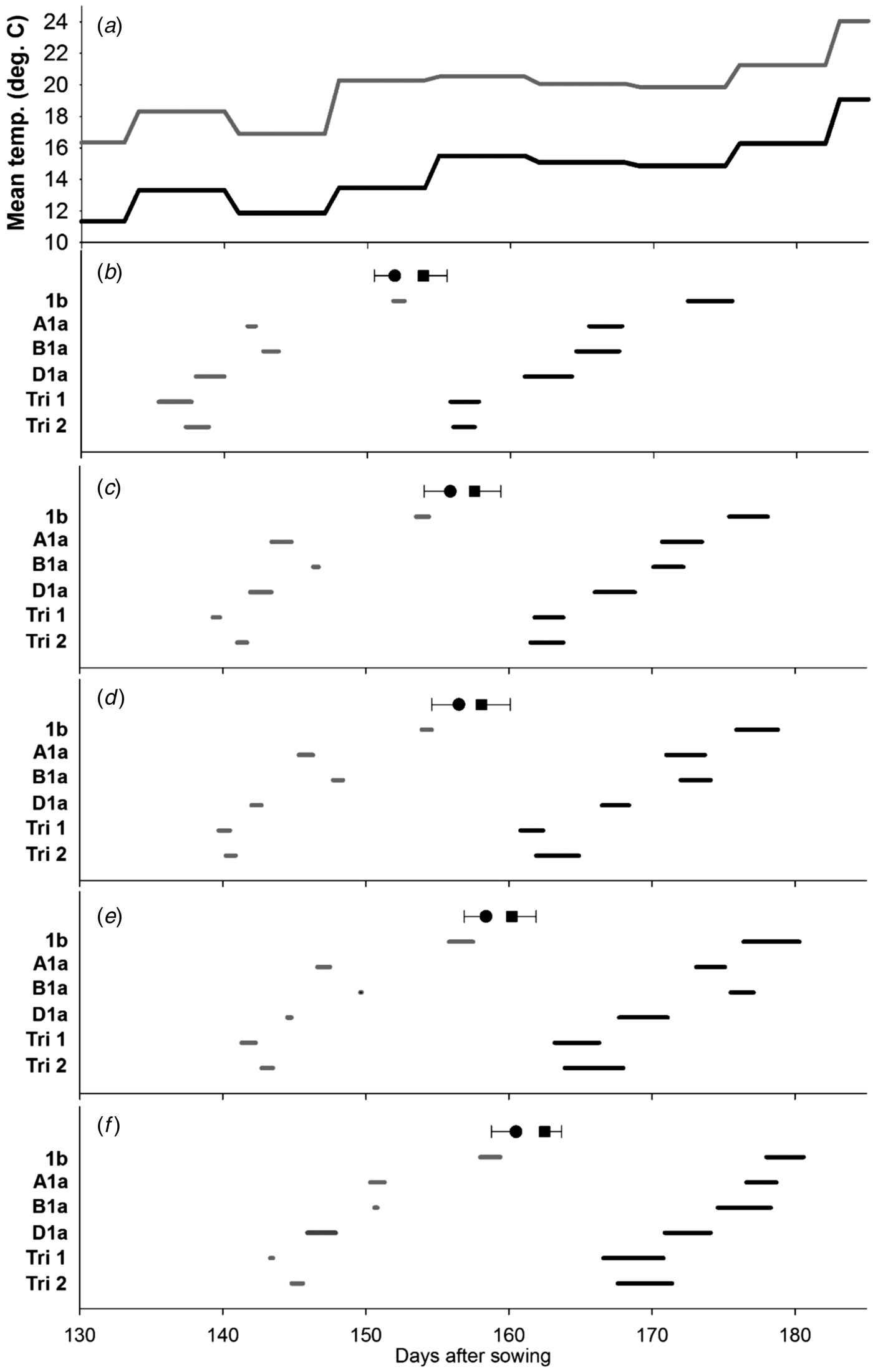
Fig. 1. Effect of Ppd-1 allele and average temperature (panel a) on timing and duration of male flowering on different tillers in the controlled experiment (CE). Panels b–f correspond to main stem, T2, T3, T4 and T5, respectively. Grey and black lines are for warm and cool environments, respectively. Error bars are one s.e.d. (80 d.f.) for comparing the start (●) and end (■) of flowering for the different alleles within each temperature and tiller. Points (●, ■) are the mean start and end of flowering for each tiller.
When all tillers were considered, there was neither evidence of a main effect of Ppd allele on duration of flowering within a spike (Table 3), nor interaction between allele and environment. Increasing temperature, however, did reduce the duration of flowering within a spike from 2·7 to 1·0 days (s.e.d. = 0·12; 4 d.f.; P < 0·001). When the whole plant was considered, flowering occurred over a shorter period for Ppd-1b than for any line carrying a PI allele. This effect of Ppd-1b is quantified in Table 3, but is also most evident by comparing the timings of flowering of the MS (Fig. 1(b)) with those of T5 (Fig. 1(f)). That is, flowering duration of a plant is dominated by the differences in start of flowering between tillers, and also the number of tillers present, rather than flowering duration within spikes. The FOI of the first three and the first five tillers to flower showed no effect of environment in contrast to the female flowering results (Table 4) or interaction between line and environment.
Table 3. Effect of Ppd-1 allele on duration of flowering in controlled environments. Results are averages over two environments

NIL, near isogenic line.
Table 4. Effect of Ppd-1 allele on the mean flowering overlap index (FOI) for male and female flowering duration for the first three and for five tillers per plant. s.e.d. is given for the interaction between environment and line

However, there was a significant (P ⩽ 0·01) effect of line for five tillers with the greatest mean overlap of tiller flowering for the Ppd-1b control, which is likely to be the dominant factor resulting in the shortest plant flowering time (Table 4). No significant difference in FOI was recorded for the male flowering for the first three tillers.
High temperature did tend to reduce the differences in flowering duration among alleles and the environment × line interaction was not particularly strong (P ⩽ 0·10). In contrast, the main effect of environment was highly significant: high temperature reduced flowering duration over plants from 13·2 to 8·3 days (s.e.d. = 0·43; d.f. = 3; P < 0·01), but this effect was dominated by reducing flowering duration within spikes (Figs 1 and 2) rather than altering the flowering overlap between tillers.
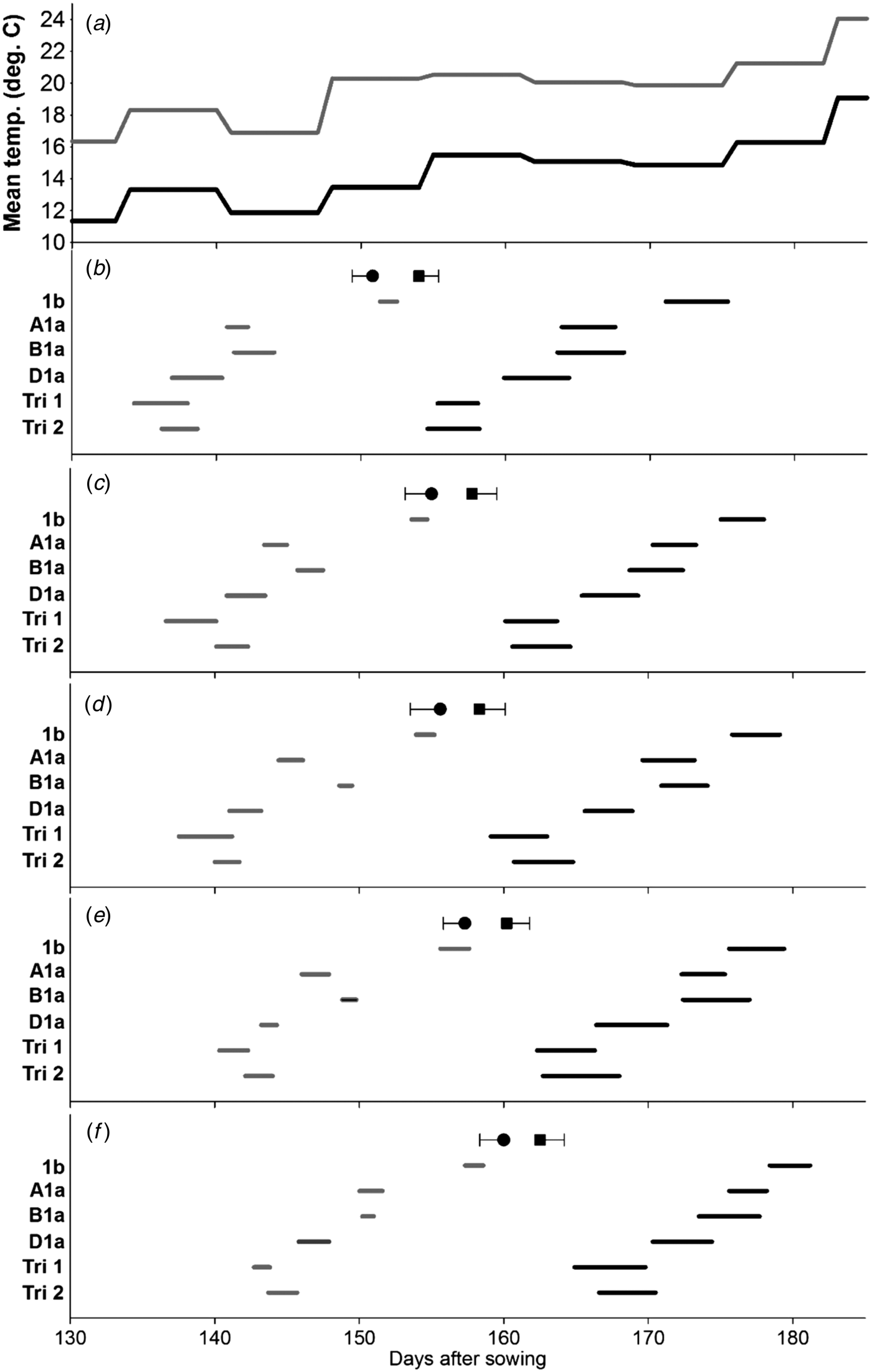
Fig. 2. Effect of Ppd-1 allele and average temperature (panel a) on timing and duration of female flowering on different tillers in the controlled experiment (CE). Panels b–f correspond to main stem, T2, T3, T4 and T5, respectively. Grey and black lines are for warm and cool environments, respectively. Error bars are one s.e.d. (80 d.f.) for comparing the start (●) and end (■) of flowering for the different alleles within each temperature and tiller. Points (●, ■) are the mean start and end of flowering for each tiller.
Female flowering
With the scoring method described, female flowering started on the same day (grand mean: days after sowing = 162·5, s.e. = 0·39) as male flowering (day = 162·7, s.e. = 0·28). The effects of Ppd-1 allele and environment on the time of female flowering were similar to those seen with male flowering (Fig. 2). Again, there were significant environment × line interactions for the start or end of flowering on the MS (Fig. 2(b)) and T4 (Fig. 2(e)). Duration of female flowering, however, tended to be longer than that for anther dehiscence (Table 3), and in contrast to the anthers, duration of female flowering within a spike was influenced by the Ppd-1 allele, i.e. significantly longer (P < 0·05) for all three lines carrying Ppd-D1a compared with the Ppd-1b line. Warm conditions reduced duration of female flowering within spikes from 3·8 to 2·0 days (s.e.d. = 0·15, d.f. = 4), and within plants from 13·8 to 9·6 days (s.e.d. = 0·64). As with the timing of pollen dehiscence, the effect of Ppd-1 allele on female flowering duration did not interact with environment. However, the FOI indicated that environment had a significant effect on the overlap between tillers (P ⩽ 0·01) for the first five tillers, with the warmer environment reducing the overlap from 0·22 to 0·18 days (s.e.d. = 0·018), no such effect was observed when considering the first three tillers only. Line had a significant effect on FOI: Ppd-1b had the greatest overlap between tillers, but it was only significantly greater than Triple 1. In contrast, considering just the first three tillers to flower (Table 4), although Ppd-1b had the greatest overlap, it was only significantly greater than Ppd-B1a (P < 0·05).
There was a highly significant relationship between duration of flowering and thermal time to the start of flowering (P < 0·001), indicating that the duration of flowering was longer in earlier flowering lines (Fig. 3). There was an effect of environment (P ⩽ 0·05), with a greater change in duration relative to start of flowering in the cooler environment. There was no significant difference in the slope between male and female flowering.

Fig. 3. Relationship between the start of flowering and the duration of flowering of whole plants varying in photoperiod insensitivity alleles. Squares and dashed lines refer to female activity and circles and solid lines refer to male activity. Open and solid symbols are cold and hot environments, respectively. Labels above female points refer to Ppd-1 allele (male alleles rank similarly for start), except T1 and T2 refer to triple combinations of A1a + B1a + D1a. Error bars are one s.e.d. for comparing female (F) and male (M) points. Fitted lines have slopes of −0·2750 d/d (s.e. = 0·0511) for cool environment and −0·0969 d/d (s.e. = 0·0270) for hot environment.
Field experiment 2013
Ppd allelic state did not influence the total amount of flowering over time (Gaussian B; Table 5). As in the CE experiment, Ppd-D1a lines were early to flower and Ppd-1b lines were late (Gaussian M; Table 5; Fig. 4). In contrast to the CE experiment, however, there was a clear distinction between the flowering time of Ppd-A1a lines, which were almost as late to flower as Ppd-1b lines, and that of Ppd-B1a lines, which were as early as Ppd-D1a lines (Fig. 4). Consistent with the CE experiment for flowering duration over plant, all PI alleles increased flowering duration in plots (Gaussian S; Table 5; Fig. 4) compared with Ppd-1b lines.

Fig. 4. Effect of Ppd-1 allele (○, solid line = Ppd-1b; ▲, long dashes = Ppd-A1a; ■, dotted line = Ppd-B1a; ●, short dashes = Ppd-D1a) on temporal flowering pattern in field grown plots. Fitted curves are Gaussian, constant omitted, parameters shown in Table 4. Horizontal bar is one s.e.d. (9 d.f.) for comparing time of peak flowering. Points are means of four blocks.
Table 5. Effect of Ppd-1 allele on amount, time and duration of flowering in field-grown spring wheat
Pot-based ambient experiment
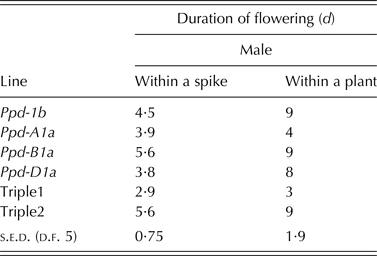
All PI lines flowered earlier than Ppd-1b, with Ppd-D1a the most potent allele to induce flowering at the lowest mean thermal time of 1281 degree days (s.e.d. = 15·51; Table 6). Under pot-based ambient conditions, Ppd-B1a line and Triple 2 had the longest male flowering duration within spikelets (P < 0·001) compared with all other lines. For the whole plant flowering duration, lines carrying Ppd-1b, Ppd-B1a, Ppd-D1a and Triple 2 flowered for about twice the duration of all other lines (P < 0·001; Table 7).
Table 6. Effect of Ppd-1a allele on thermal time from sowing to male flowering in a pot-based ambient environment. Results are a mean of 2 years

Table 7. Effect of Ppd-1a and Ppd-1b alleles on duration of flowering in a pot-based ambient environment. Results are averages over 2 years with the exception of Triple 1 and Triple 2 which are for 1 year only
DISCUSSION
The subject of the present study was the effect on flowering of single or triple Ppd-1a insensitivity alleles introgressed into a common genetic background. The relative potency of Ppd-1a insensitivity alleles in terms of flower initiation is in agreement with the previous literature: the greatest effects were consistently caused by the Ppd-D1a introgression (Worland et al. Reference Worland, Korzun, Röder, Ganal and Law1998; Bentley et al. Reference Bentley, Turner, Gosman, Leigh, Maccaferri, Dreisigacker, Greenland and Laurie2011).
In wheat, induction of flowering involves the CONSTANS (CO) gene having peak transcription under long day conditions, which activates the FLOWERING LOCUS T (FT) expression, causing flowering. The deletion upstream of the pseudo-response regulator coding region in the Ppd-D1a allele was found to be associated with expression of a floral regulation FT under short days, and has been suggested to be involved in the circadian clock (Beales et al. Reference Beales, Turner, Griffiths, Snape and Laurie2007). The potency of the Ppd-1a alleles varied in the present study, with the earliest flowering line also having the longest flowering duration. Significant cross-talk between numbers of signalling pathways is highly likely and the single deletions afforded by the Ppd-1a alleles may result in earlier flowering, but less control on the actual start of flowering, potentially leading to greater variability between tillers. The longer flowering duration in the earlier flowering lines was found to be largely attributed to smaller overlap between tillers. Indeed, the effect of Ppd-1a alleles on flowering duration was less pronounced in the warm compared with the cooler environment. In addition, it may be suggested that the FT transcripts may have a pleiotropic effect on the induction of flowering in successive tillers, potentially by cross-talk through the sugar or hormonal pathways.
The most rapid method for assessing start of flowering is to consider a population of tillers, as in a field trial, and determine the mean across that population, with 50% anthesis stated when half of all tillers are extruding anthers (Griffiths et al. Reference Griffiths, Simmonds, Leverington, Wang, Fish, Sayers, Alibert, Orford, Wingen, Herry, Faure, Laurie, Bilham and Snape2009). This method prohibits any differentiation between the MS and subsequent tillers and thus, provides a level of uncertainty relating to the variation in number of tillers per plant and therefore the duration of flowering per plant. To better characterize this variation, a fixed number of five tillers were scored per plant in a pot-based and a CE experiment. Analysis of flowering duration for individual tillers and the timing of the start of flowering between subsequent tillers has revealed a pleiotropic effect of the Ppd-1a alleles that is independent of environment. The presence of all Ppd-1b alleles led to the greatest overlap between tillers, such that at any one time during anthesis there were a greater number of open florets, whether considering dehiscing anthers or receptive stigma, compared with the presence of one or more Ppd-1a alleles. This greater overlap is likely to have been the major factor in reducing the overall time of flowering for an individual plant, with the dominant effect caused by the fourth and the fifth-flowering tiller. The relative timing of floret development and flowering between individual tillers may have particular relevance to floret survival and ultimately grain yield. González et al. (Reference González, Miralles and Slafer2011) demonstrated using multiple data sets that the initiation of floret death was linked to assimilate demand from the start of maximum stem extension, but no detail is given as to the number of tillers per plant. To provide insights into whether a greater distribution of tillers across time would be advantageous for greater floret survival, and thus seed number, experiments controlling the number of spikes per plant under different environments are needed. It can be hypothesized that the lower the floret overlap the greater the spread of assimilate demand across multiple spikes, resulting in higher seed numbers particularly under stress conditions.
In agreement with other literature (Greenup et al. Reference Greenup, Peacock, Dennis and Trevaskis2009), the Ppd-1a lines demonstrated temperature responsiveness for start of flowering, but also reduced the duration of flowering in the warmer environment. Of particular relevance is that under the warm CE regime, the difference between Ppd-1a alleles became less marked. There was a tendency for flowering duration for the whole plant to be shorter with increasing thermal time to flowering, with Ppd-1b lines flowering the latest and for the shortest duration, which was particularly pronounced in cooler conditions.
Stigma receptivity may occur over a number of days (Lukac et al. Reference Lukac, Gooding, Griffiths and Jones2012), whereas anther dehiscence can be identified to a single day. This may favour recording of anther dehiscence over the opening of florets and inspection of the stigma as a tool to score varieties. Indeed, visual assessment of the start of flowering commonly relies principally on the visible appearance of protruding anthers and has the advantage of being more rapid and not requiring the opening of individual florets compared with stigma assessments. In the CE experiments, the stigma receptivity exceeded the duration of anther dehiscence. The present analysis did not take into account the number of florets that flowered earlier, but did agree with the findings of Lukac et al. (Reference Lukac, Gooding, Griffiths and Jones2012) who identified a level of asynchronous behaviour in the maturity of stigma relative to the anther. The longer duration of stigma receptivity may have been caused by either (i) a delay in fertilization as a consequence of the stigma being receptive earlier than the time of pollen release and/or (ii) an influence of the environment which delayed the arrival of pollen on the active stigma. The relative synchrony in flowering was hypothesized by Lukac et al. (Reference Lukac, Gooding, Griffiths and Jones2012) to be linked to increased stress resilience due to greater probability of cross-pollination between heat damaged plants. The difference identified in female flowering duration relative to male duration in the present paper between all three lines carrying Ppd-D1a compared with Ppd-1b lines provides an opportunity to further test this hypothesis under extreme stress environments.
The present study allows the comparison of field-based, ambient pot-based and CE experiments. This had the advantage to allow for management of tiller number and detailed floret assessments in comparison with field-based assessment which integrates full plant development under agricultural conditions. The broad agreement between field-based and pot-based data validates data from the CE, but the pot-based work provides a means of a greater resolution of flower analysis. Furthermore, the difference in the start of flowering between Ppd-1a and Ppd-1b lines results in flowering starting on different calendar dates. In the field, this can result in genotype × environment (G × E) interaction; for example, one line that starts flowering on a warmer day would result in a shorter duration flowering than a line that starts anthesis 1 or 2 days later under cooler conditions. Under CE conditions, the G × E effect can be controlled. Furthermore, it has been suggested that the relative timing of the flowering of male compared with female floret parts may be more closely linked to environmental effects than genotype (reviewed in Waines & Hegde Reference Waines and Hegde2003). Thus, the CE experiments prevent anomalous results which are a risk in the field under conditions of drought or heat stress.
CONCLUSION
In conclusion, the present paper has quantified the differential effect of the three broad classes of PI alleles on spatial and temporal distribution of flowering in wheat. Further, as yet unexplored, variation in flowering phenology may exist for the individual haplotypes of these alleles. The work presented here is relevant for the major challenges of crop adaptation to climate stress. Based on the evidence from ‘Paragon’ introgressed with three separate Ppd-1a alleles, the authors claim that (i) Ppd-D1a is the most potent photoperiod insensitive allele; (ii) field, ambient pot-based experiments and CE experiments have their own relative merits in terms of dissecting a flowering phenotype as a consequence of the practicalities of scoring a large number of ears, and the relative control of the environment; (iii) flowering duration varies with genotype principally at the whole plant level; (iv) the Ppd-1a alleles resulted in lines flowering closer together under warmer conditions; and (v) FOI demonstrates a significant level of variation between genotypes which may interact with growth environment and can vary with the number of tillers per plant.
This work was funded by the Biotechnology and Biological Sciences Research Council (project H012176-1) with the exception of the field trials which were part of A. A.'s Ph.D. project, funded by the Saudi Government. The authors gratefully acknowledge the technical assistance of Caroline Hadley, Laurence Hansen and Richard Casebow. Data reported in this paper are available from the University of Reading Research Data Archive at http://dx.doi.org/10.17864/1947.42














