The manifestation of hyperlipidaemia, a significant risk factor for CVD, could be attributed to the consumption of a diet rich in energy content and fat (mainly SFA and n-6 fatty acids), which typically defines the Western diet(Reference Simopoulos1). Though hyperlipidaemia has been comprehensively studied in respect to CVD, its role in cognitive dysfunction (CD) needs to be explored. Cognitive decline can be accredited to genetic, environmental, nutritional and metabolic factors. It is known that chronic hyperlipidaemia increases oxidative stress and inflammation, and perennial exposure of organs to mediators of oxidative stress and inflammation may eventually cause dysfunction(Reference Ramaiyan, Bettadahalli and Talahalli2,Reference Acharya and Talahalli3) . The exposure of the brain and neuromuscular system to elevated superoxide radicals and proinflammatory mediators may subsequently manifest into CD(Reference Pistell, Morrison and Gupta4). Notably, activation of brain glial cells (microglia and astrocytes) due to oxidative stress ruptures the blood–brain barrier, causes inflammation and exaggerates the condition leading to CD. Increased proinflammatory cytokines levels affect the synaptic plasticity and exuberance of neurons (mostly hippocampal neurons), which are potential markers regulating cognitive functions. Cognitive decline can have a significant impact on affected individuals, as they compromise their ability to thinking, communication, understanding, memory and also coordination skills. Even relatively mild cognitive decline in early life due to metabolic abnormalities may cause functional dependence(Reference Lipnicki, Sachdev and Crawford5). Clinical interventions are less successful and have yielded mixed results in arresting the cognitive decline(Reference Bhome, Berry and Huntley6). Thus, preventive strategies through dietary bioactive molecules are often explored for enhanced results(Reference Spencer7).
Dietary constituents (curcumin, flavonoids, vitamins, PUFA, etc.) have been studied extensively for their effects on multiple brain processes, including regulation of synaptic transmission, membrane fluidity and signal transduction(Reference Gómez-Pinilla8,Reference Nissankara Rao, Kilari and Kola9) . Classically, fatty acids of the n-3 family, mainly long-chain n-3 fatty acids like EPA (20 : 5n-3) and DHA (22 : 6n-3), are under constant scrutiny to enhance human health and well-being, precisely due to their anti-inflammatory and immunomodulatory effects(Reference Laye, Nadjar and Joffre10). DHA is known to influence synaptic transmission and long-term potentiation in the hippocampus(Reference Connor, Tenorio and Clandinin11). DHA protects neurons from inflammatory pathogenesis in chronic degenerative diseases by maintaining neural synaptic action potentials(Reference Elinder and Liin12). However, long-chain unsaturated fatty acids like EPA + DHA are the likely victims of peroxidation damage, and its preservation from oxidative insults in cellular membranes is critical. To counter the oxidative damage, we explored the efficacy of zerumbone, a sesquiterpene present in the Zingiberaceae family. Zerumbone (2,6,9,9-tetramethyl-[2E,6E,10E]-cycloundeca-2,6,10-trien-1-one) is a monocyclic sesquiterpene with three double bonds, two conjugated and one isolated, as well as a conjugated carbonyl group, in an 11-membered ring structure(Reference Kalantari, Moniri and Boroumand13). Since hyperlipidaemia causes burst in superoxide generation, administration of zerumbone with proven anti-inflammatory(Reference Chien, Chen and Lee14,Reference Sulaiman, Perimal and Akhtar15) and antioxidant(Reference Murakami, Takahashi and Kinoshita16) properties under hyperlipidaemic conditions may augment modulatory potentials of EPA + DHA. We selected rats as a model system to establish the cognitive modulatory potentials of EPA + DHA and zerumbone under hyperlipidaemic conditions. We fed a high-fat diet to induce hyperlipidaemia, as rats are known to respond to changes in the dietary lipids. Besides, the behavioural pattern and brain anatomy in rats are well established, and results can be interpreted without ambiguity. We hypothesised that high-fat feeding to rats induces hyperlipidaemia and may result in CD, and administration of EPA + DHA and zerumbone at levels that can be rationally adopted in human dietary practices may beneficially modulate the hyperlipidaemia-induced CD. Hence, we tested the modulatory potentials of dietary EPA + DHA, zerumbone, and their combination on the cognitive parameters under hyperlipidaemic conditions in rats.
Materials and methods
Materials
Lard was purchased from the local market. Refined fish oil was procured from Janatha fish meal and products Ltd Udupi, India. Casein was obtained from Nimesh Corporation. AIN-93-based mineral mix and vitamin mixes were procured from Hi-Media Pvt Ltd. Zerumbone (99·9 % pure) was extracted from ginger as per the procedure described earlier(Reference Kumar, Negi and Manjunatha17). Briefly, the fresh rhizomes of Zingiber zerumbet (100 g) were cut into small pieces, crushed to a paste, added 500 ml of water and hydro-distilled for 15 h. The volatile oil (0·9 ml) collected was stored at –80°C for 4 h. The crystal of zerumbone was separated by decanting the oil and recrystallised from petroleum ether (60–80°C). Upon vacuum drying in a desiccator for 12 h over calcium chloride, pure crystals of zerumbone (0·53 g) were obtained and authenticated by physical and spectral studies. All the diets were stored in airtight pouches flushed with N2, kept at the refrigerated condition.
Animal model, diet, feeding and growth parameters
The experimental animal procedures followed in this study were approved (approval number CFT/IAEC/120/2018) and carefully monitored by the Institutional Animal Care and Use Committee of CSIR-Central Food Technological Research Institute. The authorised trainers gave comprehensive training to the animal handlers of this study, and extensive care was taken during the experimental procedures not to cause any distress to the animals. The animal experiment was conducted from 30 September 2018 to 30 November 2018. Thirty male Wistar rats (OUTB-Wistar, IND-cft (2C)) (Rattus norvegicus) weighing 50 ± 5 g were randomised and individually housed under 12 h light and dark cycle with water and standard chow diet available ad libitum. After acclimatisation, the rats (n 6) were assigned to the respective diet based on AIN-76 (Table 1) dietary guidelines(Reference Diet, Diet and Diet18). The feeding trial was carried out for 30 d, and after the completion of experimental procedures, the rats were killed using carbon dioxide anaesthesia. The control diet had 7 % lard, high-fat lard (HF) had 35 % lard, high-fat fish oil ((HF+F), source of EPA (10·9 mg/100 mg fat) + DHA (9·02 mg/100 mg fat)) had 17·5 % fish oil + 17·5 % lard, high-fat zerumbone (HF+Z) had 35 % lard and zerumbone (200 mg/kg body weight) and high-fat fish oil zerumbone (HF+F+Z) had 17·5% fish oil + 17·5 % lard + 200 mg zerumbone/kg body weight.
Table 1. Composition of the diets (g/kg)
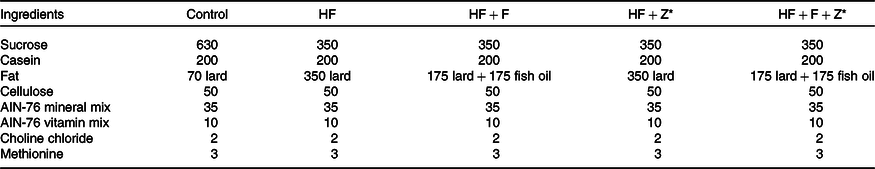
HF, high-fat; HF + F, high-fat + fish oil; HF + Z, high-fat + zerumbone; HF + F + Z, high-fat + fish oil + zerumbone; AIN, American Institute of Nutrition.
* Zerumbone was administered orally at 200 mg/kg body weight to rats in the HF + Z and HF + F + Z groups.
Diets were stored in airtight pouches, flushed with N2 and stored at refrigerated conditions to prevent oxidation. The fatty acid compositions of diet are given in Table 2. The dietary fatty acid compositions indicated that control and HF diet had a similar fatty acid composition, wherein 16 : 0, 18 : 1n-9 and 18 : 2n-6 were found to be at 25, 57 and 13 %, respectively. The HF + F diet had EPA + DHA at 19 % of total fatty acids, whereas control and HF diet did not have any EPA + DHA. Growth parameters in control experimental groups are given in Table 3. The growth parameter’s change observed in the HF + F and HF + Z compared with control was statistically not significant. However, the bodyweight of the HF + F + Z group was found to be reduced by 11 % compared with control. The decrease in the relative weight of the brain (to body weight) in HF, HF + F and HF + F + Z was found to be very minimal and not statistically significant. However, the relative weight of the brain in the HF + F + Z group was found to be increased by 12 % compared with control.
Table 2. Fatty acid composition of the diets
(Mean values of triplicate values)
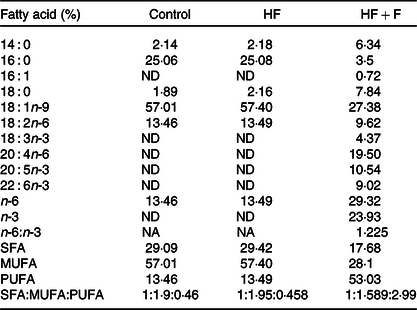
HF, high-fat; HF + F, high-fat + fish oil; ND, not detected; NA, not applicable.
Table 3. Growth parameters
(Mean values and standard deviations; n 6)
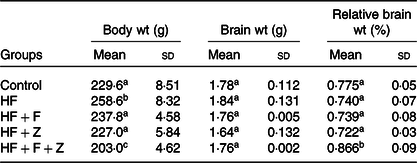
HF, high-fat; HF + F, high-fat + fish oil; HF + Z, high-fat + zerumbone; HF + F + Z, high-fat + fish oil + zerumbone.
a,b,c Mean values within a column with unlike superscript letters are significantly different (P < 0·05).
Behavioural studies
After 30 d of feeding respective diets, the rats were subjected to behavioural studies, and the behavioural tests were carried out after the acclimatisation of the animals to the testing room.
Morris water maze test
Rats were kept in a place where they cannot see the black circular pool/spatial cues prior to the beginning of the test(Reference Bromley-Brits, Deng and Song19). The circular platform (10 cm diameter) was placed in the pool with water maintained at room temperature. The pool dimension was calibrated using a camera connected to All-maze video tracking software. The pool was divided into four different quadrants, and the platform location was entered into the software. The maximum time for each trial was set to 60 s, and the parameters like escape latency, path length and time spent in each quadrant were recorded.
Elevated maze-transfer latency
Spatial learning and long-term memory were assessed using elevated maze apparatus as per the method described earlier(Reference Itoh, Nabeshima and Kameyama20). The study was conducted with a 3 min trial and one trial per d, and the behaviour was recorded to interpret transfer latency.
T-maze spontaneous alternation
T-maze test with spontaneous alteration parameters was employed to assess the spatial working memory as per the protocol described earlier(Reference Mishima, Tanoue and Tsuda21). The rats were placed at the base of the long vertical arm and allowed to explore the maze during a 5 min test session. The sequence of arm entries was recorded (arm entry was counted if both the head and the base of the tail entered the arm). A correct alternation was defined as a non-repeated entry into the arms for three consecutive entries. The spontaneous alternation percentage was calculated using the equation; ((correct alterations/total no of alterations) – 2) × 100. Each arm of the T-maze is cleaned between sessions using ethanol to remove any olfactory hints, which may affect the behaviour of the next rat tested.
The rotarod performance test
The rotarod performance test (accelerating speed) was conducted as per the method described earlier(Reference Jones and Roberts22). Briefly, the rats were placed on the horizontally rotating rod. The initial speed of the rotating arm was adjusted at 10 rpm from 0 to 30 s, 15 rpm from 30 to 60 s, 20 rpm from 60 to 120 s, 25 rpm from 120 to 160 s, 30 rpm from 160 to 220 s, 35 rpm from 220 to 280 s, 40 rpm from 280 to 300 s. Passive rotation is considered as latency fall, and the fall time was recorded.
Open field test for locomotor activity
The open field test comprehensively assesses locomotion as well as behavioural activity(Reference Tatem, Quinn and Phadke23). Rats were acclimatised in the testing room 30 min before the study and to the apparatus for a period of 5 min. The animal behaviour was monitored by All-Maze video tracking software, and the parameters, including total distance travelled, movement time and resting time, were recorded. Rearing/grooming behaviours like face washing, body and genital grooming, body and paw licking and scratching behaviours were counted manually.
Statistical analysis
The sample size (minimum n 6/group) was determined based on nutritional intervention studies reported earlier, with a statistical power of 0·8 and α error of 0·05. The data were analysed by one-way ANOVA (non-parametric), followed by Tukey’s test using Graph Pad Prism version 7.04 (GraphPad Software; www.graphPad.com), and a P value < 0·05 was considered as statistically significant. Only the mean and standard deviation for each diet group are plotted in each figure.
Results
Behavioural assessment for spatial learning and memory
Spatial learning and memory retention were assessed by the Morris water maze animal behaviour test. On day 0 for visible platform test, all five group rats (6/6) exhibited a similar latency and had identical swimming distance before escaping onto the visible platform without significant difference (data not shown). Fig. 1(a) represents the escape latency of rats in hidden platform tests (day 1 to day 4) in control and experimental groups. HF rats took significantly (P < 0·05) longer time to reach the platform compared with control, whereas compared with HF rats, experimental (HF + F, HF + Z and HF + F + Z) rats took significantly (P < 0·05) shorter time to reach the platform. Fig. 1(b) represents path length taken by rats in hidden platform tests in control and experimental groups. HF rats took significantly (P < 0·05) longer path length to reach the platform compared with control and experimental (HF + F, HF + Z and HF + F + Z) rats. Whereas administration of zerumbone with EPA + DHA reduced the path length taken by rats in hidden platform tests compared with HF + F rats. Fig. 1(c) represents passing time on day 5 in control and experimental groups. Among the groups tested, HF rats spent significantly (P < 0·05) shorter time (41·5 %) in the quadrant where the platform was previously placed. Whereas compared with HF rats, rats in HF + F, HF + Z and HF + F + Z groups spent significantly (P < 0·05) longer time (55, 62 and 80 %, respectively) in the quadrant where the platform was previously placed. Fig. 2 represents the images of the Morris water maze probe trial on day 5 without a platform for the assessment of memory retention.

Fig. 1. Morris water maze test for assessment of long-term memory. (a) Escape latency on day 1 to day 4. (b) Path length on day 1 to day 4 and (c) passing time on day 5. Values are means and standard deviations of six rats. (a, b) ![]() , Control;
, Control; ![]() , high fat (HF);
, high fat (HF); ![]() , high-fat + fish oil (HF + F);
, high-fat + fish oil (HF + F); ![]() , high-fat + zerumbone (HF + Z);
, high-fat + zerumbone (HF + Z); ![]() , high-fat + fish oil + zerumbone (HF + F + Z). (c)
, high-fat + fish oil + zerumbone (HF + F + Z). (c) ![]() , Control;
, Control; ![]() , HF;
, HF; ![]() , HF + F;
, HF + F; ![]() , HF + Z;
, HF + Z; ![]() , HF + F + Z.
, HF + F + Z.

Fig. 2. Morris water maze probe trial test without platform on day 5 for assessment of memory retention. Note: the number 3 indicates quadrant where platform was placed earlier, dotted lines indicate the passing pattern of rats in each quadrant. HF, high-fat; HF + F, high-fat + fish oil; HF + Z, high-fat + zerumbone; HF + F + Z, high-fat + fish oil + zerumbone.
Further, the learning behaviour was assessed by the transfer latency test with elevated maze apparatus for five successive days (Fig. 3). Transfer latency after day 2 was found to be significantly (P < 0·05) shorter than day 1 in control, HF + F, HF + Z and HF + F + Z rats. On day 5, HF rats took significantly (P < 0·05) longer time to reach the enclosed arm of the elevated plus-maze compared with control, HF + F, HF + Z and HF + F + Z rats. Whereas compared with HF rats, HF + F, HF + Z and HF + F + Z fed rats took significantly (P < 0·05) shorter time to reach the enclosed arm. The spatial working memory performance was assessed by the T-maze spontaneous alternation test (Fig. 4). The HF rats showed significantly (P < 0·05) poor performance (as measured by percentage alternation) in the task when compared with control, HF + F, HF + Z and HF + F + Z rats. Whereas compared with HF rats, HF + F, HF + Z and HF + F + Z rats showed significantly (P < 0·05) improved percentage alternation. Similarly, compared with HF + F rats, HF + Z and HF + F + Z rats showed significantly (P < 0·05) improved percentage alternation.

Fig. 3. Elevated maze-transfer latency test on day 1 to day 5. Values are means and standard deviations of six rats. ![]() , Control;
, Control; ![]() , high-fat (HF);
, high-fat (HF); ![]() , high-fat + fish oil;
, high-fat + fish oil; ![]() , high-fat + zerumbone;
, high-fat + zerumbone; ![]() , high-fat + fish oil + zerumbone.
, high-fat + fish oil + zerumbone.

Fig. 4. T-maze spontaneous alternation test. Values are means and standard deviations of six rats. ![]() , Control;
, Control; ![]() , high-fat (HF);
, high-fat (HF); ![]() , high-fat + fish oil (HF + F);
, high-fat + fish oil (HF + F); ![]() , high-fat + zerumbone (HF + Z);
, high-fat + zerumbone (HF + Z); ![]() , high-fat + fish oil + zerumbone.
, high-fat + fish oil + zerumbone.
Assessment for the locomotory behaviour
Impairment in motor coordination as a result of brain function deterioration was assessed by a rotating rod with accelerating speed. Fig. 5(a) describes the mean latency fall in control and experimental groups. The latency fall of HF fed rats was significantly (P < 0·05) decreased compared with control rats. Whereas compared with HF rats, HF + F, HF + Z and HF + F + Z rats had significantly (P < 0·05) increased latency to fall. Fig. 5(b) represents the average speed at fall in control and experimental rats. The speed at the fall of HF rats was significantly (P < 0·05) less compared with HF + F, HF + Z and HF + F + Z rats. Whereas compared with HF + Z rats, HF + F + Z rats had increased speed at fall.
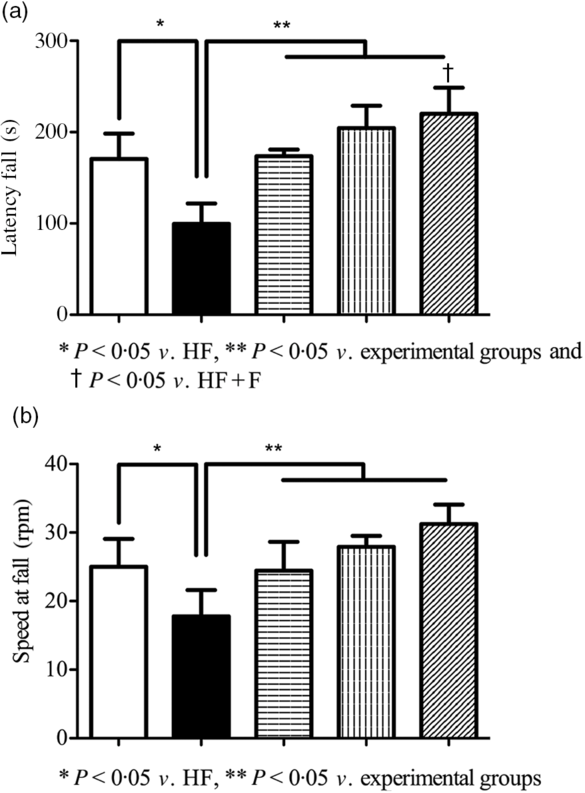
Fig. 5. Rotarod-accelerated speed test. (a) Latency fall in seconds and (b) speed at fall in rpm. Values are means and standard deviations of six rats. ![]() , Control;
, Control; ![]() , high-fat (HF);
, high-fat (HF); ![]() , high-fat + fish oil (HF + F);
, high-fat + fish oil (HF + F); ![]() , high-fat + zerumbone;
, high-fat + zerumbone; ![]() , high-fat + fish oil + zerumbone.
, high-fat + fish oil + zerumbone.
Further, the locomotion impairments were confirmed by the open field locomotion test (Fig. 6). HF rats covered significantly (P < 0·05) less distance than control rats. Whereas compared with HF rats, control and HF + F, HF + Z and HF + F + Z rats covered significantly (P < 0·05) more distance within the open filed apparatus (Fig. 6(a)). Fig. 6(b) represents movement time in a sec. HF rats showed significantly (P < 0·05) decreased movement time than control and HF + F, HF + Z and HF + F + Z rats. Whereas compared with HF rats, HF + F, HF + Z and HF + F + Z rats showed significantly (P < 0·05) increased movement time within the open field apparatus. Fig. 6(c) describes the resting time within the apparatus. HF rats spent significantly (P < 0·05) more time in resting compared with control and HF + F, HF + Z and HF + F + Z rats. Whereas compared with HF rats, HF + F, HF + Z and HF + F + Z rats showed significantly (P < 0·05) less resting time within the open field apparatus. However, there was no significant difference in grooming behaviour in any of the groups (Fig. 6(d)).

Fig. 6. Open field locomotion test. (a) Distance travelled within the open field apparatus in seconds, (b) movement time within the open field apparatus in seconds, (c) resting time within the open field apparatus in seconds and (d) grooming/rearing behaviour within the open field apparatus. HF, high-fat; HF + F, high-fat + fish oil; HF + Z, high-fat + zerumbone; HF + F + Z, high-fat + fish oil + zerumbone. Values are means and standard deviations of six rats. ![]() , Control;
, Control; ![]() , high-fat (HF);
, high-fat (HF); ![]() , high-fat + fish oil (HF + F);
, high-fat + fish oil (HF + F); ![]() , high-fat + zerumbone (HF + Z);
, high-fat + zerumbone (HF + Z); ![]() , high-fat + fish oil + zerumbone.
, high-fat + fish oil + zerumbone.
Discussion
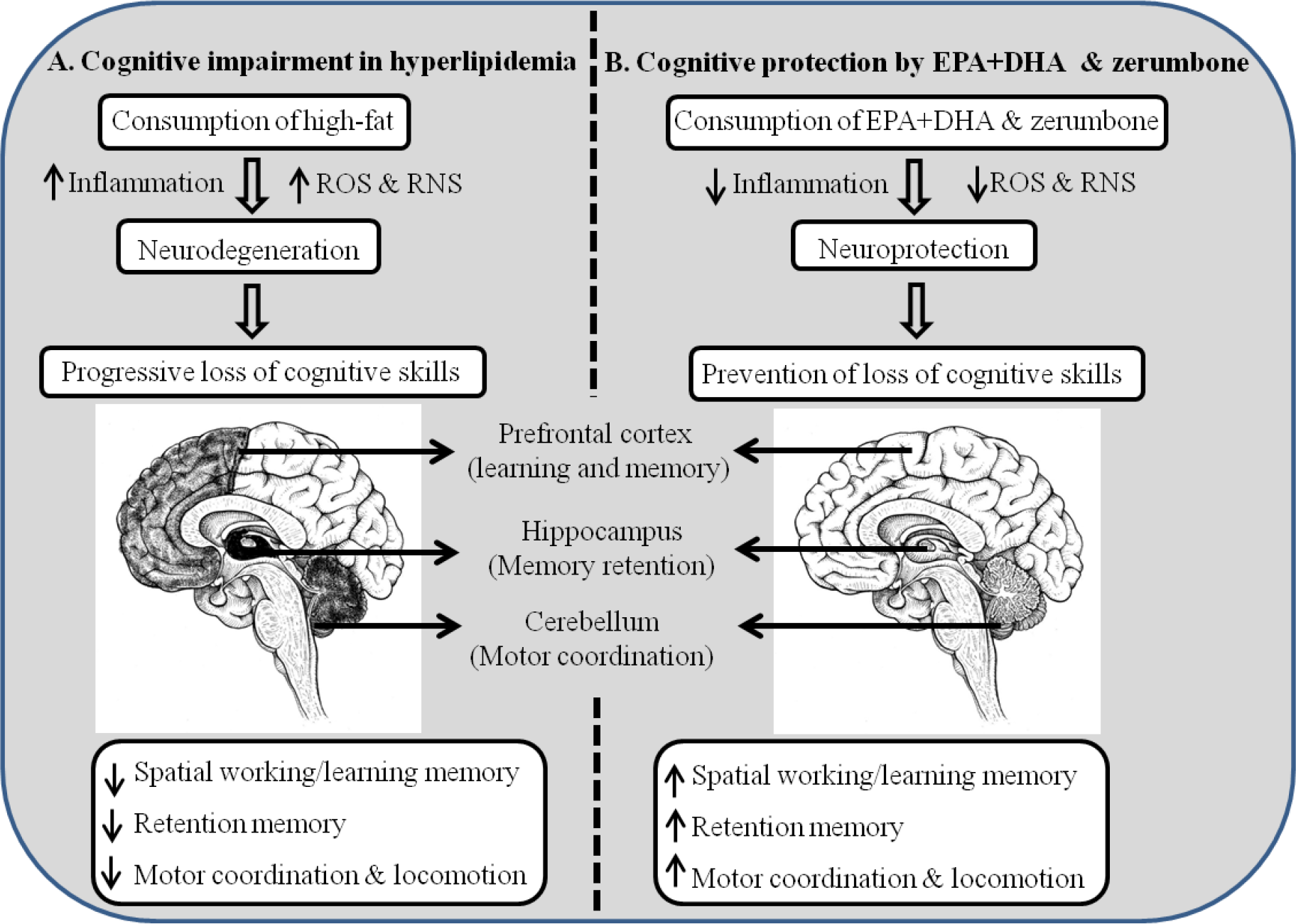
In this study, we established the link between hyperlipidaemia and cognition and showed that EPA + DHA and zerumbone ameliorate hyperlipidaemia-induced CD. Though the potentials of EPA + DHA as neural signal modulators to improve cognition is well established(Reference Cederholm, Salem and Palmblad24), its intervening efficacy on CD caused by hyperlipidaemia has never been measured. In this study, the effect of zerumbone was tested individually, and also in combination with EPA + DHA, as zerumbone is a potential antioxidant molecule and may support brain cell’s functional integrity by protecting the EPA + DHA from oxidative insults under hyperlipidaemic conditions(Reference Ramaiyan, Bettadahalli and Talahalli2,Reference Rosa, Caprioglio and Isola25) . In addition, it has been shown that zerumbone exhibits physicochemical properties suitable for higher bio-availability and blood-brain barrier (BBB) permeability(Reference Hwang, Youn and Ji26). The hippocampus plays a critical role in the consolidation of information from short-term memory to long-term memory, and in spatial memory that enables navigation. The hippocampus-dependent spatial learning and memory, as evaluated by Morris water maze for the visible platform (non-spatial memory), did not show any difference for the escape latency and path length on the training day (day 0). The above observation indicates that rats in all the groups can see the platform and the cues and can swim acceptably confirming normal vision. The decrease in escape latency and path length over the successive days (day 1 to day 4) for spatial learning in rats administered with EPA + DHA and zerumbone indicate their cognition-protective effects under hyperlipidaemic conditions. Memory retention, as measured by the target quadrant approach without a platform on day 5, revealed that HF exhibited lowest passing time, that is, the least time spent in the quadrant where previously the platform was placed. Whereas the administration of EPA + DHA and zerumbone prevented memory loss, while the EPA + DHA, together with zerumbone, resulted in maximum prevention of memory loss. Similarly, transfer latency test, an indicative of spatial learning memory, and also short-term working memory further underlined the synergistic effects of EPA + DHA and zerumbone in preventing hyperlipidaemia-induced cognitive deterioration. The poor performance from HF rats indicates the damaging effects of high-fat (in the absence of EPA + DHA and zerumbone) on the hippocampus/cortex region of the brain, which are primarily involved in learning and memory. Our results are in agreement with earlier reports that demonstrate cognitive decline due to long-term consumption of high-fat diet rich in saturated fats(Reference Lam, Stephenson and Nesbit27,Reference Almeida-Suhett, Graham and Chen28) . Motor coordination involves body movements comprising kinematic (such as spatial direction) and kinetic (force) parameters that result in the execution of an action/skill. Our study demonstrated that hyperlipidaemia dampens coordination skills, while administration of EPA + DHA and zerumbone prevented hyperlipidaemia-induced dampening of motor coordination(Reference Lange, Makulska-Gertruda and Reisinger29,Reference Janssen, Zerbi and Mutsaers30) . The poor performance of the HF group can be correlated to alterations in the normal functioning of the hippocampus, cortex, cerebellum and hypothalamus as they are mainly involved in the balanced regulation between the central and peripheral nervous system. Motor coordination deficits are a sequela of brain damage as a result of impairment in the sympathetic (thoracolumbar region) and parasympathetic pathways (craniosacral division) of the autonomous nervous system. Previous results showed that high-fat feeding causes oxidative stress in crucial areas of the brain, including the hippocampus(Reference Freeman and Keller31).
The present investigation in the rat model established a possible link between hyperlipidaemia and CD. Collectively, our data imply that hyperlipidaemia causes CD by decreasing the memory and motor coordination skills, and administration of EPA + DHA and zerumbone prevents hyperlipidaemia-induced CD (Fig. 7). The augmented effect of EPA + DHA, together with zerumbone, discloses a promising strategy for lowering the severity of CD in hyperlipidaemic conditions. The concentrations of EPA + DHA and zerumbone employed in this study may be reasonably adapted to human applications, as both the dietary molecules are present in sources (EPA + DHA and zerumbone present in marine fish and ginger, respectively) that are generally consumed across the population. The zerumbone concentration used in this study (200 mg) is achievable in the human diet through intake of dry ginger powder (8 g) over multiple dose as we could isolate 500 mg of zerumbone/100 g fresh weight(Reference Sulaiman, Perimal and Akhtar15). Since ginger contains approximately 80 % water, 8 g dry ginger powder, when incorporated in the diet, can yield the zerumbone concentration as employed in the present study. Thus, the administration of EPA + DHA and zerumbone may further benefit the clinical intervention of neurodegenerative diseases. However, in-depth studies on the brain profiling for oxidative stress and inflammatory mediators that have a bearing on neurodegeneration under hyperlipidaemic conditions and the mechanistic role played by EPA + DHA and zerumbone in modulating the cognitive parameters need to be studied.

Fig. 7. Graphical representation of cognitive function modulatory potentials of EPA + DHA and zerumbone in hyperlipidaemic conditions in rats. (a) Cognitive impairment induced under hyperlipidaemia and (b) cognitive protection by EPA + DHA and zerumbone. ROS, reactive oxygen species; RNS, reactive nitrogen species.
Acknowledgements
V. U. acknowledged DBT, New Delhi, for the award of his Research Fellowship.
A part of this work was financially assisted by GAP-462 Nutrition Biology.
V. U. was responsible for the execution of the experiment; B. B. K. was responsible for the zerumbone extraction; P. A. was responsible for data assessment with V. U.; R. R. T. was responsible for the research question, design, data assessment and manuscript writing. All the authors contributed to this manuscript and approved the final version.
The authors declare that there are no conflicts of interest.